Abstract
Induction of smooth muscle differentiation from bladder mesenchyme depends on signals that originate from the urothelium. We hypothesize Sonic hedgehog (Shh) is the urothelial signal that promotes bladder mesenchymal proliferation and induces bladder smooth muscle differentiation.
Pregnant FVB mice were euthanized on embryonic day (E) 12.5 and fetal bladders were harvested. Two experimental protocols were utilized:
Bladder mesenchyme (BLM) was isolated by incubating intact bladders (IB) in 0.02 M EDTA and then removing the urothelium by microdissection. IB and BLM were cultured in Shh-deficient media or BLM was cultured in Shh-supplemented (480 nM) media for 72 h.
IB were cultured for 72 h in media containing different concentrations of Shh (0, 48, and 480 nM).
Specimens were sized by serial sectioning. Cell counts were performed after trypsin digestion. Immunohistochemistry was performed to detect smooth muscle-specific protein expression. α-Actin expression was quantified using Western blot.
All specimens were viable at 72 h. BLM cultured without Shh survived but did not grow or undergo smooth muscle differentiation. IB cultured without Shh and BLM cultured with Shh grew and expressed smooth muscle proteins at 72 h. IB cultured with Shh were larger and contained more cells than IB cultured without Shh (all p <0.05). Increasing Shh concentration from 48 to 480 nM did not change bladder size, cell counts, or the level of α-actin expression. Prior to culture, IB did not express α-actin. After culture of IB in Shh-deficient media, α-actin was detected throughout the mesenchyme except in the submucosal layer. The IB submucosa was thinner after culture with 48 nM Shh and smooth muscle completely obliterated the submucosa after culture with 480 nM Shh.
In fetal mouse bladders, urothelium-derived Shh is necessary for mesenchymal proliferation and smooth muscle differentiation. Shh concentration affects mesenchymal proliferation and patterning of bladder smooth muscle.
Keywords: Bladder, Urothelium, Sonic hedgehog, Smooth muscle differentiation
1. Introduction
The induction of smooth muscle differentiation from bladder mesenchyme depends on signals that originate in the urothelium (Baskin et al., 1996; DiSandro et al., 1998). The full thickness of bladder mesenchyme, from the submucosal to the sub-peritoneal layers, has the potential to develop into smooth muscle (Cao et al., 2008). During normal development, bladder smooth muscle forms in the peripheral mesenchyme and is separated from the urothelium by a submucosal layer, which is mostly devoid of smooth muscle. The location of the urothelium on the luminal surface of the bladder is critical to this patterning of the bladder wall (Cao et al., 2008).
Previous work has demonstrated that Sonic hedgehog (Shh) is necessary for bladder development and may be the factor responsible for bladder smooth muscle patterning (Yucel et al., 2004; Shiroyanagi et al., 2007). Shh has been shown in other organ systems to pattern tissue during embryogenesis; in the nervous system, cells from different areas in the developing brain differentiate into distinct cell types upon exposure to Shh-producing cells (Ericson et al., 1995a, 1995b)). Recent work by Cheng and others demonstrated that Gli2, a transcription factor induced by Shh, mediates the radial patterning of smooth muscle seen in the bladder, possibly through regulation of BMP4 (Cheng et al., 2008). However, other proteins are known to be potent activators of Gli2 and therefore the exact role of Shh in bladder smooth muscle patterning is not known (Dennler et al., 2007). Furthermore, the role of Shh in maintenance and proliferation of bladder mesenchyme is not known (Haraguchi et al., 2001).
We hypothesize that Shh is the urothelial-specific protein that induces bladder smooth muscle differentiation and that Shh is essential for bladder mesenchymal proliferation. We tested these hypotheses by culturing fetal mouse intact bladders and isolated bladder mesenchyme (BLM) procured before smooth muscle differentiation in media containing different concentrations of Shh. The goal was to determine the effect of Shh on bladder growth and smooth muscle differentiation and patterning using morphometric analysis, cell count, immunohistochemistry, and Western blot.
2. Materials and methods
This study was approved by the UCSF institutional animal care and use committee. Timed pregnant friend virus B-type (FVB) mice (normal gestation ∼20 days) were used for all experiments. Noon on the day of the vaginal plug was designated as embryonic day 0.5 (E0.5). Smooth muscle develops in the mouse urinary bladder at E13.5 (Shiroyanagi et al., 2007). Pregnant mice were euthanized on E12.5, and fetal bladders were harvested by microdissection. Two experimental protocols were utilized (see below). Each protocol included 8–12 specimens per group. At the end of the culture period, the specimens were harvested, mounted whole, photographed, and then specimens from each groups were processed separately for cell count, immunohistochemistry (IHC), and Western blot (see below). After the culture period described above, specimens from each of the experimental groups were fixed in formalin and embedded in paraffin. Sections were cut at 5 μm. The total number of sections obtained from each specimen was recorded and were used to determine bladder size. ANOVA analysis with Scheffe correction for multiple comparisons was performed to test for a difference in bladder explant sizes between experimental groups. p-Values less than 0.05 were considered statistically significant. Statistical analysis was performed using Stata 11 SE (StataCorp, College Station, TX, USA).
