Alterations in thrombosis and fibrinolysis comprise important parts of stroke pathophysiology. A key step in the fibrinolytic process includes the tissue-type plasminogen activator (tPA)-mediated conversion of the proenzyme plasminogen into the active protease plasmin, which in turn degrades the fibrin structure of intravascular thrombi. There are a number of review articles well summarizing molecular mechanisms of the fibrinolytic system.1,2 Inhibition of the fibrinolytic system may occur at the level of plasminogen activation, mainly by a direct inhibition of tPA by plasminogen activator inhibitor 1 (PAI-1) or indirectly by thrombin-activatable fibrinolysis inhibitor and, at the level of plasmin, by α2-antiplasmin. The roles of these 3 inhibitors are complementary in thrombolysis.3
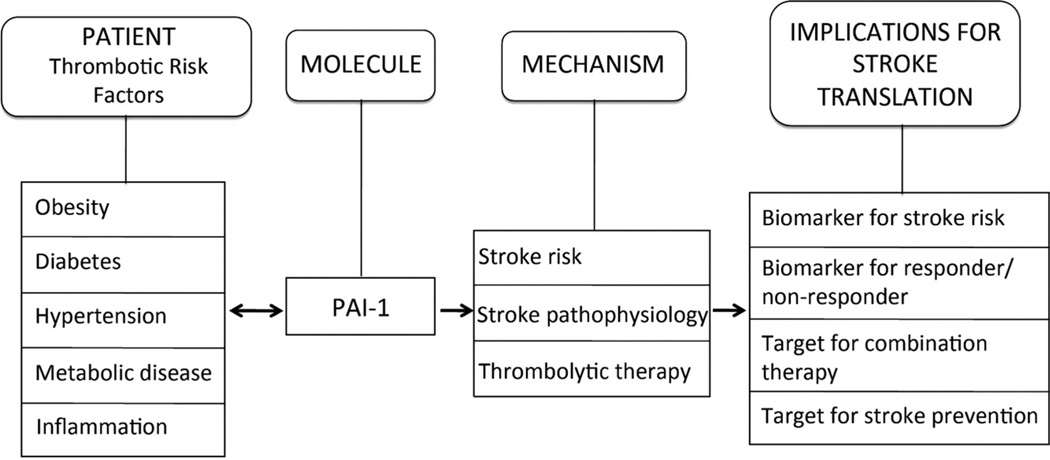
PAI-1 has become recognized as a central molecule linking pathogenesis and progression of thrombotic vascular events including stroke. As a main endogenous inhibitor of tPA, PAI-1 might be related to reperfusion efficacy and hemorrhagic risk of tPA thrombolytic therapy. Moreover, a clear association has been observed between elevated PAI-1 plasma levels and prothrombotic disease conditions such as hypertension, obesity, insulin resistance, and diabetes.4–7 Clinical and experimental studies show that PAI-1 deficiencies cause accelerated fibrinolysis and bleeding, whereas elevated PAI-1 plasma levels are associated with vascular thrombosis.8 Concentrations of active PAI-1 in newly formed thrombi can be several thousandfold greater than active PAI-1 concentrations in normal plasma9 and thus high enough to inhibit doses of tPA used for clinical thrombolysis. Furthermore, beyond its effects in modulating the activity of tPA and the functionally related urokinase-type plasminogen activator, which predominantly catalyzes plasmin formation in the extravascular space, PAI-1 also plays diverse roles in metabolic and vascular disease and may participate in the evolution of brain damage and recovery after stroke.4,10,11 In this translational review, we briefly survey the molecular mechanisms of PAI-1 and propose that it may serve as a critical crosstalk molecule, potential biomarker, and target that links risk factors to stroke mechanisms and response to thrombolytic and neuroprotective strategies.
