Abstract
The concept of the neurovascular unit emphasizes that common signals and substrates underlie the physiology and pathophysiology of neuronal and endothelial compartments in brain. Recent data suggest that activation of the integrin-associated protein CD47 promotes neuronal cell death. Is it possible that CD47 may also negatively affect cerebral endothelial cells? Exposure of wild-type primary mouse cerebral endothelial cells to the CD47 ligand thrombospondin 1 (TSP-1) induced an increasing amount of cell death, whereas cytotoxicity was significantly decreased in cerebral endothelial cells derived from CD47 knockout mice. The specific CD47-activating peptide, 4N1K, similarly induced cell death in human brain microvascular endothelial cells. Promotion of inflammation was also involved because lower TSP-1 was able to up-regulate the adhesion molecules inter-cellular adhesion molecule-1 and vascular cell adhesion molecule-1. Finally, CD47 signaling may suppress angiogenesis because 4N1K significantly inhibited endothelial cell migration and tube formation in vitro. We conclude that CD47 signaling can negatively affect the viability and function of cerebral endothelial cells, further supporting the notion that CD47 may be a potential neurovascular target for stroke and brain injury.
Keywords: CD47, cell death, inflammation, angiogenesis, endothelial cell
CD47 (also known as integrin-associated protein) is a member of the immunoglobulin superfamily that plays an important role in blood cell homeostasis and inflammation. By interacting with various beta integrins, CD47 modulates monocyte motility, leukocyte adhesion and migration, phagocytosis, and platelet activation (Brown and Frazier, 2001). Furthermore, by interacting with various ligands, including thrombospondins (TSPs) and signal regulatory proteins, CD47 can also trigger cell death mechanisms in T cells (Manna and Frazier, 2003), monocytes and dendritic cells (Johansson et al., 2004), and leukemia cells (Mateo et al., 1999, 2002; Saumet et al., 2005; Bras et al., 2007).
Recently, CD47 has been found to also play a critical role in the central nervous system. Activation of CD47 signaling induced novel caspase-dependent and caspase-independent cell death mechanisms in neurons (Koshimizu et al., 2002; Xing et al., 2009). Hence, in the context of stroke and brain injury, overactivation of the CD47 pathway may lead to multiple deleterious effects in brain. These would potentially include promotion of inflammation by enhancing neutrophil infiltration, secondary thrombosis by augmenting platelet aggregation, and parenchymal damage by triggering cell death in neurons. Recently, we showed that CD47 gene knockout reduced infarction after focal cerebral ischemia (Jin et al., 2009). Because CD47 can be found on endothelial cells, we decided to ask whether CD47 may also contribute to neurovascular dysfunction relevant to stroke and brain injury. Specifically, we assessed the role of CD47 signaling in cell death, inflammation and angiogenesis in cerebral endothelial cells in culture.
MATERIALS AND METHODS
Reagents
Dulbecco’s Modified Eagle Medium (DMEM)/nutrient mixture F-12 (F-12), RPMI 1640 media, 0.05% trypsin-EDTA, L-glutamine, fetal bovine serum, horse serum, sodium pyruvate, minimum essential medium (MEM) nonessential amino acids, MEM vitamins, and antibiotics for cell culture were from Invitrogen (Carlsbad, CA). Growth factor-reduced Matrigel Matrix, collagen I, endothelial cell growth supplement, and NuSerum were purchased from BD Biosciences (San Jose, CA). TSP-1 was purchased from Calbiochem (San Diego, CA). 4N1K (KRFYVVMWKK) was from Sigma Genosys (The Woodlands, Texas), and was dissolved in sterile ddH2O at a concentration of 100 mg/ml as a stock solution. This stock was formed into aliquots and stored at −80°C. Cytotoxicity Detection Kit LDH (lactate dehydrogenase) was from Roche Diagnostics (Mannheim, Germany). Enzyme-linked immunosorbent assay (ELISA) kits of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin were purchased from Thermo-Scientific/Pierce (Rockfold, IL). Heparin, MTT (3-(4,5-dime-thylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), and thymidine were from Sigma-Aldrich (St. Louis, MO).
