Abstract
Background and Purpose
The use of lithium as a neuroprotective agent has been demonstrated using various models in which improvements in infarct size, DNA damage, and neurological function were reported. We further investigated neurohemodynamic aspects of the treatment-associated recovery by assessing the therapeutic efficacy of delayed chronic lithium treatment using functional MRI.
Methods
Ipsilesional functional MRI activations in the somatosensory cortex, acquired 2 weeks after the 90-minute transient middle cerebral artery occlusion, were compared between lithium- and saline-treated rats. Specifically, MRI signal changes based on blood oxygenation level dependence and functional cerebral blood volume responses were examined using electrical stimulation of forelimbs. Additional immunohistochemical assays were performed.
Results
The ratio of ipsilesional to contralesional blood oxygenation level dependence response magnitudes significantly improved with lithium treatments. In contrast, the increase of the functional cerebral blood volume response magnitude ratio was not statistically significant. Nonetheless, the lithium treatment induced significant enhancements of total functional MRI activation (defined as a product of activation volume and response magnitude) for both blood oxygenation level dependence and functional cerebral blood volume methods. Increased cerebral blood volume in periinfarct tissues suggests a possible stroke-induced vascular transformation in both saline- and lithium-treated rats; however, other MRI-derived vascular parameters (vascular size index and microvascular volume) and immunohistochemical staining (CD31, glia fibrillary-associated protein, and matrix metalloproteinase-9) may imply that the neoformation of vasculature was differently affected by the lithium treatment.
Conclusions
The delayed chronic lithium treatment enhanced the blood oxygenation level dependence functional MRI response magnitude in the absence of neurological improvement and influenced vascular formation in poststroke animal models.
Keywords: BOLD, CBV, fMRI, focal ischemia, neuroprotective agents, rat, treatment
Lithium has been used as a therapy option mainly for treating mood disorders. However, recent recognition of lithium as a neuroprotective agent prompted experimental studies of lithium treatment for managing neurodegenerative diseases and cerebral ischemia.1,2 Previous reports demonstrated that lithium treatment effectively reduces the severity of stroke damage. In particular, lithium treatment protects against ischemic damage of central nervous system neurons resulting from glutamate-induced cell death,2,3 and such lithium-based neuroprotection likely involves inhibition of abnormal activity of glycogen synthase kinase-3, which is responsible for the regulation of neurodegeneration.4–6
Previously, using an extended treatment scheme and concentration range commonly used for human bipolar illnesses, the efficacy of pre- and poststroke lithium treatment was demonstrated in animal models.1,4,7,8 The effectiveness of such treatment strategies is clinically important for designing pharmacological intervention of stroke-induced impairment.2 In general, therapy assessments of lithium treatment have been established by measuring the final infarct volume and behavioral scoring, which indicated enhanced restoration of overall neurological function as well as limiting structural degradation. However, neurohemodynamic aspects of such improvements, which may be important to understand the underlying mechanisms, have not been documented until now. In this study, we report the use of functional MRI (fMRI), which is based on MRI signal changes due to neurohemodynamic activities to assess the efficacy of delayed chronic lithium treatment in rat stroke models.
Recently, we reported the alteration of fMRI responses to electrical forelimb stimulation using rat models with ischemic infarcts induced by transient middle cerebral artery occlusion.9 The study demonstrated that the mean blood oxygenation level dependence (BOLD) response magnitude ratio between ipsilesional and contralesional somatosensory cortices was significantly smaller than the mean ratio acquired using the task-related functional change of cerebral blood volume (fCBV), suggesting an intrinsic alteration of fMRI hemodynamic responses. Similarly in the present study, we examined both BOLD and fCBV fMRI responses to assess the long-term effects of lithium treatment using rat stroke models. Various MRI-derived parameters were used to quantify structural damage and alteration of fMRI responses in both saline- and lithium-treated stroke models. Our goal was to show the effects of protracted lithium treatment and to investigate whether combined fMRI and structural MRI parameters could be used to assess stroke recovery.
Materials and Methods
Animal Preparation and Treatment Protocol
Experimental protocols were institutionally approved in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Twelve male Sprague-Dawley rats (Charles River, Wilmington, Mass), weighing 250 to 350 g, were kept under diurnal lighting condition. After 90 minutes of focal cerebral ischemia with an intraluminal filament,10 rats received a subcutaneous injection of either LiCl (Sigma-Aldrich, St. Louis, Mo) dissolved in normal saline (1 mmol/L LiCl/kg rat: n=6) or the same volume of normal saline (0.9% NaCl: n=6) 12 hours after the operation. These injections were repeated daily for 14 days. Neurological scoring was performed in the same sequence and at approximately the same time of day. All behavioral testing and subsequent MRI signal analyses were performed by an investigator blinded to the treatment assignment of each animal.
Neurological Scoring
At 24 hours (day 1), 3, 7, 11, and 14 days after onset of stroke, the animal’s neurological status was evaluated using a modified grading system based on those previously described by others.11–13 Total neurological score was a composite of motor (muscle status, abnormal movement), sensory (tactile and proprioceptive), and reflex tests. The sum of partial scores gave the total neurological score, which is graded on a scale of 0 to 20 points (normal score 0; maximal deficit score 20).