Mechanisms, Regulation, and Sources of PAI-1
PAI-1 is a member of the serine protease inhibitor (serpin) superfamily.12 It is always synthesized in an active configuration but spontaneously converts into an inactive state with a half-life of approximately 1 to 2 hours in physiological environments.13 In plasma, the active form of PAI-1 is stabilized by binding to vitronectin, thereby increasing its half-life severalfold. The rate of inactivation is also dramatically reduced at lower pH such as in the ischemic tissues, thus potentially increasing the capacity to inhibit fibrinolysis further. Under some in vitro conditions, inactive PAI-1 can be reactivated by denaturants such as sodium dodecyl sulfate, guanidine HCl, and urea.14 Whether reactivation of PAI-1 takes place in vivo is uncertain, although it has been suggested that negatively charged phospholipids exposed on the surface of activated platelets could reactivate PAI-1.15 Binding and inactivation of tPA by PAI-1 is very fast with a second-order rate constant between 106 and 107m−1 • s−1 and the approximately 110 kDa tPA–PAI-1 complex is stable under physiological conditions.16
PAI-1 has 3 potential sites for N-linked glycosylation— N232, N288, andN352—and different states of glycosylation may affect the stability of active PAI-1.17 Furthermore, glycosylated PAI-1 may have a stronger inhibitory activity on tPA and urokinase-type plasminogen activator than nonglycosylated PAI-1.18 It is therefore necessary to further study the biological importance of glycosylation by evaluating the pattern and degree of PAI-1 glycosylation in various cell types and tissues in different conditions.
The PAI-1 promoter contains a common −675 4G/5G polymorphism that may affect both basal and inducible PAI-1 expression. However, clinical studies have shown divergent results.19,20 In vivo, elevated plasma PAI-1 antigen and activity levels are associated with increased body mass index and with features of the insulin resistance syndrome like obesity, hyperlipidemia, and hyperinsulinemia.6,7,21 Lipoproteins, insulin, and glucose have been shown to increase PAI-1 synthesis in several cell types in vitro.22 Moreover, PAI-1 is well recognized as an acute-phase reactant and its expression can increase rapidly in response to inflammatory cytokines, transforming growth factor-β,23,24 angiotensin II,25 and hypoxia.26 There is also a pronounced circadian variation in PAI-1 plasma levels with the highest concentration present in the morning and lowest in the late afternoon.27
Under normal conditions, PAI-1 is present in plasma at low concentrations (5–20 ng/mL). It is cleared by the liver with a half-life of approximately 5 minutes, indicating a high biosynthetic rate. The concentration of PAI-1 can change rapidly in response to a number of stimuli, demonstrating a dynamic regulation. However, the origin of circulating PAI-1, ultimately responsible for the hemostatic–fibrinolytic balance in blood, remains to be fully defined.
The largest pool of PAI-1 in blood is present in platelet α granules, which contain approximately 90% of the circulating PAI-1, and platelet count is correlated with plasma PAI-1 concentrations.28 Nevertheless, the platelet contribution to PAI-1 plasma levels is controversial, because the majority of PAI-1 in platelets has been reported to be inactive compared with plasma PAI-1, which is mainly active. However, this observation could be due to preparation artifacts, and the majority of platelet PAI-1 may be active.29 Additionally, emerging data suggest that there is a constitutive de novo synthesis of active PAI-1 in platelets and the synthesis rate in vitro is approximately 35-fold greater than that required to maintain steady-state plasma levels.30 Interestingly, the glycosylation fingerprint of plasma PAI-1 implies a platelet origin.31
PAI-1 can be produced by many cell types in culture and is widely distributed in many tissues in vivo. In addition to platelets, liver, endothelial cells, macrophages, and adipocytes may all contribute to plasma PAI-1 in humans. Under pathological conditions such as vascular disease, sepsis, inflammation, and metabolic disorders such as obesity and diabetes, PAI-1 may be differentially regulated in all these various cell types. Because PAI-1 may display cell type-specific glycosylation and activity, the glycosylation patterns of PAI-1 may serve as potential biomarkers to predict cellular dysfunction and thrombotic risk in humans.31
Human Plasma PAI-1 Levels and Assessments
The concentration of PAI-1 in human plasma varies in relation to a large number of factors. In a young healthy population, the levels range from a few nanograms per milliliter up to >100 ng/mL in an obese diabetic population. Lifestyle variables and age influence the PAI-1 level but the strongest affecting factor is insulin resistance.7 Analysis of PAI-1 requires some important considerations. Like with any other protein released from activated platelets, careful sampling is a prerequisite for correct assessment. Evidently this is particularly important for blood sampling in conditions with hyperreactive platelets such as in diabetes. Furthermore, in view of the circadian variation in plasma PAI-1 levels,27 the timing of sampling is critical.