Cerebral Endothelial Cell Cultures
All experiments were approved by Institutional Animal Care and Use Committee following the NIH Guide for the Use and Care of Laboratory Animals. Standard procedures were used to obtain mouse primary cerebral endothelial cells (Guo et al., 2008). Briefly, primary mouse brain microvascular endothelial cells were cultured from the cerebrum of 3–5-week-old male wild-type or CD47 knockout mice, purified with anti-murine CD31 precoated M-450 Dynabeads (Dynal), and plated onto tissue culture dishes coated with collagen I in complete culture medium (DMEM/F-12 containing 10% fetal bovine serum [FBS], 10% horse serum, 0.1 mg/ml endothelial cell growth supplement, 0.1 mg/ml heparin, and 100 units/ml penicillin/streptomycin). Mouse endothelial cells were split at a ratio of 1:2 and used at passages 2–3. To determine the effects of CD47 signaling, wild-type or CD47 knockout endothelial cells were incubated with different doses of TSP-1 (100, 500, and 1,000 ng/ml) for 24 hr. Cytotoxicity was evaluated with MTT assay (at least three times in triplicate). We also tested a human brain microvascular endothelial cell line, previously confirmed to express brain endothelial phenotypes (Callahan et al., 2004; Guo et al., 2008). Endothelial cells were cultured in RPMI 1640 supplemented with 10% FBS, 10% NuSerum, 1mM sodium pyruvate, MEM nonessential amino acids, MEM vitamins, and 100 units/ml penicillin/streptomycin, and used to study the effects of the CD47-spe-cific peptide 4N1K. Endothelial cells were incubated with different amounts of 4N1K (25, 50, and 100 μg/ml) for 24 hr, and cytotoxicity was evaluated by the standard LDH release assay (at least three times in triplicate).
Adhesion Molecule ELISA Assays
Human brain microvascular endothelial cells were incubated with 500 ng/ml TSP-1 for various times (2, 4, or 8 hr). The levels of ICAM-1, VCAM-1, and E-selectin in culture supernatant were determined by ELISA kits, according to the manufacturer’s instructions.
In Vitro Angiogenesis Assays
Cell migration and tube formation tests were used as indirect in vitro assays to assess angiogenesis. The human brain endothelial cell line was plated on 60-mm culture dishes at 90% confluence. A wound was made across the cells with a razor blade, then the cultures were washed with serum-free medium and further incubated in RPMI 1640 with 1% FBS, 1 mM thymidine, with or without 4N1K (100 μg/ml). Cells were allowed to migrate for 24 hr, then rinsed with PBS, followed by fixing with methanol and staining with Giemsa. The numbers of endothelial cells that moved across the injury line were counted as a standard marker of migration. Data were analyzed as a percentage of migration in untreated endothelial cells. The standard Matrigel assay was used to assess the spontaneous formation of capillary-like structures by the human brain endothelial cell line. Cells (6 × 104 cells/well) were seeded in 24-well plates previously coated with growth factor reduced Matrigel, and incubated at 37°C for 18 hr with or without 4N1K. The degree of tube formation was determined by counting the number of tubes in four random fields from each well. Data were analyzed as a percentage of the tube numbers in untreated control wells.
Statistical Analysis
All data were expressed as mean ± SD. Three to five separate experiments were performed for each study. Data were analyzed by Student’s t test or ANOVA with Tukey post hoc tests (SPSS version 11.5; SPSS, Chicago, IL). Statistical significance was set at P < 0.05.
RESULTS
CD47 Mediates TSP-induced Cytotoxicity in Cerebral Endothelial Cells
Cytotoxic effects of CD47 were evaluated with standard assays for MTT and LDH release. Exposure to the CD47 ligand TSP-1 (100 to 1,000 ng/ml) for 24 hr induced increasing amounts of cell death in primary cultured wild-type cerebral endothelial cells. Compared with this wild-type response, levels of endothelial cytotoxicity were significantly reduced in CD47 knockout mouse cells (Fig. 1A). Of course TSP-1 can bind other receptors besides CD47. So we sought to further confirm our findings with 4N1K, a CD47-specific activating peptide. Exposure to 4N1K (25 to 100 μg/ml) for 24 hr similarly induced a cytotoxic response in human brain endothelial cells (Fig. 1B). However, we failed to detect a clear dose response in this case. Only the highest concentration of 4N1K (100 μg/ml) yielded a statistically significant cytotoxicity.
Fig. 1.