Protocol 1
Effect of Shh on isolated bladder mesenchyme.
E12.5 intact bladders were incubated in 0.02 M EDTA for 20 min at 37 °C. The urothelium was then separated from the bladder mesenchyme (BLM) by microdissection. The specimens (see groups 1–3 below) were incubated for 72 h at 37 °C in serum-free DMEM media containing insulin (0.01 mg/ml), transferrin (0.55 × 10−2 mg/ml), gentamicin (10 μg/ml), and penicillin (100 U/ml):
E12.5 intact bladder+culture media without Shh (positive control).
E12.5 BLM+culture media without Shh (negative control).
E12.5 BLM+culture media with 480 nm Shh.
Protocol 2
Effect of increasing concentrations of Shh on intact bladder.
Intact bladders were cultured for 72 h at 37 °C in the same supplemented serum-free DMEM media described in protocol 1 and different concentrations of Shh (0, 48, and 480 nM). (R&D systems, 1845-SH-025.)
2.1. Immunohistochemistry
IHC staining was performed after paraffin embedding and sectioning (5 μm) of the specimens. Tissue sections were deparaffinized in Histo-clear (National Diagnostic, Atlanta, GA, USA) and hydrated in graded alcoholic solutions and distilled water. Antigen retrieval was carried out by microwave heating for 30 min in an antigen unmasking solution (Vector Laboratories, Foster City, CA, USA). Endogenous peroxidase activity was blocked with 0.5% hydrogen peroxide in methanol for 30 min followed by washing in phosphate-buffered saline (PBS) at pH of 7.4. Superb-lock blocking buffer (Thermo Fisher Scientific, Rockford, IL, USA) was applied to the sections for 30 min to bind nonspecific sites. The sections were then incubated with the primary antibodies overnight at 4 °C. Sections were then washed and incubated with biotinylated goat anti-mouse or goat anti-rabbit immunoglobulin (Sigma) diluted with PBS at 1:200 for 30 min at room temperature. The following antibodies were used α-actin (Sigma), smooth muscle myosin heavy chain and smooth muscle 22 α-actin (abcam), calponin, and caldesmon (Santa Cruz), the nuclear proliferation marker Ki67 (Novocastra), and uroplakin (Nichirel Bioscience). Sections were then washed and incubated with biotinylated goat anti-mouse or goat anti-rabbit immunoglobulin (Sigma) diluted with PBS at 1:200 for 30 min at room temperature. After incubation with the secondary antibody, sections were washed in PBS and then incubated with the avidin–biotin complex (Vector Laboratories, Foster City, CA, USA) for 30 min at room temperature. Sections were then washed in PBS before visualizing using 3′,3′-diaminobenzidine (DAB) and 0.03% hydrogen peroxide in PBS. Sections were counterstained with hematoxylin and dehydrated in alcohol.
All BLM samples were stained for uroplakin to rule out urothelial contamination. Any BLM specimens containing residual bladder urothelium were excluded from the analysis.
2.2. Cell count
Two to three specimens from each experimental group were collected and digested with 0.25% trypsin at 37 °C for 2 h and stained with trypan blue to assess for cell viability. Unstained cells were counted using a hemocytometer. Three independent observers manually counted the cells. The mean number of cells was calculated.
2.3. Western blot analysis
Following culture of intact bladders and BLMs, specimens were lysed with lysis buffer 1% Nonident P40, 0.1% sodium dodectyl sulfate (SDS), 150mM NaCl, 50 mM Tris/pH 7.4, 10 mM EDTA, 10 mM p-nitrophenylphosphate, 250 U/L aprotinin, 40g/ml leupeptin, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 40 mM glcerolphosphate, and plus 1 × Protein Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN, USA). Cell lysates were stirred for 30 min and centrifuged for 10 min at 4 °C. The supernatants were used for electrophoresis and the protein concentration was determined using the Bradford assay (Pierce, Rockford, IL, USA). Twenty micrograms protein per lane were loaded into a 10% Precast SDS-PAGE Gel (BioRad Laboratories, Hercules, CA, USA), followed by electrophoresis and subsequent transfer of the proteins onto Immuno-Blot PVDF membranes (BioRad). Ponceau S staining was performed to detect total protein. The membrane was blocked overnight at 4 °C with 5% powered milk in PBST (phosphate buffer solution with 1% Tween 20) and washed five times, 5 min each with PBST. This was followed by incubating the membrane at room temperature with mouse anti-α-actin (Sigma, St. Louis, MO, USA) at 1:1000 for 1 h. The membrane was then washed as above and then incubated with rabbit anti-mouse horseradish peroxidase antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 1:5000 for 1 h. Protein detection was performed with SuperDura chemiluminescence reagent as per the manufacturer's instructions (Pierce, Rockford, IL, USA) and exposed to X-ray film (Sigma). Data reported as relative smooth muscle α-actin levels normalized by internal control β-actin.