Due to the conformational changes of the PAI-1 molecule depending on its state, detection and quantification of PAI-1 is intricate and care should be taken when choosing immunochemical assays. Evaluated enzyme-linked immunosorbent assays detecting all molecular forms with similar affinity have to be used for PAI-1 antigen determination. The standard used in the enzyme-linked immunosorbent assay is crucial for correct assessment of absolute concentrations and should preferably originate from the same source to ensure identical detection.17,32
Analyzing PAI-1 activity also requires some considerations. Due to the thermodynamic instability of the active molecule and the short half-life, it is crucial that the molecule is captured in its active form and that spontaneous inactivation during the preparatory procedure is prevented for correct assessment. Plasma PAI-1 is generally stabilized by lowering the pH using acid citrate. However, analysis of intracellular PAI-1 or PAI-1 released in cell culture is more intricate. Common methods for cell lysis (sonication, freezing–thawing, and Triton X-100) without immediate capture of the active molecule may cause inactivation.29 Furthermore, multicenter evaluations have shown the difficulties in assessing PAI-1 activity and the majority of assays failed to correctly determine the true activity of prepared samples.33 For clinical studies, standardization of sampling and assessment protocols is necessary.
PAI-1 in the Brain
When dissecting the role and function of PAI-1 in stroke pathophysiology, it is important to keep in mind that in the brain, the roles of tPA and PAI-1 extend beyond that of regulating vascular patency. In the central nervous system, PAI-1 is mainly expressed by astrocytes, whereas the expression of tPA is more widely distributed.34,35 Besides the clearly beneficial role of tPA as a thrombolytic molecule in ischemic stroke, tPA may also play detrimental roles at the blood–brain interface, where it can mediate blood–brain barrier leakage, edema, and hemorrhagic transformation.36
Conversely, PAI-1, derived from astrocytes, can reduce excitotoxicity and neuronal cell death by limiting excessive tPA activity in the brain parenchyma. Transforming growth factor-β may enhance this neuroprotective effect by upregulating astrocytic PAI-1 expression.37 In experimental models of permanent middle cerebral artery occlusion, in which the inhibitory role of PAI-1 in intravascular fibrinolysis is precluded, a reduced infarct volume is observed after overexpression or intracranial injection of PAI-1,38,39 whereas mice deficient in PAI-1 display exacerbated brain damage.40 PAI-1 may positively regulate blood–brain barrier function by directly enhancing endothelial tight junction properties.41 Moreover, PAI-1 has been shown to have an antiapoptotic role in neurons independent of its protease inhibitor properties.42 However, the involvement of PAI-1 in the late recovery phase after stroke remains unknown.
Interestingly, the PAI-1–675 4G/5G polymorphism has been shown to affect PAI-1 transcriptional activity in human astrocytes under both basal and transforming growth factor-β-stimulated conditions.43 A recent meta-analysis suggests that the PAI-1 4G high expression allele may have a protective effect in ischemic stroke, whereas the same allele may be associated with an increased risk of myocardial infarction.20 While lending further support for a neuroprotective role of PAI-1 in the central nervous system, these findings clearly point to a more complex role for tPA and PAI-1 in stroke pathophysiology compared with other thrombotic diseases, thus making the design of PAI-1-targeted treatments more challenging.
PAI-1 and Pathogenic Factors in Cerebrovascular Disease
Perturbations in PAI-1 and impairment of the fibrinolytic cascade are associated with a wide range of thrombotic conditions and risk factors.8 However, because many cardiovascular risk factors are known to influence plasma PAI-1, the predictive ability of PAI-1 in these epidemiological studies is strongly reduced after adjusting for other risk factors, especially the markers of the metabolic syndrome (body mass index, triglycerides, high-density lipoprotein cholesterol, hyperglycemia, and hyperinsulinemia).6,8,44 This suggests that the metabolic syndrome might be a required pathological condition for the increased plasma PAI-1 levels in patients at risk of vascular thrombosis. Thus, the pathophysiological mechanisms underlying development and progression of vascular thrombotic disease and the role of PAI-1 in these processes need to be further elucidated.