MRI Preparation and Protocols
On day 15, fMRI was performed to detect differences in functional recovery responses in both groups. Both femoral veins and right femoral arteries were catheterized. Additionally, animals were tracheotomized and mechanically ventilated with 1.5% halothane in O2/air (1:1), during which arterial blood was sampled for blood gas analysis. Before MRI experiments, the anesthetic regimen was switched from a halothane gas mixture to continuous infusion of α-chloralose (25 mg/kg/h) and pancuronium (≈1.25 mg/kg/h) as described in the previous study.9 Sufficient time (≈1 hour) before fMRI experiments was allowed for the anesthetic transition.
The fMRI activation of both BOLD and fCBV (gradient echo planar imaging, TR/TE=3700/15 ms for BOLD, TR/TE=3700/11 ms for fCBV, field of view=2.5×2.5 cm2, 9 1-mm slices, and 80×80 matrix zero filled to 128×128) was acquired on a horizontal bore 9.4T Bruker/Magnex system equipped with a home-built rat head surface coil. The details of the fMRI paradigm were previously published.9 Because the order of BOLD and fCBV acquisitions were not randomized (ie, BOLD was always obtained before fCBV), temporal stability of measurements for each stroke rat was assessed and confirmed by comparing the BOLD and fCBV activation magnitudes acquired from the contralesional somatosensory cortex with the previously collected activation magnitude in normal healthy rats.9
Before the contrast agent administration, T2 and T2* maps were created by conventional gradient echo and spin echo pulse sequences with multiple echoes in which TR/TE=1000/4, 7, 10, 13 ms and TR/TE=3000/15, 30, 45, 60, 75, 90, 105, and 120 ms were used, respectively. For further structural analysis, apparent diffusion coefficient (ADC) maps were created with a diffusion-weighted echo planar image pulse sequence with TR/TE=3700/40 ms and b=5, 300, 800, and 1200 sec/mm2. To obtain the fractional anisotropy (FA),14 diffusion tensor imaging was performed with an approximate b value of 1200 sec/mm2. After intravenous monocrystalline iron oxide nanocolloid (MION) administration, T2 and T2* maps were again created as described previously for the calculation of blood volume and vessel size index (VSI).15
Functional MRI Analysis
Functional activation maps were computed as described previously.9 The statistical threshold for significant activation response was P<0.0001 with a Bonferroni correction for multiple comparisons throughout the measured brain volume. For the fMRI signal change analyses in the somatosensory cortex, regions of interest (ROI) were determined from the significantly activated volume with the fCBV technique for each rat. For the analyses of thalamic activation, ROIs were placed over anatomically defined areas.9
The changes in the T2* relaxation rates (ΔR2*) in the somatosensory cortex were acquired for the BOLD and fCBV responses. The Mean_ΔR2* for the BOLD response was defined (see Equation 1) as a temporally averaged R2* change for a duration of 20 seconds starting 5 seconds after the initiation of each contralateral stimulus, whereas for the fCBV responses, the temporally averaged blood volume change was measured for 20 seconds starting 15 seconds after the initiation of stimulus for the fCBV responses, unless specified otherwise (eg, Mean_ΔR2*(t 1–t2); t 1–t2 =time period used for averaging signal intensity in an activation epoch).
The averaging window for fCBV responses was delayed with respect to the BOLD window to account for the slower fCBV response.16 The acquired fMRI responses were analyzed using the responses that were contralateral to the stimulated forepaw (ie, contrastimulus). To reduce the influence of individual variations on the quantification of fMRI activity, the ipsilesional activation magnitude was normalized to the contralesional activation by taking a ratio. The Mean_ΔR2*ratios (rΔR2*) between contralesional and ipsilesional cortices were calculated as follows:
Lesion volumes were calculated from the multislice T2 data sets in which T2 lesion volume was defined as the ipsilateral parenchymal volume with T2 values that differ by +2 SDs from the mean T2 values of the contralateral tissue. For the comparison of MRI-derived structural parameters, each ROI within a slice was analyzed by comparing the ipsilesional value with the contralesional counterpart to minimize any possible variations across slices. Percent differences (ie, (contralesional-ipsilesional)/contralesional) of ADC, FA, CBV, VSI, and microvascular volume (MVV) were calculated for the somatosensory cortex and periinfarct area.14,15,17,18 All the statistical analyses for the ROIs presented in the study were performed using a 2-tailed t test. For correlation analyses, we applied the Pearson product moment correlation test (and linear regression). All the numerical data were presented as averages ±1 SD. Statistical significance was accepted at a confidence level of 0.95.
Immunohistochemistry
We transcardially perfused rats with ice-cold phosphate-buffered saline (pH 7.4) and 4% paraformaldehyde. Brains were fixed in 4% paraformaldehyde solution and cytoprotected in 30% sucrose at 4°C overnight. We stained cryostat sections (20 µm) with antibodies against matrix metalloproteinase-9 (MMP-9), glia fibrillary-associated protein, and CD31 and used sections treated without primary antibodies as controls, whereas observers were blinded to the experimental groups. We selected brain sections at +0.7 and −1.3 mm from bregma (axial sections 3 and 4) for qualitative comparisons because these represented locations of maximal cortical vascular changes.19 In addition, fluorescence density was used for the quantification of CD31 and MMP-9 signal in the periinfarct areas using standard ImageJ software.
Results
Neurological Scoring
Both the lithium-treated group and the saline control group showed a strong neurological deficit immediately after the surgical operation (data not shown). In follow-up tests, gradually decreasing total neurological scores (ie, positive sensorimotor recovery) were observed. The neurological scores 14 days after middle cerebral artery occlusion were similar between the lithium-treated and the saline-treated control groups. Specifically, recovery in postural signs and contralateral forelimb placing indicates that the neurological improvement is still ongoing 14 days after the middle cerebral artery occlusion.