3. Results
3.1. Urothelium is the likely source of Shh and is necessary for bladder growth and smooth muscle differentiation
Specimens from all experimental protocols were viable after 72 h of culture as demonstrated by exclusion of Trypan blue in the cell-count analysis and expression of the nuclear proliferation marker Ki67 on IHC. BLM specimens cultured in media containing 480 nM Shh were similar in size and morphology to intact bladders incubated in Shh-deficient media, both of which were larger than BLM specimens cultured in Shh-deficient media (Fig. 1). BLM at time of harvest and after incubation in Shh-deficient media for 72 h did not express α-actin (Figs. 2 and 3). In contrast, intact bladders incubated in Shh-deficient media and BLM incubated with 480 nM of Shh expressed α-actin after 72 h of culture (Fig. 3). Homogenous α-actin staining was noted throughout the explant in the BLM incubated with 480 nM of Shh. This pattern of α-actin staining was similar to that seen in the peripheral zone of intact bladders incubated in Shh-deficient media (Fig. 3). Additionally, BLM cultured with Shh and intact bladders cultured without Shh expressed markers of advanced smooth muscle differentiation (myosin heavy chain, calponin, caldesmon and SM22 alpha actin) (Fig. 4).
Fig. 1.
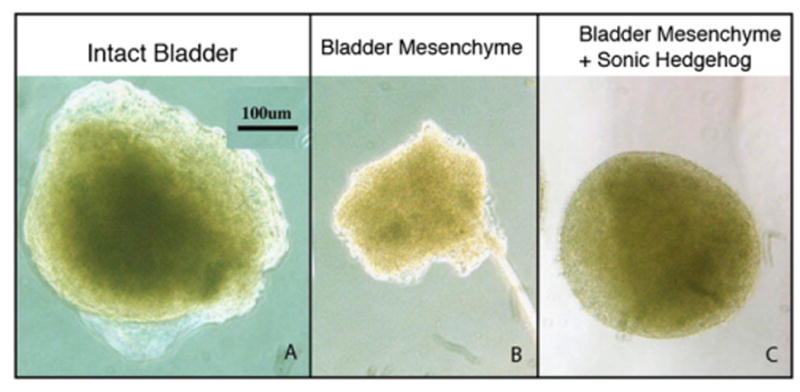
Urothelium and Shh support BLM growth. After 72 h, (A) intact bladders incubated without Shh (375 μm) were larger than (B) BLM incubated without Shh (200 μm). In contrast, (C) BLM incubated with 480 nM of Shh (350 μm) were over twice as large as BLM incubated without Shh and were comparable in size to intact bladders. All images at 10 × magnification.
Fig. 2.
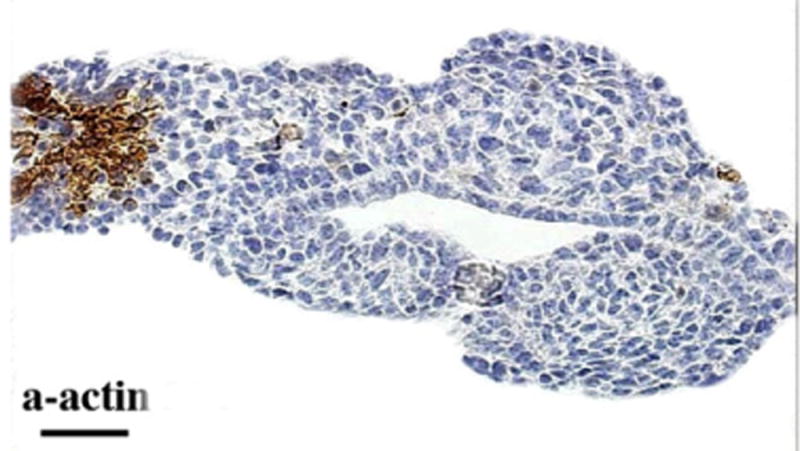
The E12.5 fetal mouse bladder does not express smooth muscle. Note α-actin staining of umbilical artery and absence of staining of bladder mesenchyme.
Fig. 3.
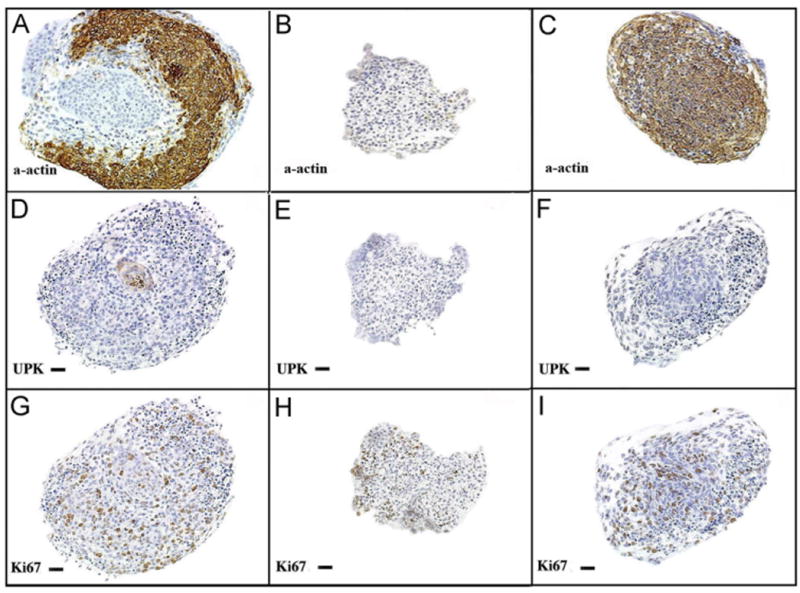
Urothelium and Shh regulates BLM smooth muscle differentiation. Immunohistostaining of explants after 72 h of incubation in serum free DMEM (20 ×). (A, D, G=intact bladders incubated without Shh) (A) Intact bladders incubated without Shh demonstrated strong α-actin staining of smooth muscle. (D) The urothelium, which stained for UPK, is seen at the center of the section (G) Diffuse expression of Ki67 was detected, indicating specimen viability. (B, E, H=BLM incubated in Shh-free media) (B) Neither smooth muscle nor (E) urothelium were detected. (H) K67 expression was detected, indicating viability. (C, F, I=BLM cultured with 480 nM of Shh). (C) α-actin was expressed homogenously throughout the explant, indicating robust smooth muscle differentiation. (F) Uroplakin was not detected, demonstrating an absence of urothelial contamination of the specimen. (I) Ki67 expression was present. α-actin=smooth muscle α-actin, UPK=uroplakin, Ki67=nuclear proliferation marker.