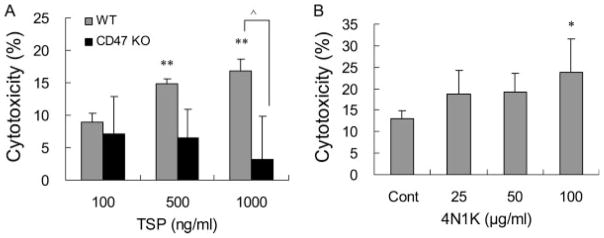
CD47-mediated TSP-1-induced cytotoxicity in endothelial cells. A: Exposure to CD47 ligand TSP-1 for 24 hr induced an increasing amount of cell death in wild-type cerebral endothelial cells. TSP-1-induced cytotoxicity was significantly decreased in brain endothelial cells from CD47 knockout mice. B: CD47-specific activating peptide 4N1K similarly induced a cytotoxic response in human brain endothelial cells. *P < 0.05, **P < 0.01 compared with the controls; ●P<0.01 between wild-type and CD47 knockout groups.
TSP Up-regulated the Inflammatory Markers ICAM-1 and VCAM-1
To assess the effects on inflammation, we measured responses of three representative endothelial cell adhesion molecules: ICAM-1, VCAM-1 and E-selectin. Exposure of human brain endothelial cells to low concentrations of TSP-1 (500 ng/ml) triggered a rapid and clear up-regulation in ICAM-1 and VCAM-1 within 2 to 8 hr (Fig. 2). However, no changes are detected for E-selectin (Fig. 2).
Fig. 2.
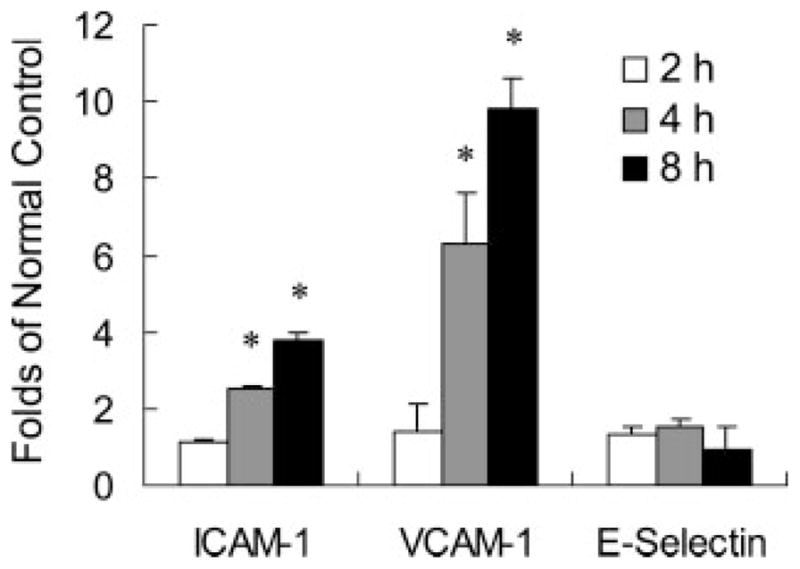
TSP-1 up-regulates the expression of ICAM-1 and VCAM-1 in brain endothelial cells. Exposure of human brain endothelial cells to a low dose of the CD47 ligand TSP-1 (500 ng/ml) triggers an up-regulation in the inflammatory markers ICAM-1 and VCAM-1 within 2 to 8 hr. No changes are detected for E-selectin; n = 3 independent experiments in triplicate. *P < 0.05 compared with controls.