Functional MRI
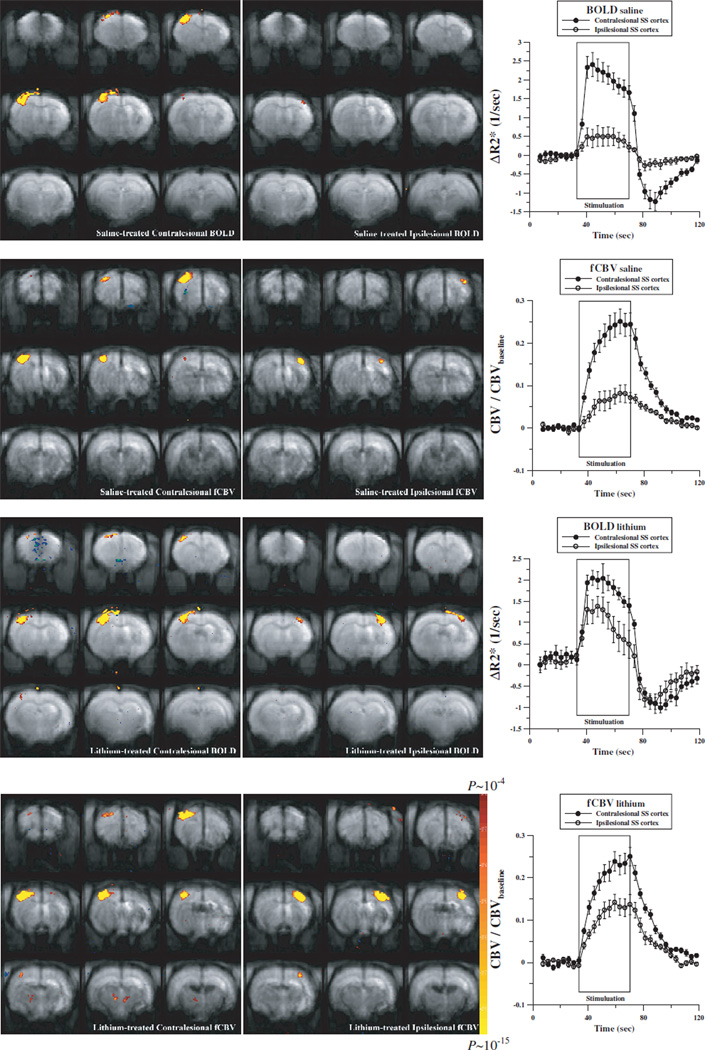
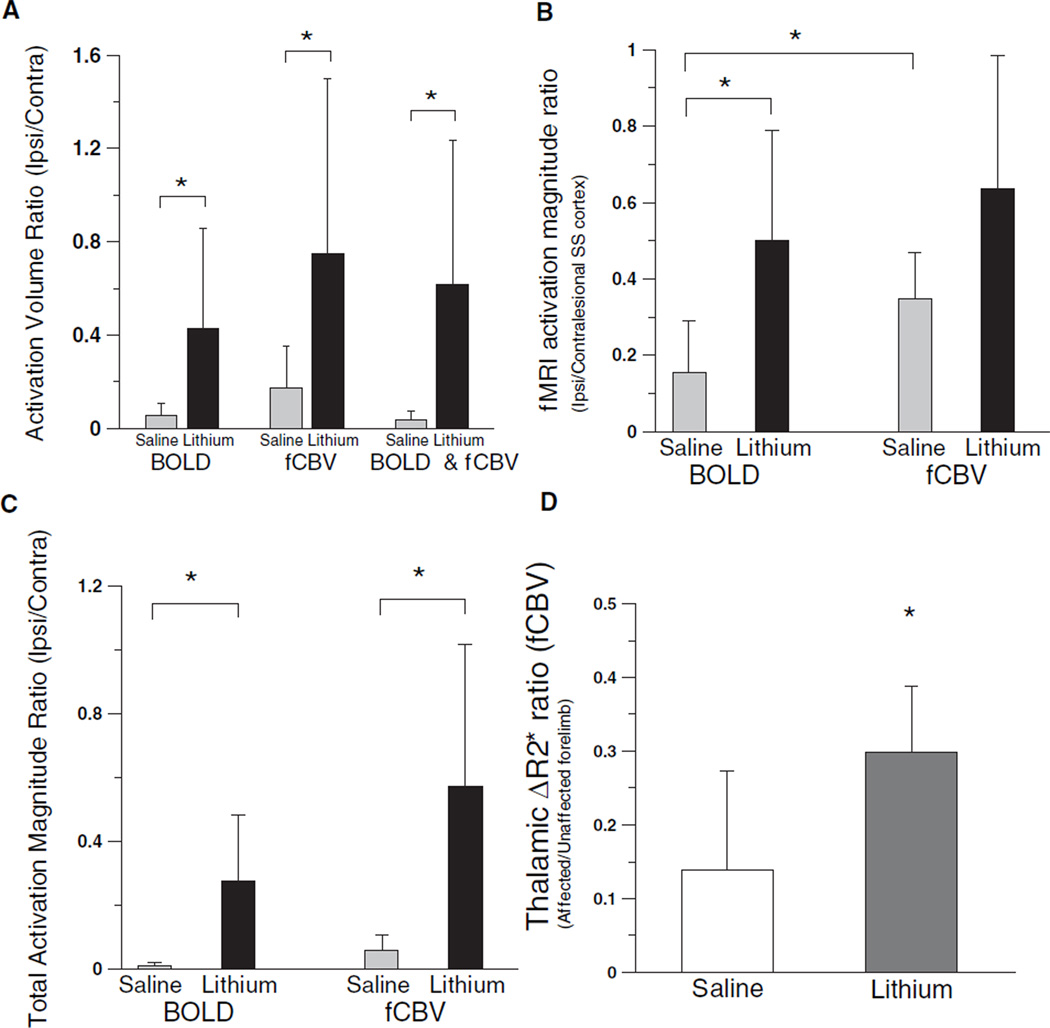
Varying degrees of ipsilesional fMRI responses were observed in both lithium- and saline-treated groups. Affected limb stimulation induced smaller overall fMRI responses compared with contralateral normal limbs for both BOLD and fCBV techniques (see Figure 1). The mean activated volume ratio between ipsilesional and contralesional cortices was significantly higher for the lithium-treated rats than the saline-treated rats (Figure 2A). For the fMRI signal magnitude, the mean activation magnitude improved for both fCBV and BOLD responses; however, only BOLD responses were significantly improved with the lithium treatment compared with the saline controls. The somatosensory BOLD activation signal magnitude ratio of lithium-treated rats was significantly higher than that of saline-treated rats (Figure 2B). The fCBV response ratio was also elevated with the lithium treatment; however, the difference was not statistically significant. When accounting for both the activation volume and the fMRI signal magnitude, the total activation magnitude acquired by multiplying the significantly activated volume by the mean fMRI activation magnitude over this volume in the ipsilesional hemispheres was significantly elevated for both fMRI methods with the lithium treatment (Figure 2C). In addition, fCBV bithalamic activations were mainly observed during the stimulation of unaffected forelimbs.9 As shown in Figure 2D, the lithium treatment significantly enhanced bithalamic activation during the stimulation of the affected forelimb relative to the unaffected forelimb.