Fig. 4.
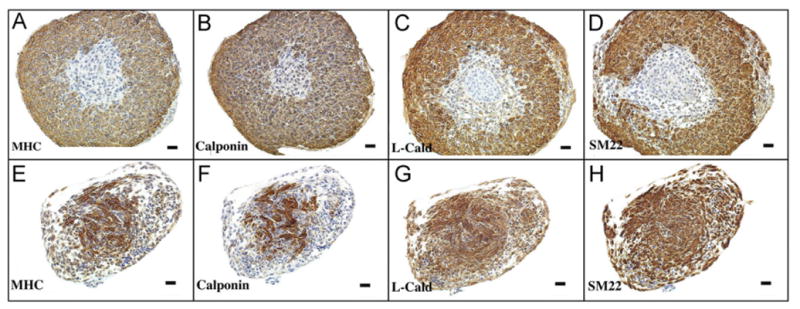
Urothelium and Shh induce expression of smooth muscle markers in BLM. Markers of smooth muscle differentiation were expressed in intact bladders cultured without Shh (top panel) and in BLM cultured in media containing 480 nM of Shh (bottom panel) after 72 h of incubation (10 ×). MHC=myosin heavy chain, calponin, L-Cald=caldesmon, SM22 = smooth muscle actin-22.
3.2. Shh promotes cell proliferation and increases bladder size
The average number of sections obtained from intact bladder explants at the time of harvest and after culture with 0, 48, and 480 nM Shh were 53.5, 47.5, 68.8, and 76.9, respectively. There was a significant difference in the average bladder size between experimental groups (F=30.83). Intact bladders at the time of harvest were similar in size to those bladders after incubation in Shh-deficient media for 72 h (p=0.5). Intact bladders incubated in Shh-containing media (48 and 480 nM) were larger than bladders at time of harvest and bladders after incubation in Shh-deficient media for 72 h (all p < 0.01). However, intact bladders cultured with 480 nM Shh were not larger than those cultured with 48 nM Shh (p=0.08) (Fig. 5). Shh had the same effect on bladder cell count as it did on bladder size: intact bladders cultured in media with exogenous Shh contained significantly greater numbers of cells than untreated specimens; however, Shh concentrations above 48 nM did not further increase bladder cell count (Fig. 5).
Fig. 5.
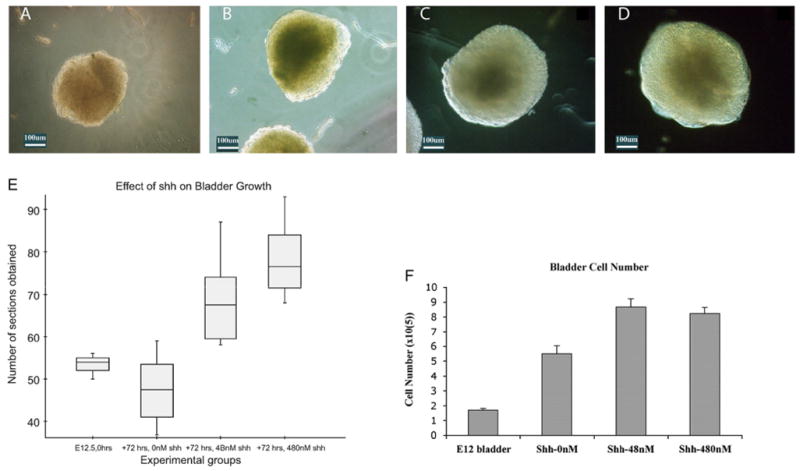
Shh regulates bladder mesenchyme proliferation. Light microscopy (10 ×) images of (A) E12.5 intact bladder prior to organ culture. (B) Intact bladder after 72 h of incubation in serum-free DMEM without Shh. The bladders incubated without Shh were smaller than (C) the bladders incubated with 48 nM of Shh and (D) the bladders incubated with 480 nM of Shh. (E) Box plot representation of the number of sections obtained from intact bladders at harvest and after 72 h of incubation in 0, 4.8, and 480 nM of Shh. The box is the graphical representation of the distribution of data between the first and third quartiles and the line within the box represents the median bladder size. The “whiskers” represent 1.5 times the interquartile range of the pertinent data set. There were no outliers. (F) Cell counts (× 105) of intact bladders at harvest (E12.5) and after 72 h of incubation with increasing concentrations of Shh. After 72 h, the cell counts of intact bladders incubated without Shh nearly tripled relative to preculture levels. Intact bladders cultured with Shh had a greater number of cells than bladders cultured without Shh although there was no difference in the cell counts of intact bladders incubated in 48 nM versus those incubated in 480 nM of Shh.