4N1K Inhibits Endothelial Cell Migration and Tube Formation In Vitro
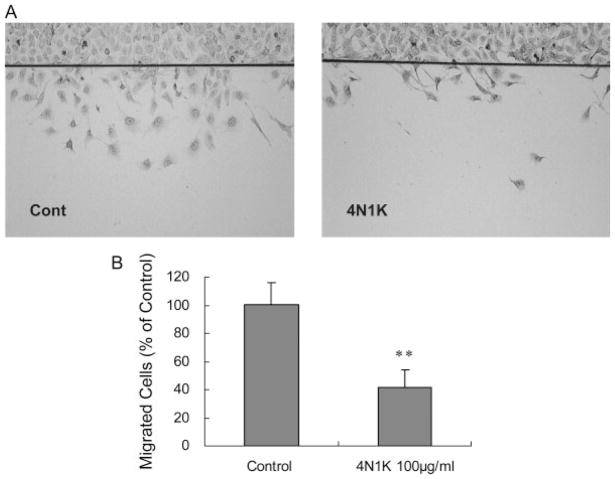
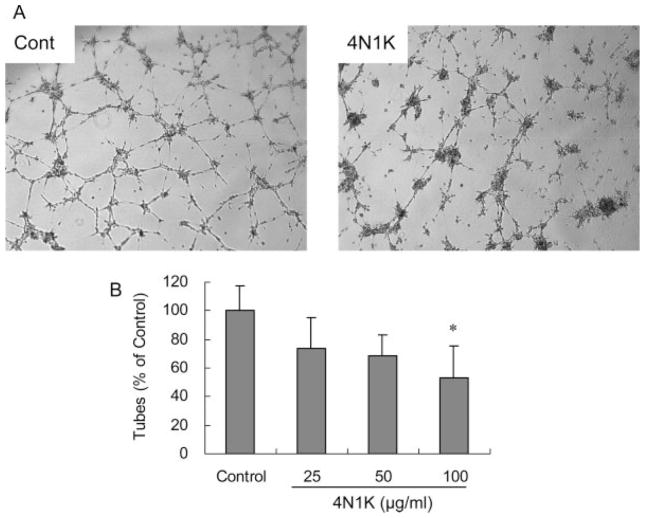
Confluent monolayers of human brain endothelial cells were subjected to scratch wounds, and then incubated with the CD47-specific peptide 4N1K (100 μg/ml) for 24 hr. Compared with untreated controls, 4N1K significantly suppressed endothelial cell migration by almost 50% (Fig. 3A, B). On a standard Matrigel assay, the human brain endothelial cells underwent spontaneous alignment over 18–20 hr, and fused into continuous tubes with distinct lumens to form capillary-like structures (Fig. 4A). Compared with untreated cultures, 4N1K significantly reduced the formation of tubes by about 40%–50% (Fig. 4B).
Fig. 3.
The CD47 activating peptide 4N1K inhibits cell migration in brain endothelial cells. A: Representative photographs showed decreased migration of human brain endothelial cells across a wound scratch line after treatment with 4N1K (100 μg/ml). B: Quantified cell counts showed a significant difference in cell migration between controls and 4N1K-treated cells. **P < 0.01.
Fig. 4.
The CD47 activating peptide 4N1K inhibits Matrigel tube formation in vitro. A: Representative photographs of human brain endothelial cells seeded on Matrigel-coated wells after 18 hr. Untreated cells formed the connected tubular networks. 4N1K (100 μg/ml) attenuated network formation. B: Quantified cell counts showed a significant difference in tube formation between controls and 4N1K-treated cells. Data are expressed as a percentage of the number of tubes in untreated wells. *P < 0.05 compared with the controls.
DISCUSSION
The neurovascular unit provides a conceptual framework wherein stroke is investigated as an integrative pathophysiology of both vascular and neuronal compartments (Lo et al., 2003; Iadecola, 2004; Zlokovic, 2005; Abbott et al., 2006; Lok et al., 2007). The importance of this concept has been supported by many recent studies showing that common signals and substrates underlie neuronal and vascular biology (Mazzone and Carmeliet, 2008). And after cerebral ischemia, most neuronal mediators can also participate in vascular responses and vice versa (Greenberg and Jin, 2005; Lazarovici et al., 2006). Here, we propose that the integrin-associated protein CD47 may play such a dual role as well. CD47 is known to induce cell death in neurons (Koshimizu et al., 2002; Xing et al., 2009). In the present study, we demonstrated that CD47 signaling triggered cell death in brain endothelial cells, promoted the inflammatory up-regulation of cell adhesion molecules, and suppressed angiogenesis in vitro.