Figure 1.
Representative fMRI activation maps and time courses of saline-treated (top 2 rows) and lithium-treated (bottom 2 rows) brains 2 weeks after the transient middle cerebral artery occlusion acquired by stimulating unaffected (left column) and affected fore-limbs (middle column) using BOLD (first and third rows) and fCBV responses (second and fourth rows).
Figure 2.
Ratios between contralesional and ipsilesional hemispheres activated volumes, in which a voxel is considered activated with a threshold of P<0.0001 (A), activation signal ratio between ipsi-/contralesional cortices (B), total activation magnitude ratio between ipsilesional and contralesional SS cortices (C), and thalamic fCBV activation amplitude ratio acquired from the entire thalamus between affected and unaffected forelimbs (D) for the lithium-treated (n=6) and saline-treated (n=6) rats (*P<0.05).
Structure–Activation Correlation
When comparing the relationship between various fMRI parameters and structural MRI measurements (see Figures 1 and 3), the ADC and FA values that were acquired from each somatosensory cortex were found to be significantly correlated with rΔR2*fCBV only for the lithium-treated rats as shown in the Table. This is probably due to the increase dynamic range of the fMRI responses for the correlation analysis, suggesting that the recovered fMRI activity is modulated by the degree of structural degradation.
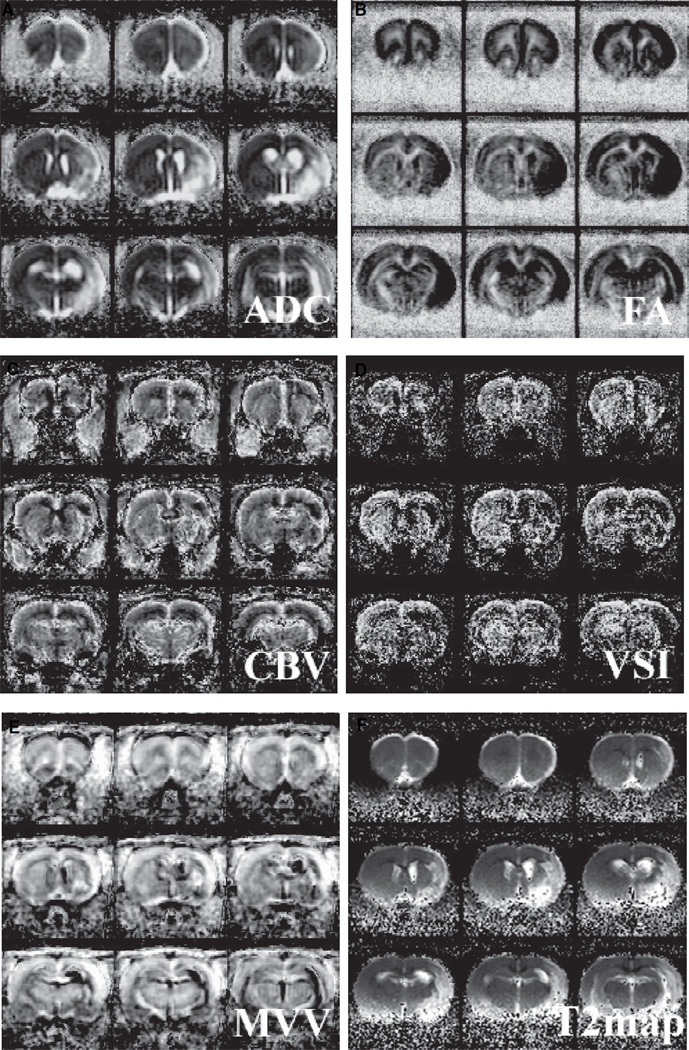
Figure 3.