3.3. Shh induces differentiation and regulates patterning of smooth muscle in the bladder
Prior to culture, E12.5 intact bladders did not express α-actin (Fig. 2). At the end of 72 h, intact bladders cultured in Shh-free media demonstrated smooth muscle differentiation, as shown by expression of α-actin in the peripheral mesenchyme (Fig. 6). The submucosa of intact bladders incubated without Shh remained devoid of α-actin staining. This smooth muscle pattern was also seen in bladders cultured with 4.8 nM of Shh (results not shown). However, the submucosal layer became thinner but was still recognizable in bladders grown in media containing 48 nM of Shh (Fig. 6). Smooth muscle formed immediately deep to the urothelium and completely obliterated the submucosa of bladders grown in media containing 480 nM of Shh (Fig. 6).
Fig. 6.
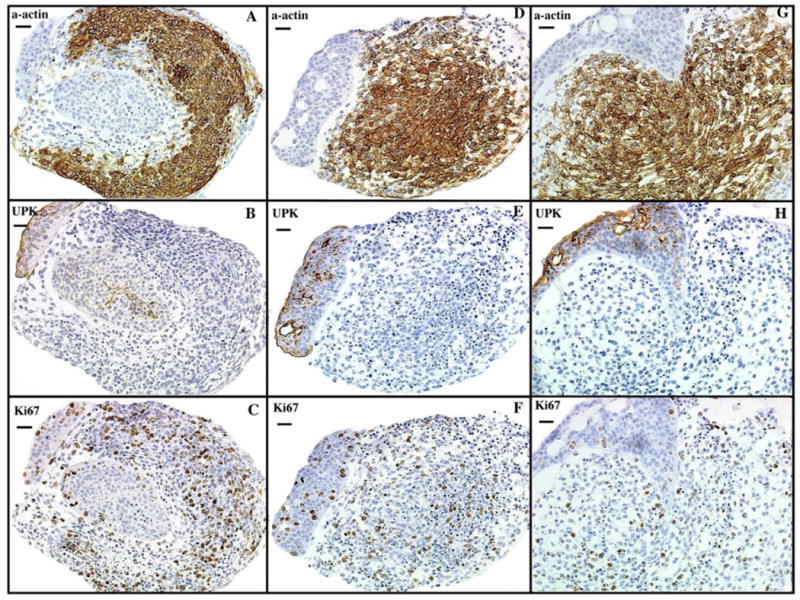
Shh concentration affects bladder smooth muscle patterning. Immunohistostaining of intact mouse fetal bladders after 72 h of incubation in serum free DMEM (20 ×). (A, B, C) Intact bladders incubated without Shh express (A) α-actin which is separated from the urothelium, stained with (B) UPK, by a thin submucosal layer. (D, E, F) Intact bladders incubated with 48 nM of Shh demonstrate similar staining patterns as the bladder incubated in Shh-free media with the exception that the submucosal layer is thinner. (G, H, I) Intact bladders incubated in 480 nM of Shh demonstrated robust α-actin expression, which obliterated the submucosal layer. Specimens were viable as indicated by homogenous Ki67 expression. α-actin=smooth muscle α-actin, UPK=uroplakin, Ki67=nuclear proliferation marker.
After 72 h of incubation, α-actin expression in the peripheral mesenchyme of intact bladders cultured in 48 nM Shh was greater than those cultured in Shh-deficient medium as assessed by IHC (Fig. 6) and Western blot analysis (Fig. 7). However, α-actin expression did not significantly change with an increase in Shh concentration from 48 to 480 nM (Fig. 7). An additional concentration of 4.8 nM Shh was also used; however, there was no significant difference in the cell counts, explant size, or smooth muscle patterning noted between 0 and 4.8 nM (results are not shown).
Fig. 7.