Historically, brain endothelial cells, unlike neurons, were thought to be relatively resistant to ischemic challenge (Gobbel et al., 1994; Plateel et al., 1995). However, accumulating data now suggest that endothelial cell death can occur after cerebral ischemia, and these vascular events play critical roles in the progression of brain damage after stroke (Lee and Lo, 2003; Zhang et al., 2005; Fisher, 2008). We recently showed that CD47 may trigger caspase-dependent and caspase-independent neuronal death (Xing et al., 2009). Here, we found that CD47 signaling similarly mediated cell death in brain endothelial cells. This broader neurovascular ability of CD47 to trigger cell death is consistent with the literature. Others have reported that the activation of CD47 by the endogenous ligand TSP-1 or the peptide 4N1K triggers complex cell death pathways in a wide range of cell types such as T cells, leukemia cells, monocytes, and breast tumor cells (Mateo et al., 1999; Manna and Frazier, 2003, 2004; Johansson et al., 2004; Saumet et al., 2005). Because CD47-mediated cell death occurs via multiple pathways, one might speculate that blocking the CD47 signal can productively ameliorate a wide spectrum of cell death mechanisms.
CD47 is well recognized as a multifactorial mediator in blood physiology, especially as a mediator of leukocyte and neutrophil transmigration across endothelial barriers (Cooper et al., 1995; Lindberg et al., 1996; Demeure et al., 2000; Hagnerud et al., 2006). In the context of stroke, this property may significantly contribute to the neuroinflammation that is thought to amplify secondary damage after ischemia and reperfusion. Here, we showed that activation of CD47 very rapidly (within 2–8 hr) up-regulated two important markers of endothelial inflammation, i.e. ICAM-1 and VCAM-1. This finding is consistent with previous studies showing that CD47 signaling increased levels of adhesion molecules in endothelial cells (Narizhneva et al., 2005), but with one difference. In human umbilical vein endothelial cells (HUVECS) and aortic endothelial cells, the expressions of ICAM-1, VCAM-1 and selectins were all elevated by CD47 activation. In contrast, E-selectin was unchanged in our brain endothelial cells. This may point to interesting differences between the routes taken by inflammatory cells when crossing brain endothelial barriers vs. other endothelial cells in the body. Nevertheless, our findings here suggest that CD47 may be a potential target for preventing neurovascular inflammation in stroke.
The concept of the neurovascular unit may apply not only during acute stages of stroke and brain injury, but also during delayed phases of stroke recovery (Lo, 2008a). Brain ischemia stimulates a coordinated response in angiogenesis and neurogenesis within days to weeks after stroke (Chen et al., 1994; Parent, 2003; Greenberg and Jin, 2005). A close interaction between remodeling vessels and plastic neuronal networks is central to stroke recovery (Ohab et al., 2006; Slevin et al., 2006; Thored et al., 2007). In this context, the CD47 ligand TSP-1 may be especially important. TSPs are a family of multi-domain, calcium-binding extracellular glycoproteins and comprise five genes encoding proteins, TSP-1 through TSP-5 (Adams and Lawler, 2004). TSP-1 plays a major role in cell-matrix and cell-cell interactions that influence platelet function, angiogenesis, tumor biology, wound healing, and vascular disease (Esemuede et al., 2004). TSP-1 is a potent inhibitor of angiogenesis (Lawler and Detmar, 2004). But, traditionally, the inhibition of angiogenesis and the induction of apoptosis in microvascular endothelial cells by TSP-1 were thought to be mediated via the CD36 receptor (Dawson et al., 1997; Jimenez et al., 2000). Our findings here suggest that CD47 plays a key role in these antiangiogenic and endothelial–toxic responses as well. More recently, other labs have reported several parallel pathways. CD47 appears to be necessary for inhibition of nitric oxide-stimulated vascular cell responses by TSP-1 (Isenberg et al., 2006). And the proliferation of microvascular endothelial cells can be inhibited by the interaction of TSP-1 with very low density lipoprotein receptor (VLDLR) (Oganesian et al., 2008). In contrast to these antiangiogenic phenomena, TSP-1 may also bind ApoER2 (apolipoprotein E receptor 2) and VLDLR to promote neuronal migration (Blake et al., 2008). Thus, TSP-1 signaling might yield opposite effects in vascular vs. neuronal compartments. Nevertheless, our present study is consistent with vasculosuppressive mechanisms of TSP-1-CD47 signaling. The CD47-activating peptide 4N1K clearly inhibited the migration of brain endothelial cells, and suppressed Matrigel tube formation in vitro. Because endogenous angiogenesis is important in remodeling brain, CD47 pathways can potentially interfere with stroke recovery as well.