Averaged ADC, FA, CBV, VSI, MVV, and T2 (A, B, C, D, E, and F, respectively) maps of the lithium-treated rats (n=6).
Table.
Correlation Coefficient(r) Between MRI-Measured Structural Parameters and Functional Activities (CS-contrastimulus) in (A) Saline Control and (B) Lithium-Treated Rats
| ADC | FA | CBV | VSI | MVV | |
|---|---|---|---|---|---|
| A. | |||||
| rΔR2*CS (fCBV) | −0.395 | 0.090 | 0.352 | 0.269 | 0.684 |
| rΔR2*CS (BOLD) | −0.452 | −0.290 | −0.087 | 0.246 | 0.228 |
| B. | |||||
| rΔR2*CS (fCBV) | 0.847 | −0.929 | −0.104 | −0.112 | −0.022 |
| rΔR2*CS (BOLD) | 0.471 | −0.785 | −0.170 | −0.234 | −0.001 |
Bold correlation coefficients represent significant correlation (P<0.05).
Structural Parameters
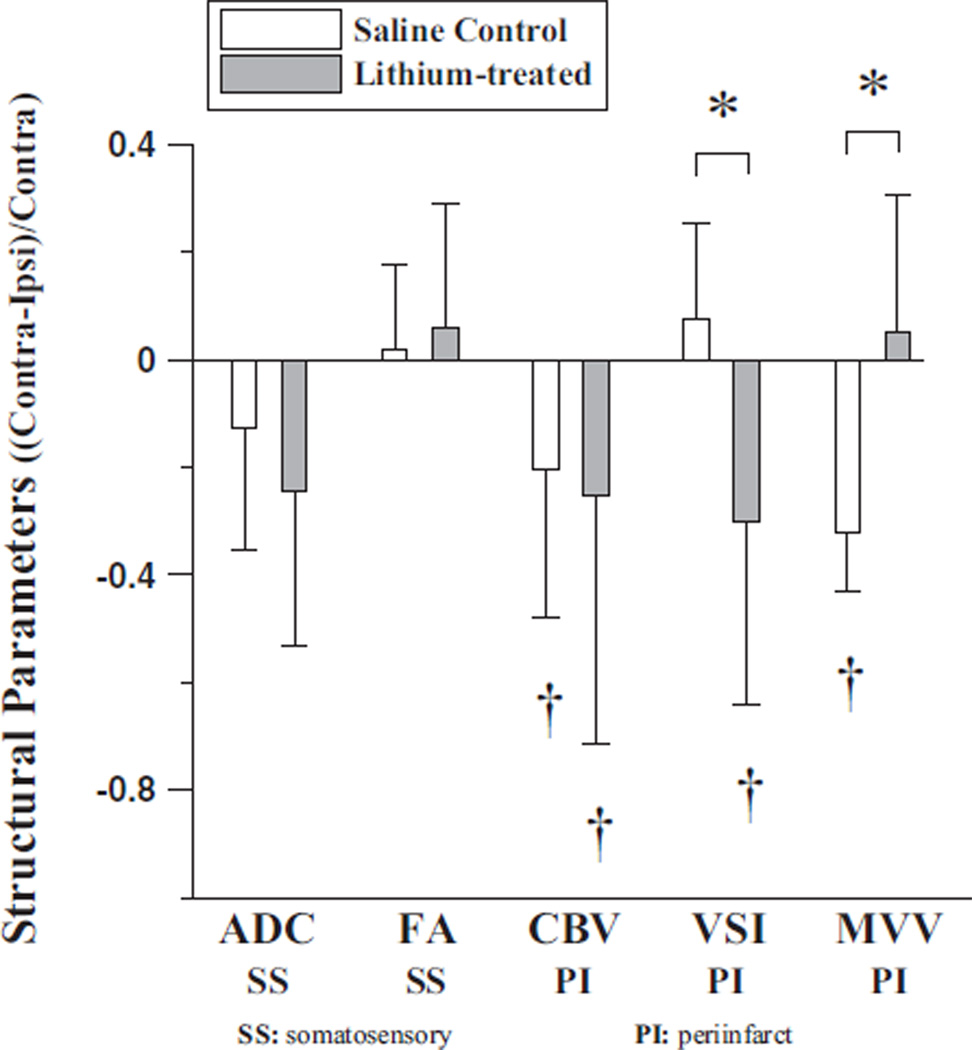
Various MRI-derived structural parameters were quantified by measuring the tissue water diffusion (ADC and FA) and vascular morphology (CBV, MVV, and VSI). In addition to frequently acquired parameters such as ADC and CBV, FA was measured in this study to assess the integrity of white matter track, whereas MVV and VSI were collected to reveal the blood volume pertaining only to microvessels and the mean vessel diameter, respectively (see Figure 3). The T2 lesion volume (Figure 3) was not different between saline-and lithium-treated rats. From the acquired ADC maps, ex vacuo dilation of the right lateral ventricles was identified for most rats. The mean ADC significantly increased for both animal groups at this late stage of stroke progression, whereas the mean FA decreased only slightly in the ipsilesional somatosensory cortices without statistical significance (see Figure 4).
Figure 4.
>MRI-derived structural parameters of saline control (n=6) and lithium-treated rats (n=6) (*P<0.05). For ADC and FA analyses, ROIs were chosen over somatosensory cortex and for CBV, VSI, and, MVV; the ROIs were chosen over the periinfarct area (≈0.2 mm of the lesion border defined from T2-weighted images). †P<0.05 significantly different from zero.