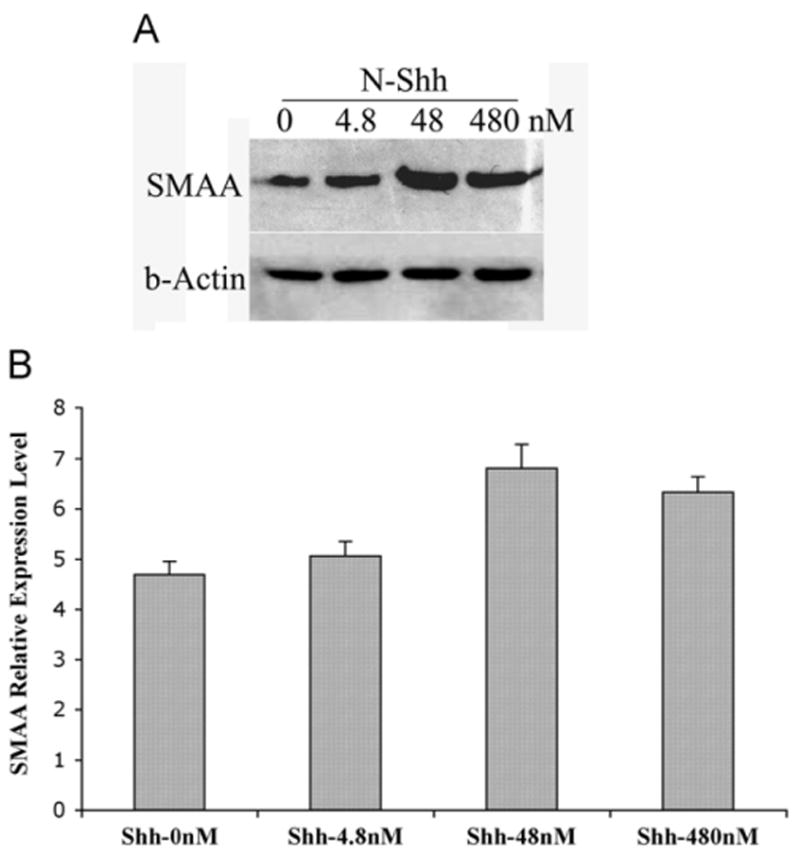
Shh concentration affects expression of α-actin in fetal mouse bladders: (A) Western blot analysis of whole mouse fetal bladders after 72 h of incubation in serum free DMEM and increasing concentrations of Shh. (B) Expression of smooth muscle α-actin in intact bladders relative to β-actin incubated with various concentration of Shh after 72 h. There was no significant difference in the level of α-actin expression between bladders cultured in 480 nM of Shh and those cultured in 48 nM of Shh. SMAA=smooth muscle α-actin, β-actin=control.
4. Discussion
We have shown that Shh is necessary for bladder mesenchymal proliferation, is critical for the differentiation of bladder mesenchyme into smooth muscle, and plays a significant role in the patterning of bladder smooth muscle. Our results support the hypothesis that the urothelium is source of Shh and further corroborates Shh's well-established role in tissue differentiation during organ development (Bitgood and McMahon, 1995).
Previously, we have shown that urothelium is critical for bladder smooth muscle patterning and have proposed that Shh is the urothelium-derived factor that induces smooth muscle differentiation (Cao et al., 2008). The experiments in the first protocol demonstrated that an intact urothelium or supplementation of media with Shh is necessary for growth of and smooth muscle differentiation in the embryonic mouse bladder. In the absence of urothelium, Shh is sufficient to support specimen growth and smooth muscle differentiation. The converse is also true: in the absence of Shh, urothelium is sufficient to support bladder growth and smooth muscle differentiation. These findings support the hypothesis that the urothelium is the source of Shh and that Shh promotes bladder growth and regulates smooth muscle differentiation (Shiroyanagi et al., 2007; Cheng et al., 2008).
The experiments from the second protocol demonstrated that, within limits, increasing Shh concentration increased bladder size, cell counts, and expression of α-actin. It is known that Shh acts as a mitogen during development. Shh expands and maintains cell subpopulations within the somite (Marcelle et al., 1999) and has been shown to promote mesenchymal proliferation in diverse tissues, including developing tooth, kidney, and ureter (Wu et al., 2003; Yu et al., 2002). It seems likely that this mechanism is preserved in the bladder. However, there appears to be a limit to the proliferative effect of Shh on bladder mesenchyme. This was suggested by the finding that there was no significant difference in the explant size or cell counts of bladders incubated in 48 nM of Shh versus those incubated in 480 nM of Shh. Shh concentration had a similar effect on the level of α-actin expression in intact bladders. There was no significant difference in the level of α-actin expression between bladders incubated in 48 and 480 nM of Shh.
One hypothesis is that all the observed effects of varying Shh concentrations on bladder size, cell count, and α-actin expression are due to its graded proliferative effect on bladder mesenchyme. Smooth muscle differentiation occurs after mesenchymal expansion during bladder development. Consequently, the level of α-actin expression may be a direct reflection of the population of mesenchymal cells available to undergo differentiation into smooth muscle.
However, this theory does not entirely explain the effect of increasing Shh concentrations on smooth muscle patterning in the developing bladder. Exogenous Shh and urothelium had significant effects on the expression pattern of highly differentiated smooth muscle markers. Whereas calponin, SM-MHC, caldesmon, and SM22 were expressed most highly in the periphery of intact bladders cultured without Shh, this pattern was reversed in BLM cultured in high concentrations of Shh. This is consistent with previous reports that these markers are most highly expressed in the areas of the mesenchyme furthest away from the Shh source (Liu et al., 2010). Additionally, as Shh concentrations increased, the size of the submucosal layer (“zone of inhibition”), decreased. Whereas there was a distinct sub-mucosal layer in the intact bladders incubated without or at low concentrations of Shh, this layer was obliterated in the bladders exposed to 480 nM of Shh. Thus, it appears that increasing Shh concentrations continues to change smooth muscle patterning even when the limits of its effects on mesenchymal proliferation and actin expression are reached.