Taken together, our data suggest that CD47 induces multiple deleterious effects in brain endothelial cells that may contribute to the pathophysiology of stroke. However, several caveats and potential limitations must be acknowledged. First, this is clearly a proof-of-concept study in cells. Further in vivo data must be obtained to support these cellular findings. Second, although we demonstrate that the CD47 signaling induces endothelial death, we do not know the precise pathways involved. In neurons, nonoverlapping caspase and free radical mechanisms are involved (Xing et al., 2009). Further studies are warranted to dissect the mechanisms of CD47-mediated endothelial cytotoxicity. A third limitation relates to the role of other receptors. As discussed endogenous mediators such as TSP-1 can activate multiple receptors including CD47 and CD36. How these receptors work together remains to be carefully elucidated. Finally, our study cannot provide a complete activation paradigm. CD47 can be activated by multiple ligands such TSP or signal regulatory proteins. Soluble TSP-1 is a major component of platelet granules and is released upon platelet activation (Mosher et al., 1992). Which mediators are important in stroke? Are there both autocrine and/or paracrine pathways? How these signals interact will have to be sorted out rigorously.
An emerging consensus in the field is that saving neurons alone may not suffice for stroke. An optimal stroke therapy must comprise simultaneous targeting of multiple pathways in multiple cell types. Functional interactions between all cell types in the neurovascular unit must be salvaged, including neuronal, glial and vascular elements (Dewar et al., 2003; Rossi et al., 2007; Lo, 2008b; Zlokovic, 2008). Indeed, many ongoing efforts in this field are now pursuing multi-target and multi-pathway therapies. Examples may include minocycline (Yong et al., 2004), activated protein C (Griffin et al., 2002; Thiyagarajan et al., 2008), erythropoietin (Brines and Cerami, 2005), hypothermia (Yenari et al., 2008), and other diverse methods to induce ischemic tolerance and preconditioning (Gidday, 2006).
In this context, we propose that CD47 might represent an interesting neurovascular target for stroke therapy because it may play critical roles at multiple steps in the ischemic cascade. CD47-mediated platelet aggregation may worsen thrombosis and lead to additional ischemia. Activated platelets can release soluble TSP-1, which goes on to trigger even more CD47 pathways. CD47 on affected brain endothelium may up-regulate ICAM-1, VCAM-1 and allow the infiltration of inflammatory cells. Once the signals reach the brain, CD47 can trigger cell death in both endothelium as well as neurons. Finally, CD47 may also suppress stroke recovery because it inhibits angiogenesis. Thus, one might speculate that successful and carefully titrated blockade of deleterious CD47 signals may influence both acute and delayed outcomes.
In conclusion, our study suggests that CD47 can induce cell death, promote inflammation, and suppress angiogenic responses in brain endothelial cells. Further studies to dissect and validate CD47 mechanisms in vivo are warranted.
Acknowledgments
This study is supported in part by grants R37-NS37074, R01-NS53560, R01-AI64569, K23-NS51588, R21-NS52498, and P01-NS55104.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake SM, Strasser V, Andrade N, Duit S, Hofbauer R, Schneider WJ, Nimpf J. Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions in postnatal neuronal migration. EMBO J. 2008;27:3069–3080. doi: 10.1038/emboj.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras M, Yuste VJ, Roue G, Barbier S, Sancho P, Virely C, Rubio M, Baudet S, Esquerda JE, Merle-Beral H, Sarfati M, Susin SA. Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. Mol Cell Biol. 2007;27:7073–7088. doi: 10.1128/MCB.02116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- Callahan MK, Williams KA, Kivisakk P, Pearce D, Stins MF, Ransohoff RM. CXCR3 marks CD4+ memory T lymphocytes that are competent to migrate across a human brain microvascular endothelial cell layer. J Neuroimmunol. 2004;153:150–157. doi: 10.1016/j.jneuroim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Chen HH, Chien CH, Liu HM. Correlation between angiogenesis and basic fibroblast growth factor expression in experimental brain infarct. Stroke. 1994;25:1651–1657. doi: 10.1161/01.str.25.8.1651. [DOI] [PubMed] [Google Scholar]