In general, all the MRI-derived structural parameters were nearly identical between the saline- and lithium-treated animals when measurements were made from the ROI anatomically placed over the somatosensory cortex (Figure 4). On the other hand, the periinfarct CBV was significantly elevated in both animal groups, and several MRI-derived parameters (eg, VSI and MVV) measured from periinfarct areas also responded to the lithium therapy. The periinfarct VSI was significantly more elevated in the ipsilesional cortex than in the contralateral cortex for the lithium-treated rats, whereas the MVV appeared uninfluenced by the lithium therapy. In contrast, the ipsilesional VSI in the saline-treated controls was similar to the contralateral VSI values and MVV was significantly greater in the ipsilesional cortex (Figure 4).
Figure 5 shows both qualitative and quantitative comparisons of immunohistochemical assays (CD31, glia fibrillary-associated protein, and MMP-9) acquired from periinfarct areas of saline- and lithium-treated rat brains. The staining using CD31 suggests increased distribution and size of microvasculature for the lithium-treated brains in comparison to the saline-treated (left column in Figure 5A). Similarly, the glia fibrillary-associated protein staining also shows the highly enhanced astrocytic signals with the lithium treatment (left column in Figure 5B). MMP-9 signals in these periinfarct cortical areas were also noted, most likely corresponding to neurovascular remodeling in recovering brain tissue. Increased MMP-9 levels were prominently observed in the lithium-treated brains, which showed colocalization of MMP-9 staining with CD31 and glia fibrillary-associated protein-positive structures (middle and right columns in Figures 5A and 5B). Figure 5C shows an increased level of CD31 and MMP-9 signals obtained from periinfarct regions of lithium-treated rat brains when compared with the contralesional counterpart. However, the periinfarct CD31 and MMP-9 signal of the saline-treated rat brains was equivalent to those of the contralesional counterpart.
Figure 5.
Immunohistochemically stained periinfarct regions using CD31 and MMP-9 (A), and glia fibrillary-associated protein and MMP-9 (B) antibodies. Top row: saline-treated; bottom row: lithium-treated rats. Comparison of quantified fluorescence density acquired from peri-infarct areas: *P<0.05 (C).
Discussion
The ipsilesional response amplitude as well as the activation volume in the responsive ipsilesional somatosensory (SS) cortex improved with the lithium treatment in comparison to the saline-treated controls. Similar to the previously shown results using untreated ischemic rats,9 the response magnitude of ipsilesional BOLD activation, when normalized to the contralesional cortex, was generally smaller than the fCBV response magnitude in both saline- and lithium-treated rats. This particular result (ipsilesional BOLD–fCBV activation mismatch) was more pronounced in the saline-treated rats (Figure 2B), in which the difference between ipsilesional BOLD and fCBV activation magnitudes was statistically significant.9 The difference between BOLD and fCBV activation magnitude ratios in the saline-treated group cannot be explained using a linear model, which assumes intact neurohemodynamic coupling. This suggests that the basic neurohemodynamic coupling was possibly disrupted, implying that unusual changes occur in the cerebral metabolic rate of oxygen and/or CBV during the electrical stimulation in the saline-treated animal group.20
We demonstrated that the difference between BOLD and fCBV activation magnitude ratios diminished with the lithium treatment. Specifically, both the BOLD and fCBV responses positively responded to the lithium treatment, whereas only the BOLD response improved significantly (Figure 2B). It is also notable that the increase of the ipsilesional fCBV response in the lithium-treated rats was not statistically significant, matching the unresponsive behavioral measurements. These findings suggest a possibility that a qualitative transformation of fMRI activity may have occurred within the SS cortex and that an increase of BOLD activity may be due to factors other than neurological improvements such as reduced oxygen consumption rate and increased baseline CBF.20 On the other hand, when we quantified the overall fMRI activity (ie, total activation) by multiplying the fMRI activation volume by the fMRI response magnitude (Figure 2C), the total activation value significantly increased for both BOLD and fCBV measurements, revealing that the restoration of fMRI activity was quantitative and highly differentiable between the 2 animal groups. As such, the lithium treatment elevated the ipsilesional fMRI responses, in which the presumably damaged neurohemodynamic linkage underwent a transformational process. Additionally, although highly reduced in magnitude compared with those of healthy rats,9 the bithalamic activation during the stimulation of affected forelimbs was significantly enhanced with the lithium treatment, implying improved connectivity through thalamic pathways.
The ipsilesional activation volume increase with the lithium treatment was statistically significant for both fMRI techniques. Despite the statistically significant activation volume ratio increase for the fCBV method, the fCBV activation magnitude ratio in the lithium-treated rats was similar to that of saline-treated rats (Figure 2B). This mismatched enhancement between the ipsilesional activation volume and magnitude in the lithium-treated rats shows that the fMRI activation density has been altered during fCBV responses, suggesting that the lithium treatment might have affected the fCBV response through a spatial co-option of hemodynamic activity.