How can we reconcile the positive effect we observed that Shh had on smooth muscle differentiation and expansion with the findings of others who have shown that high concentrations of Shh inhibits smooth muscle differentiation in the gut, ureter, and bladder? (Cheng et al., 2008; Sukegawa et al., 2000). Investigations into mesenchymal development and differentiation in the genitourinary system and other organs have revealed that T-box transcription factors are important in early mesenchymal migration and patterning (Airik et al., 2006; Bussen et al., 2004; Ludtke et al., 2009). It is possible that high Shh concentrations in our experiments interfered with the mesenchymal response to these signaling pathways and caused the smooth muscle pattern changes we observed. Another possibility is that disruption of the precisely controlled secretion of Shh from the urothelium to the adjacent mesenchyme altered the normal radial patterning of the bladder. Discs-large homolog 1 (Dlgh1), which is highly expressed in urothelium, is critical for the regulation of secretion of soluble factors from the urothelium to the smooth muscle cells in the developing ureter; Dlgh1-null mice lack a suburothelial layer and have misaligned ureteral smooth muscle (Mahoney et al., 2006). It is plausible that a similar mechanism of regulation might exist in the bladder and that Shh is the regulated “soluble factor”. Should that be so, high levels of Shh in the culture medium may have overridden the regulated secretion of Shh from the epithelium and altered the normal radial differentiation of mesenchyme into smooth muscle. Ultimately, it is likely that culturing bladder explants in medium containing high concentrations of Shh affected early patterning of bladder mesenchyme, which is necessary for the radial smooth muscle pattern seen in normal development.
Overall, our results demonstrated that Shh has a dual function in the developing mouse bladder: Shh is necessary for both mesenchymal proliferation and bladder smooth muscle differentiation and patterning. It is uncertain to what extent smooth muscle differentiation is dependent upon mesenchymal proliferation. Yu postulated that smooth muscle differentiation begins only when mesenchymal precursors reach a specific density (Yu et al., 2002). Our finding that BLM cultured without urothelium or Shh, in which mesenchymal proliferation and cell growth is limited, does not express any smooth muscle markers is consistent with this theory. However, there is also evidence that smooth muscle differentiation and patterning are distinct from mesenchymal proliferation and are mediated through different pathways (Cheng et al., 2008; Yu et al., 2002; Caubit et al., 2008). For example, while bladders of Gli2 null mice have a normal number of proliferating mesenchymal cells; these cells fail to pattern appropriately (Cheng et al., 2008). Additionally, certain transcription factors, such as Tbx18, are active early in bladder development during mesenchymal proliferation (Airik et al., 2006) whereas others, such as BMP4 and Tshz3, seem to be restricted to mediating smooth muscle differentiation (Shiroyanagi et al., 2007; Caubit et al., 2008; Wang et al., 2009).
In the broader context of the gastrointestinal and genitourinary systems in which smooth muscle patterning is a prominent feature, it seems likely that both hypotheses are true and that distinct but linked signaling pathways mediate the different effects of Shh (Ramalho-Santos et al., 2000; Jones et al., 2006; Madison et al., 2005, 2009). We propose that Shh is the common secreted factor that links mesenchymal proliferation and smooth muscle differentiation during normal bladder development. First, Shh acts as an autocrine growth factor and promotes mesenchymal proliferation, which has been shown in other organ systems (Yang et al., 2008; Koleva et al., 2005; Mimeault and Batra, 2007; Shiroyanagi et al., 2007; Haraguchi et al., 2007). Then, once a critical mass of mesenchymal cells has been achieved, Shh acts along the well-described pathway through the Gli proteins and BMP4 to induce smooth muscle differentiation. Further work should be done to elucidate what is assuredly a complex interaction between Shh and various transcription factors, during the fluid process of bladder development.
Acknowledgments
Role of the funding source: NIH R01 DK073449. The study sponsor had no role in the study design; the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Contributor Information
Mei Cao, Email: mcao@urology.ucsf.edu.
Gregory Tasian, Email: gtasian@urology.ucsf.edu.
Gerald Cunha, Email: cunhag@urology.ucsf.edu.
Laurence Baskin, Email: lbaskin@urology.ucsf.edu.