PAI-1 in Atherosclerosis
PAI-1, as the principal inhibitor of fibrinolysis, may play an important role in cerebrovascular diseases by promoting vascular atherosclerosis and thrombosis.45 The presence of increased PAI-1 expression in atherosclerotic lesions and atheroma strongly suggests a critical role in atherogenesis. For example, at 3 months after the first transient ischemic attack or stroke caused by an intracranial atherostenosis, high blood levels of C-reactive protein and PAI-I were identified as significant predictors of intracranial large artery atherosclerosis progression and risk of recurrent ischemic strokes.46 However, experimental studies have shown both beneficial and deleterious properties of PAI-1, which makes its role and causal effects on the atherogenic process controversial. The complex vascular functions of PAI-1 may depend on the vascular bed, type of lesion, the experimental/clinical conditions, and its interactions with different molecules. For instance, when vascular injury is associated with activation of the coagulation system and fibrin formation, PAI-1 may have atherogenic properties by stabilizing fibrin.47 In the absence of fibrin, PAI-1 may inhibit cell migration within the vascular wall, thus inhibiting formation of intimal hyperplasia.48 Although many unresolved issues remain, PAI-1 may be a significant contributor to the mechanisms of atherosclerosis.
PAI-1 in Obesity
There is a strong correlation between PAI-1 and body mass index and clinical studies have demonstrated an association of obesity with impaired fibrinolysis. Because obesity is considered a low-grade inflammatory state, elevated inflammatory cytokines could induce the increased PAI-1 expression.44,49 In both nutritionally induced and ob/ob mice, infarct size after focal stroke was significantly larger compared with lean wild-type controls. In both models, obesity was associated with markedly elevated circulating PAI-1 levels. This study suggests that PAI-1 may contribute to the deleterious effect of obesity in stroke risk and outcome.4 Nevertheless, uncertainties remain about whether observed associations between elevated levels of PAI-1 and prothrombotic, metabolic and inflammatory parameters are correlative or causative. Contributions of platelet activation to the link between PAI-1 and thrombosis will have to be carefully assessed in obese individuals. Additionally, it would be clinically important to determine whether measuring PAI-1 level/activity has a role in diagnostic or therapeutic decision-making for obese and nonobese individuals with or without evidence of atherosclerotic disease.
PAI-1 in the Metabolic Syndrome and Type 2 Diabetes
Elevated plasma PAI-1 is a common feature of patients with metabolic syndrome and diabetes.7,21,50 Results from a large cohort of healthy nondiabetic subjects showed that increased levels of PAI-1 at baseline were predictive of incident diabetes over 5 years of follow-up.51 Subsequently, elevated PAI-1 levels during diabetes may increase the likelihood of developing atherosclerotic lesions and occlusive intravascular thrombi. For example, approximately 30% of patients with stroke have diabetes.7,52 Emerging evidence suggests that a decreased fibrinolytic activity or increased PAI-1 levels could play a critical role in the development of vascular diseases in patients with diabetes, because enforced weight loss and decreased plasma concentration of PAI-1 may lower the risk of thrombosis in patients with insulin resistance and a manifested cluster of risk factors for vascular diseases.50 Reducing glucose or the incidence of diabetes through an insulin-sensitizing strategy also reduces circulating PAI-1 and lowers progression of diabetes development, suggesting that PAI-1 inhibition might reduce progression to diabetes and related vascular complications in high-risk populations.53
PAI-1 in Hypertension
Hypertension is a major risk factor of thrombotic vascular diseases including ischemic stroke.54 Accelerated atherosclerosis and incidence of plaque rupture may increase the risk of ischemic events in hypertension, but it is becoming increasingly apparent that hypertension may also tilt the hemostatic balance toward a prothrombotic or hypercoagulable state.5 Increased PAI-1 and decreased tPA activity in plasma and endothelium have been found in patients with hypertension, but the underlying mechanisms have not yet been clarified. One potential pathway is the renin–angiotensin system, which plays an important role in controlling fibrinolytic function in the vasculature.55 Independent of its hemodynamic biological effects, angiotensin II can inhibit fibrinolysis by causing a release of PAI-1, whereas bradykinin may promote fibrinolysis by inducing tPA release. More investigations are needed to define whether these effects of angiotensin II may directly and indirectly contribute to the pathogenesis and progression of fibrinolysis impairment in hypertensive patients. Finding ways to synergistically lower blood pressure and normalize fibrinolytic balance may provide optimal targets for hypertension and stroke.