In the current scheme of delayed chronic lithium administration, the improved fMRI responses did not translate into the improvement of behavioral recovery, which was previously observed by other lithium-based therapy studies that used the administration during the acute stage1 or the extended pre- and poststroke lithium treatment.7 The current study used daily subcutaneous administration of lithium starting 12 hours after the onset of stroke. In addition to such model differences, absence of neurological improvement may be due to the incompleteness of neurohemodynamic (ie, fMRI) recovery, spatially variable ischemic damage across models, and/or the difficulty of accurately assessing sensorimotor functions of the rats recovering from stroke with variable severity of infarct. In particular, the mismatched relationship between the fMRI activity in the SS cortex and behavioral measures may be caused by the fact that the general neurological status is likely to be affected by the function of other structures (eg, basal ganglia, motor cortex, thalamus), some of which were within the area affected by ischemia (Figure 3). Therefore, it still remains a question whether the improvement of local fMRI activity represents the actual functional restoration with a relevance to identifying the stroke-induced morbidity or that the measured fMRI parameters are only sensitive to drug-induced changes at the cellular level.1 Nonetheless, we posit that, for animal stroke model studies, fMRI may provide a sensitive tool that allows the detection of restoration in sensorimotor neurohemodynamic and metabolic correlates20 and demonstrated the efficacy of lithium treatment. However, because we have not fully explored the entire gamut of behavioral tests in our rat model of recovering stroke, we cannot be sure whether, in the end, the lack of correlation lies with our inadequate testing or with the behavioral relevance of the MRI parameters per se.
Significant increases in ipsilesional SS ADC values show that the structural degradation at the cellular level is evident at the 2-week stage of stroke progression. Similar to the previous study, we did not observe either temporal changes in signal intensity or signal voids after the administration of monocrystalline iron oxide nanocolloid, which suggest an intact blood–brain barrier.9 It was interesting to observe that the MRI-derived structural parameters of the periinfarct area showed an apparent vascular transformation with the chronic lithium treatment. In particular, the periinfarct VSI values of the lithium-treated rats and the periinfarct MVV of the salinetreated rats was significantly elevated. For both the lithium- and saline-treated rats, the total resting blood volume of the periinfarct areas also increased in comparison to the contralateral counterpart. Such increases in blood volume were probably caused by the increased neovascular formation as was previously demonstrated with the increased periinfarct vascular endothelial growth factor activity in saline-treated rats by Zhao et al.19
Because MRI-derived vascular parameters indicate a qualitative difference, one can infer that the increase of blood volume in the control rats is due to the elevated microvascular volume, whereas the CBV increase in the lithium-treated rats was probably due to the increased number of relatively large vessels. Taken together, it can be suggested that the significant increase of blood volume in both rat groups resulted from a prolonged angiogenic processes at a different progression rate and/or that the nature of this vascular formation was differently affected between the 2 rat groups. Increased blood volume detected at the vicinity of the infarct core in the lithium-treated rats is consistent with the fact that lithium inhibits the activation of glycogen synthase kinase-3β, which is known to inhibit capillary formation.21,22 Our histological data also revealed that the microvascular structure and MMP activity may be associated with lithium treatment, particularly at the periinfarct zone (see Figure 5). Moreover, lithium’s ability to modulate glycogen synthase kinase-3β activity may also downregulate the neurodegenerative processes, which may in turn influence the overall neuronal function.23 Along with the increased fMRI activity, the findings show that both structural and possible neurofunctional changes due to the lithium administration can be detected by the MRI-derived parameters. However, our study cannot conclusively say whether fMRI (ie, BOLD) responses were directly mediated by the lithium-affected vascular transformation. A major limitation of our study is that we did not obtain vascular immunostaining in the same brains as those used for fMRI. Therefore, although the quantified vascular staining suggests a lithium effect, we cannot directly conclude that these signals truly explain the fMRI findings.
In summary, overall BOLD and fCBV activities (activation magnitude and volume) in the ipsilesional cortex increased with chronic lithium treatment. However, the enhanced fMRI activations indicate only a partial restoration of neurohemodynamic activity. To investigate the integrity of the fMRI responses, we compared the ipsilesional BOLD and fCBV activities in which the BOLD–fCBV coupling appeared to be altered in the ipsilesional cortex of recovering brains. The mismatch between ipsilesional BOLD and fCBV responses, which was present in the control animal group, was mitigated with the prolonged lithium treatments. In particular, the delayed chronic lithium treatment enhanced the BOLD–fMRI response magnitude without invoking behavioral improvement. Discrepant results using BOLD and fCBV methods, demonstrated by the inconclusive restoration of hemodynamic measurements of the current experiments, warrant future studies for understanding the evolution of underlying metabolic processes during the recovery phase of stroke. Additionally, the lithium treatment appeared to affect the vascular morphology, which was different from the control group. Because fMRI signals were derived from the neurohemodynamic activities, it will be interesting to investigate the possible relationship between the abnormal vascular transformation and the affected fMRI activity from such vascular transformations. In conclusion, the delayed chronic lithium treatment enhanced the overall fMRI activity in the ipsilesional SS cortex despite the absence of neurological improvement during the recovery phase of focal ischemia.
Acknowledgments
We thank Dr Christian Farrar for reviewing the manuscript.
Sources of Funding
This study was supported in part by a Bugher award from the American Heart Association and grants from the National Institutes of Health: 5R01EB002066-17 R01-EB002066, R01-NS37074, R01-NS48422, R01-NS56458, P50-NS10828, and P01-NS55104.
Footnotes
Disclosures
None.