References
- Airik R, Bussen M, Singh MK, Petry M, Kispert A. Tbx18 regulates the development of the ureteral mesenchyme. J Clin Invest. 2006;116:663–674. doi: 10.1172/JCI26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin LS, Hayward SW, Sutherland RA, DiSandro MJ, Thomson AA, Goodman J, Cunha GR. Mesenchymal–epithelial interactions in the bladder. World J Urol. 1996;14:301–309. doi: 10.1007/BF00184602. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell–cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Bussen M, Petry M, Schuster-Gossler K, Leitges M, Gossler A, Kispert A. The T-box transcription factor Tbx18 maintains the separation of anterior and posterior somite compartments. Genes Dev. 2004;18:1209–1221. doi: 10.1101/gad.300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Liu B, Cunha G, Baskin L. Urothelium patterns bladder smooth muscle location. Pediatr Res. 2008;64:352–357. doi: 10.1203/PDR.0b013e318180e4c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caubit X, Lye CM, Martin E, Core N, Long DA, Vola C, Jenkins D, Garratt AN, Skaer H, Woolf AS, Fasano L. Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development. 2008;135:3301–3310. doi: 10.1242/dev.022442. [DOI] [PubMed] [Google Scholar]
- Cheng W, Yeung CK, Ng YK, Zhang JR, Hui CC, Kim PC. Sonic hedgehog mediator Gli2 regulates bladder mesenchymal patterning. J Urol. 2008;180:1543–1550. doi: 10.1016/j.juro.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Dennler S, Andre J, Alexaki I, Li A, Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F, Mauviel A. Induction of Sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- DiSandro MJ, Li Y, Baskin LS, Hayward S, Cunha G. Mesenchymal–epithelial interactions in bladder smooth muscle development: epithelial specificity. J Urol. 1998;160:1040–1046. doi: 10.1097/00005392-199809020-00022. discussion 1079. [DOI] [PubMed] [Google Scholar]
- Ericson J, Muhr J, Jessell TM, Edlund T. Sonic hedgehog: a common signal for ventral patterning along the rostrocaudal axis of the neural tube. Int J Dev Biol. 1995a;39:809–816. [PubMed] [Google Scholar]
- Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995b;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128:4241–4250. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, Nakagata N, Moon A, Yamada G. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development. 2007;134:525–533. doi: 10.1242/dev.02736. [DOI] [PubMed] [Google Scholar]
- Jones RG, Li X, Gray PD, Kuang J, Clayton F, Samowitz WS, Madison BB, Gumucio DL, Kuwada SK. Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J Cell Biol. 2006;175:505–514. doi: 10.1083/jcb.200602160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleva M, Kappler R, Vogler M, Herwig A, Fulda S, Hahn H. Pleiotropic effects of Sonic hedgehog on muscle satellite cells. Cell Mol Life Sci. 2005;62:1863–1870. doi: 10.1007/s00018-005-5072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Feng D, Lin G, Cao M, Kan YW, Cunha GR, Baskin LS. Signalling molecules involved in mouse bladder smooth muscle cellular differentiation. Int J Dev Biol. 2010;54:175–180. doi: 10.1387/ijdb.082610bl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke TH, Christoffels VM, Petry M, Kispert A. Tbx3 promotes liver bud expansion during mouse development by suppression of cholangiocyte differentiation. Hepatology. 2009;49:969–978. doi: 10.1002/hep.22700. [DOI] [PubMed] [Google Scholar]
- Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–289. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- Madison BB, McKenna LB, Dolson D, Epstein DJ, Kaestner KH. FoxF1 and FoxL1 link hedgehog signaling and the control of epithelial proliferation in the developing stomach and intestine. J Biol Chem. 2009;284:5936–5944. doi: 10.1074/jbc.M808103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney ZX, Sammut B, Xavier RJ, Cunningham J, Go G, Brim KL, Stappenbeck TS, Miner JH, Swat W. Discs-large homolog 1 regulates smooth muscle orientation in the mouse ureter. Proc Natl Acad Sci USA. 2006;103:19872–19877. doi: 10.1073/pnas.0609326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelle C, Ahlgren S, Bronner-Fraser M. In vivo regulation of somite differentiation and proliferation by Sonic hedgehog. Dev Biol. 1999;214:277–287. doi: 10.1006/dbio.1999.9389. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Interplay of distinct growth factors during epithelial mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann Oncol. 2007;18:1605–1619. doi: 10.1093/annonc/mdm070. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- Shiroyanagi Y, Liu B, Cao M, Agras K, Li J, Hsieh MH, Willingham EJ, Baskin LS. Urothelial Sonic hedgehog signaling plays an important role in bladder smooth muscle formation. Differentiation. 2007;75:968–977. doi: 10.1111/j.1432-0436.2007.00187.x. [DOI] [PubMed] [Google Scholar]
- Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, Iba H, Yasugi S, Fukuda K. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development. 2000;127:1971–1980. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Brenner-Anantharam A, Vaughan ED, Herzlinger D. Antagonism of BMP4 signaling disrupts smooth muscle investment of the ureter and ureteropelvic junction. J Urol. 2009;181:401–407. doi: 10.1016/j.juro.2008.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Shimo T, Liu M, Pacifici M, Koyama E. Sonic hedgehog functions as a mitogen during bell stage of odontogenesis. Connect Tissue Res. 2003;44(Suppl. 1):92–96. [PubMed] [Google Scholar]
- Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, Li YX, Diehl AM. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–5312. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]
- Yucel S, Liu W, Cordero D, Donjacour A, Cunha G, Baskin LS. Anatomical studies of the fibroblast growth factor-10 mutant, Sonic Hedge Hog mutant and androgen receptor mutant mouse genital tubercle. Adv Exp Med Biol. 2004;545:123–148. doi: 10.1007/978-1-4419-8995-6_8. [DOI] [PubMed] [Google Scholar]