- Cooper D, Lindberg FP, Gamble JR, Brown EJ, Vadas MA. Transendothelial migration of neutrophils involves integrin-associated protein (CD47) Proc Natl Acad Sci U S A. 1995;92:3978–3982. doi: 10.1073/pnas.92.9.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombo-spondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeure CE, Tanaka H, Mateo V, Rubio M, Delespesse G, Sarfati M. CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J Immunol. 2000;164:2193–2199. doi: 10.4049/jimmunol.164.4.2193. [DOI] [PubMed] [Google Scholar]
- Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- Esemuede N, Lee T, Pierre-Paul D, Sumpio BE, Gahtan V. The role of thrombospondin-1 in human disease. J Surg Res. 2004;122:135–142. doi: 10.1016/j.jss.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Fisher M. Injuries to the vascular endothelium: vascular wall and endothelial dysfunction. Rev Neurol Dis. 2008;5(Suppl 1):S4–S11. [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Gobbel GT, Chan TY, Gregory GA, Chan PH. Response of cerebral endothelial cells to hypoxia: modification by fructose-1,6-bisphosphate but not glutamate receptor antagonists. Brain Res. 1994;653:23–30. doi: 10.1016/0006-8993(94)90367-0. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- Griffin JH, Zlokovic B, Fernandez JA. Activated protein C: potential therapy for severe sepsis, thrombosis, and stroke. Semin Hematol. 2002;39:197–205. doi: 10.1053/shem.2002.34093. [DOI] [PubMed] [Google Scholar]
- Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, Stins MF, Wang X, Dedhar S, Lo EH. Neuroprotection via matrix–trophic coupling between cerebral endothelial cells and neurons. Proc Natl Acad Sci U S A. 2008;105:7582–7587. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagnerud S, Manna PP, Cella M, Stenberg A, Frazier WA, Colonna M, Oldenborg PA. Deficit of CD47 results in a defect of marginal zone dendritic cells, blunted immune response to particulate antigen and impairment of skin dendritic cell migration. J Immunol. 2006;176:5772–5778. doi: 10.4049/jimmunol.176.10.5772. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- Jin G, Tsuji K, Xing C, Yang YG, Wang X, Lo EH. CD47 gene knockout protects against transient focal cerebral ischemia in mice. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.02.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson U, Higginbottom K, Londei M. CD47 ligation induces a rapid caspase-independent apoptosis-like cell death in human monocytes and dendritic cells. Scand J Immunol. 2004;59:40–49. doi: 10.1111/j.0300-9475.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- Koshimizu H, Araki T, Takai S, Yokomaku D, Ishikawa Y, Kubota M, Sano S, Hatanaka H, Yamada M. Expression of CD47/integrin-associated protein induces death of cultured cerebral cortical neurons. J Neurochem. 2002;82:249–257. doi: 10.1046/j.1471-4159.2002.00965.x. [DOI] [PubMed] [Google Scholar]
- Lawler J, Detmar M. Tumor progression: the effects of thrombo-spondin-1 and -2. Int J Biochem Cell Biol. 2004;36:1038–1045. doi: 10.1016/j.biocel.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Lazarovici P, Marcinkiewicz C, Lelkes PI. Cross talk between the cardiovascular and nervous systems: neurotrophic effects of vascular endothelial growth factor (VEGF) and angiogenic effects of nerve growth factor (NGF)-implications in drug development. Curr Pharm Des. 2006;12:2609–2622. doi: 10.2174/138161206777698738. [DOI] [PubMed] [Google Scholar]
- Lee SR, Lo EH. Interactions between p38 mitogen-activated protein kinase and caspase-3 in cerebral endothelial cell death after hypoxia–reoxygenation. Stroke. 2003;34:2704–2709. doi: 10.1161/01.STR.0000096540.40826.BA. [DOI] [PubMed] [Google Scholar]
- Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008a;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- Lo EH. Experimental models, neurovascular mechanisms and translational issues in stroke research. Br J Pharmacol. 2008b;153(Suppl 1):S396–S405. doi: 10.1038/sj.bjp.0707626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, Lo EH. Cell–cell signaling in the neurovascular unit. Neurochem Res. 2007;32:2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- Manna PP, Frazier WA. The mechanism of CD47-dependent killing of T cells: heterotrimeric Gi-dependent inhibition of protein kinase A. J Immunol. 2003;170:3544–3553. doi: 10.4049/jimmunol.170.7.3544. [DOI] [PubMed] [Google Scholar]
- Manna PP, Frazier WA. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004;64:1026–1036. doi: 10.1158/0008-5472.can-03-1708. [DOI] [PubMed] [Google Scholar]
- Mateo V, Lagneaux L, Bron D, Biron G, Armant M, Delespesse G, Sarfati M. CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat Med. 1999;5:1277–1284. doi: 10.1038/15233. [DOI] [PubMed] [Google Scholar]
- Mateo V, Brown EJ, Biron G, Rubio M, Fischer A, Deist FL, Sarfati M. Mechanisms of CD47-induced caspase-independent cell death in normal and leukemic cells: link between phosphatidylserine exposure and cytoskeleton organization. Blood. 2002;100:2882–2890. doi: 10.1182/blood-2001-12-0217. [DOI] [PubMed] [Google Scholar]
- Mazzone M, Carmeliet P. Drug discovery: a lifeline for suffocating tissues. Nature. 2008;453:1194–1195. doi: 10.1038/4531194a. [DOI] [PubMed] [Google Scholar]
- Mosher DF, Misenheimer TM, Stenflo J, Hogg PJ. Modulation of fibrinolysis by thrombospondin. Ann N Y Acad Sci. 1992;667:64–69. doi: 10.1111/j.1749-6632.1992.tb51598.x. [DOI] [PubMed] [Google Scholar]
- Narizhneva NV, Razorenova OV, Podrez EA, Chen J, Chandrasekharan UM, DiCorleto PE, Plow EF, Topol EJ, Byzova TV. Thrombo-spondin-1 up-regulates expression of cell adhesion molecules and promotes monocyte binding to endothelium. FASEB J. 2005;19:1158–1160. doi: 10.1096/fj.04-3310fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oganesian A, Armstrong LC, Migliorini MM, Strickland DK, Bornstein P. Thrombospondins use the VLDL receptor and a nonapoptotic pathway to inhibit cell division in microvascular endothelial cells. Mol Biol Cell. 2008;19:563–571. doi: 10.1091/mbc.E07-07-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Plateel M, Dehouck MP, Torpier G, Cecchelli R, Teissier E. Hypoxia increases the susceptibility to oxidant stress and the permeability of the blood–brain barrier endothelial cell monolayer. J Neurochem. 1995;65:2138–2145. doi: 10.1046/j.1471-4159.1995.65052138.x. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saumet A, Slimane MB, Lanotte M, Lawler J, Dubernard V. Type 3 repeat/C-terminal domain of thrombospondin-1 triggers caspase-independent cell death through CD47/alphavbeta3 in promyelocytic leukemia NB4 cells. Blood. 2005;106:658–667. doi: 10.1182/blood-2004-09-3585. [DOI] [PubMed] [Google Scholar]
- Slevin M, Kumar P, Gaffney J, Kumar S, Krupinski J. Can angiogenesis be exploited to improve stroke outcome? Mechanisms and therapeutic potential. Clin Sci (Lond) 2006;111:171–183. doi: 10.1042/CS20060049. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan M, Fernandez JA, Lane SM, Griffin JH, Zlokovic BV. Activated protein C promotes neovascularization and neurogenesis in postischemic brain via protease-activated receptor 1. J Neurosci. 2008;28:12788–12797. doi: 10.1523/JNEUROSCI.3485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- Xing C, Lee S, Kim WJ, Jin G, Yang YG, Ji X, Wang X, Lo EH. Role of oxidative stress and caspase 3 in CD47-mediated neuronal cell death. J Neurochem. 2009;108:430–436. doi: 10.1111/j.1471-4159.2008.05777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari M, Kitagawa K, Lyden P, Perez-Pinzon M. Metabolic down-regulation: a key to successful neuroprotection? Stroke. 2008;39:2910–2917. doi: 10.1161/STROKEAHA.108.514471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM. The promise of minocycline in neurology. Lancet Neurol. 2004;3:744–751. doi: 10.1016/S1474-4422(04)00937-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang X, Park TS, Gidday JM. Cerebral endothelial cell apoptosis after ischemia–reperfusion: role of PARP activation and AIF translocation. J Cereb Blood Flow Metab. 2005;25:868–877. doi: 10.1038/sj.jcbfm.9600081. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]