References
- 1.Ren M, Senatorov VV, Chen RW, Chuang DM. Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. Proc Natl Acad Sci U S A. 2003;100:6210–6215. doi: 10.1073/pnas.0937423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chuang DM. Neuroprotective and neurotrophic actions of the mood stabilizer lithium: can it be used to treat neurodegenerative diseases? Crit Rev Neurobiol. 2004;16:83–90. doi: 10.1615/critrevneurobiol.v16.i12.90. [DOI] [PubMed] [Google Scholar]
- 3.Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Rev Mol Med. 2004;6:1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
- 4.Bhat RV, Shanley J, Correll MP, Fieles WE, Keith RA, Scott CW, Lee CM. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci U S A. 2000;97:11074–11079. doi: 10.1073/pnas.190297597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhat RV, Budd Haeberlein SL, Avila J. Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem. 2004;89:1313–1317. doi: 10.1111/j.1471-4159.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 6.Chuang DM, Chen RW, Chalecka-Franaszek E, Ren M, Hashimoto R, Senatorov V, Kanai H, Hough C, Hiroi T, Leeds P. Neuroprotective effects of lithium in cultured cells and animal models of diseases. Bipolar Disord. 2002;4:129–136. doi: 10.1034/j.1399-5618.2002.01179.x. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Culman J, Blume A, Brecht S, Gohlke P. Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death. Stroke. 2003;34:1287–1292. doi: 10.1161/01.STR.0000066308.25088.64. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Scholz A, Rosch N, Blume A, Unger T, Kreutz R, Culman J, Gohlke P. Low-dose lithium combined with captopril prevents stroke and improves survival in salt-loaded, stroke-prone spontaneously hypertensive rats. J Hypertens. 2005;23:2277–2285. doi: 10.1097/01.hjh.0000189868.48290.d8. [DOI] [PubMed] [Google Scholar]
- 9.Kim YR, Huang IJ, Lee SR, Tejima E, Mandeville JB, van Meer MP, Dai G, Choi YW, Dijkhuizen RM, Lo EH, Rosen BR. Measurements of BOLD/CBV ratio show altered fMRI hemodynamics during stroke recovery in rats. J Cereb Blood Flow Metab. 2005;25:820–829. doi: 10.1038/sj.jcbfm.9600084. [DOI] [PubMed] [Google Scholar]
- 10.Dijkhuizen RM, Ren J, Mandeville JB, Wu O, Ozdag FM, Moskowitz MA, Rosen BR, Finklestein SP. Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc Natl Acad Sci U S A. 2001;98:12766–12771. doi: 10.1073/pnas.231235598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 12.Menzies SA, Hoff JT, Betz AL. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery. 1992;31:100–106. doi: 10.1227/00006123-199207000-00014. discussion 106–107. [DOI] [PubMed] [Google Scholar]
- 13.Reglodi D, Tamas A, Lengvari I. Examination of sensorimotor performance following middle cerebral artery occlusion in rats. Brain Res Bull. 2003;59:459–466. doi: 10.1016/s0361-9230(02)00962-0. [DOI] [PubMed] [Google Scholar]
- 14.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 15.Dennie J, Mandeville JB, Boxerman JL, Packard SD, Rosen BR, Weisskoff RM. NMR imaging of changes in vascular morphology due to tumor angiogenesis. Magn Reson Med. 1998;40:793–799. doi: 10.1002/mrm.1910400602. [DOI] [PubMed] [Google Scholar]
- 16.Mandeville JB, Marota JJ, Kosofsky BE, Keltner JR, Weissleder R, Rosen BR, Weisskoff RM. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med. 1998;39:615–624. doi: 10.1002/mrm.1910390415. [DOI] [PubMed] [Google Scholar]
- 17.Zaharchuk G, Yamada M, Sasamata M, Jenkins BG, Moskowitz MA, Rosen BR. Is all perfusion-weighted magnetic resonance imaging for stroke equal? The temporal evolution of multiple hemodynamic parameters after focal ischemia in rats correlated with evidence of infarction. J Cereb Blood Flow Metab. 2000;20:1341–1351. doi: 10.1097/00004647-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen AG, Wu O, Copen WA, Davis TL, Gonzalez RG, Koroshetz WJ, Reese TG, Rosen BR, Wedeen VJ, Weisskoff RM. Human acute cerebral ischemia: detection of changes in water diffusion anisotropy by using MR imaging. Radiology. 1999;212:785–792. doi: 10.1148/radiology.212.3.r99se24785. [DOI] [PubMed] [Google Scholar]
- 19.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 20.Mandeville JB, Marota JJ, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR. MRI measurement of the temporal evolution of relative CMRO(2) during rat forepaw stimulation. Magn Reson Med. 1999;42:944–951. doi: 10.1002/(sici)1522-2594(199911)42:5<944::aid-mrm15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Kim HS, Skurk C, Thomas SR, Bialik A, Suhara T, Kureishi Y, Birnbaum M, Keaney JF, Jr, Walsh K. Regulation of angiogenesis by glycogen synthase kinase-3beta. J Biol Chem. 2002;277:41888–41896. doi: 10.1074/jbc.M206657200. [DOI] [PubMed] [Google Scholar]
- 22.Zhang F, Cheng J, Hackett NR, Lam G, Shido K, Pergolizzi R, Jin DK, Crystal RG, Rafii S. Adenovirus e4 gene promotes selective endothelial cell survival and angiogenesis via activation of the vascular endothelial-cadherin/AKT signaling pathway. J Biol Chem. 2004;279:11760–11766. doi: 10.1074/jbc.M312221200. [DOI] [PubMed] [Google Scholar]
- 23.Liang MH, Chuang DM. Regulation and function of glycogen synthase kinase-3 isoforms in neuronal survival. J Biol Chem. 2007;282:3904–3917. doi: 10.1074/jbc.M605178200. [DOI] [PubMed] [Google Scholar]