PAI-1 in Antiplatelet Therapy
Since the 1970s, antiplatelet therapy with aspirin has greatly benefited patients who are at high risk of occlusive vascular events. However, the effectiveness of currently available antiplatelet agents is variable.56 Clinical evidence showed that for patients with a prior ischemic attack or stroke, the incidence of aspirin resistance was significantly higher and aspirin nonresponders had a 10-fold increase in the risk of recurrent vascular events as compared with aspirin-sensitive patients.57 Therefore, finding ways to optimize antiplatelet therapy has become one of the highest priorities in the prevention of thrombotic vascular complications.
In this context of antiplatelet agents in stroke, PAI-1 should be considered a target molecule that deserves more attention. Elevated PAI-1 is associated with most prethrombotic disease conditions and is also directly associated with thrombotic risk.8 Importantly, platelets may serve as a major source of circulating PAI-1,29,30 and it is likely that the majority of the PAI-1 in thrombi is released from activated platelets. In line with this, clinical data suggest that long-term antiplatelet therapy, for example, with aspirin and/or clopidogrel, is basically effective to reduce plasma PAI-1 levels.58 In platelet-rich arterial thrombi, the release of PAI-1 from activated platelets completely outweighs the PAI-1 levels in plasma. Proper platelet inhibition and direct PAI-1 inhibition approaches are thus likely to reduce the inhibitory effect of PAI-1 on fibrinolysis and might be able to enhance thrombolytic efficacy if combined with thrombolytics for reperfusion therapy. However, associations between PAI-1 levels and long-term prevention efficacy of different anti-platelet therapies have not been established. It would be clinically important to seek potential approaches in terms of optimizing antiplatelet therapies that protect against platelet-mediated atherothrombosis. Thus, plasma PAI-1 levels and activity might be usable biomarkers to help identify patients and select antiplatelet medications that potentially can lower plasma PAI-1 levels for better effects in stroke prevention.
PAI-1 in Stroke Pathophysiology
The evolution of cerebral injury after focal ischemia involves a dynamic interplay of vascular thrombosis, ischemic brain damage, vascular and cellular inflammation, tissue recovery, and remodeling. Many of these pathways can be influenced by PAI-1 activity and signaling in different cell types. After focal cerebral ischemia induced by mechanical occlusions, infarction volumes in PAI-1 overexpressing transgenic mice were smaller than wild-type controls. However, if focal ischemia was induced through thrombosis, infarctions in PAI-1 overexpressing mice were larger than wild-types.38 This study indicates the complexity of PAI-1 in the cerebral ischemia intravascularly and inside the brain. In the vascular compartment, PAI-1 may be detrimental because it can augment persistence and formation of occlusive thrombi after vascular injury. In contrast, PAI-1 expressed in brain cells may be protective by ameliorating tPA-induced excitotoxicity and neuronal apoptosis. However, the true roles of PAI-1 in human stroke are controversial given the rarity of PAI-1-deficient humans and the inconclusiveness of PAI-1 polymorphism studies.8 Further studies are required to rigorously dissect the roles of PAI-1 in multiple cell types within the entire neurovascular unit.
PAI-1 in tPA Thrombolytic Therapy
Biologically, PAI-1, as the main and potent endogenous tPA inhibitor, is supposed to interfere with the exogenously administered tPA and affect its therapeutic outcomes. Concentrations of active PAI-1 in newly formed thrombi can be several thousandfold greater than active PAI-1 concentrations in normal plasma.9 These concentrations are high enough to inhibit doses of tPA used for clinical thrombolysis. However, clinical investigation is largely lacking. A few studies have investigated the association between admission plasma PAI-1 levels and the PAI-1 to 675 4G/5G polymorphism with symptomatic hemorrhagic transformation, recanalization resistance, and brain vessel reocclusion in patients with stroke treated with tPA reperfusion therapy.10,59,60 The results of these studies were indicative of a link between PAI-1 and tPA stroke therapy outcome, but further investigations are warranted to confirm and define this potential association and the underlying molecular mechanisms. Furthermore, the relationship with other mediators such as thrombin-activatable fibrinolysis inhibitor, antiplasmin, and perhaps even other forms of PAI also requires more investigation.
The potential influence of PAI-1 may be considered after antiplatelet therapies as well. In a clinical study in which 30% of patients with acute stroke had pretreatment with antiplatelet agents, which may lower plasma PAI-1 levels, no association was observed between antiplatelet therapy and increased risk of tPA-induced hemorrhage. This suggests that prior antiplatelet treatment might not be considered a contraindication to tPA stroke thrombolysis.61 Because metabolic syndrome is associated with the increased plasma PAI-1 levels in patients at risk of atherothrombosis, the predictive ability of PAI-1 has to be readjusted and carefully interpreted with other risk factors of atherothrombosis and the preexisting vascular comorbidities in most patients with stroke.62 Taken together, the roles and mechanisms of circulating PAI-1 to the variable reperfusion efficacy and hemorrhagic transformation risk after tPA thrombolytic stroke therapy remain to be further elucidated.
As discussed earlier, PAI-1 in experimental models may be protective against ischemia and tPA-mediated neurotoxicity. However, whether PAI-1 functions in a similar manner in human stroke like in the mouse brain remain largely unexplored. Because tPA can penetrate into the brain parenchyma by crossing the blood– brain barrier, a better understanding of how PAI-1 is expressed inside the brain, how its expression is altered, its role in the development of ischemic brain damage, and its response to exogenous tPA administration would be important for improving tPA stroke therapy.
Targeting PAI-1 for Potential Therapies
Clinically, increased understanding of the pathobiology of thrombotic and vascular disorders has helped researchers to target novel pathways. Most of these studies for potential modifiers of the fibrinolytic process have used PAI-1 as a marker of primary interest in the evaluation of the success or failure of the proposed interventions.63 In this context, numerous drugs exhibited effects of indirectly increasing fibrinolytic activity by reducing plasma PAI-1 levels, including hypoglycemic agents, angiotensin-converting enzyme inhibitors, hypolipidemizing drugs, insulin-sensitizing agents, and hormone replacement therapy in women.63–66 Because stroke risk is not homogeneous and varies with associated morbidities and different risk factors, plasma PAI-1 levels reflect a complex of genetic factors, hormonal, metabolic, and inflammatory stimuli, and body mass, and all have to be categorized into classes based on a combination of risk factors of vascular thrombosis.63,67,68
Although available data are very preliminary, a few studies have shown that the antithrombotic effects of PAI-1 inhibition are achieved by enhancing endogenous fibrinolytic activity without directly affecting blood coagulation and platelet function.69,70 Prevention or treatment of thrombotic vascular diseases is considered the most predictable application for PAI-1 antagonists. In view of the potential causal link between PAI-1 and development of obesity as well as Type 2 diabetes, a wider application of PAI-1 inhibitors in selected risk populations may also be beneficial.63 PAI-1 deficiency in both humans and mice suggests manifestations of PAI-1 deficiency are generally restricted to abnormal bleeding, but they may have a spectrum of bleeding including intracranial and joint bleeding after mild trauma and delayed surgical bleeding. Therefore, PAI-1 antagonists should be constrained to reducing PAI-1 excess rather than to completely eliminating PAI-1 activity to avoid severe bleeding and other potential side effects.63 Novel PAI-1 inhibitors in development as a new class of antithrombotic drugs might have a wider therapeutic index and overcome some of the limitations associated with conventional antiplatelet and anticoagulant agents. However, both efficacy and safety need to be carefully evaluated in preclinical studies.52,63,71 In addition, when aiming at targeting PAI-1, we need to keep in mind that the roles of PAI-1 in the intravascular space differ from its role in the brain parenchyma under both physiological and pathological conditions.20 Thus, an ideal PAI-1 antagonist for long-term intervention should only target intravascular PAI-1 without crossing the blood– brain barrier to avoid potential inhibition of PAI-1 activity in the brain parenchyma.
Conclusions
Most of our knowledge and therapies in stroke are now based on the plasminogen activator system. However, plasminogen activators do not work alone. Biologically, there is a dynamic balance and homeostasis between plasminogen activators and their endogenous inhibitors. In this context, PAI-1 may be a critical crosstalk molecule that links mechanisms to outcomes (Figure). PAI-1 signaling can be affected by a wide range of stroke risk factors, including hypertension, diabetes, obesity, metabolic disease, and vascular inflammation. PAI-1 may affect stroke mechanisms in multiple ways by affecting the risk of stroke, acute tissue injury during cerebral ischemia, and the subsequent response to thrombolytic therapies. Further translational studies are warranted to investigate the clinical roles for PAI-1 as a biomarker for stroke risk, biomarker for differentiating responders and nonresponders to reperfusion therapies, and potential target for stroke prevention and acute combination treatments.
Figure.
A schematic overview of plasminogen activator inhibitor 1 (PAI-1) as a molecular link that connects major stroke risk factors to vascular and cerebral mechanisms that influence outcomes after stroke.
Acknowledgments
Sources of Funding
The present study was supported in part by the Swedish Research Council (grant 524-2011-7563), the Swedish Society of Medicine, the Göteborg Foundation for Neurological Research, the Rune and Ulla Amlöv, Edit Jacobson and Åke Wiberg Foundations (to A.T.-W.); the Swedish Heart-Lung Foundation and Magn Bergvalls Foundation (to B.H.); National Institute of Health grants R37-NS37074 (to E.H.L.) and R01-NS065998 (to X.W.).
Footnotes
Disclosures
None.
References
- 1.Rijken DC, Lijnen HR. New insights into the molecular mechanisms of the fibrinolytic system. J Thromb Haemost. 2009;7:4–13. doi: 10.1111/j.1538-7836.2008.03220.x. [DOI] [PubMed] [Google Scholar]
- 2.Booth NA. Fibrinolysis and thrombosis. Baillieres Best Pract Res Clin Haematol. 1999;12:423–433. doi: 10.1053/beha.1999.0034. [DOI] [PubMed] [Google Scholar]
- 3.Mutch NJ, Thomas L, Moore NR, Lisiak KM, Booth NA. TAFIA, PAI-1 and alpha-antiplasmin: complementary roles in regulating lysis of thrombi and plasma clots. J Thromb Haemost. 2007;5:812–817. doi: 10.1111/j.1538-7836.2007.02430.x. [DOI] [PubMed] [Google Scholar]
- 4.Nagai N, Van Hoef B, Lijnen HR. Plasminogen activator inhibitor-1 contributes to the deleterious effect of obesity on the outcome of thrombotic ischemic stroke in mice. J Thromb Haemost. 2007;5:1726–1731. doi: 10.1111/j.1538-7836.2007.02631.x. [DOI] [PubMed] [Google Scholar]
- 5.Lip GY, Blann AD. Does hypertension confer a prothrombotic state? Virchow’s triad revisited. Circulation. 2000;101:218–220. doi: 10.1161/01.cir.101.3.218. [DOI] [PubMed] [Google Scholar]
- 6.Alessi MC, Poggi M, Juhan-Vague I. Plasminogen activator inhibitor-1, adipose tissue and insulin resistance. Curr Opin Lipidol. 2007;18:240–245. doi: 10.1097/MOL.0b013e32814e6d29. [DOI] [PubMed] [Google Scholar]
- 7.Grant PJ. Diabetes mellitus as a prothrombotic condition. J Intern Med. 2007;262:157–172. doi: 10.1111/j.1365-2796.2007.01824.x. [DOI] [PubMed] [Google Scholar]
- 8.Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther. 2010;28:e72–e91. doi: 10.1111/j.1755-5922.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fay WP, Murphy JG, Owen WG. High concentrations of active plasminogen activator inhibitor-1 in porcine coronary artery thrombi. Arterioscler Thromb Vasc Biol. 1996;16:1277–1284. doi: 10.1161/01.atv.16.10.1277. [DOI] [PubMed] [Google Scholar]
- 10.Ribo M, Montaner J, Molina CA, Arenillas JF, Santamarina E, Quintana M, et al. Admission fibrinolytic profile is associated with symptomatic hemorrhagic transformation in stroke patients treated with tissue plasminogen activator. Stroke. 2004;35:2123–2127. doi: 10.1161/01.STR.0000137608.73660.4c. [DOI] [PubMed] [Google Scholar]
- 11.Alessi MC, Juhan-Vague I. Metabolic syndrome, haemostasis and thrombosis. Thromb Haemost. 2008;99:995–1000. doi: 10.1160/TH07-11-0682. [DOI] [PubMed] [Google Scholar]
- 12.Dupont DM, Madsen JB, Kristensen T, Bodker JS, Blouse GE, Wind T, et al. Biochemical properties of plasminogen activator inhibitor-1. Front Biosci. 2009;14:1337–1361. doi: 10.2741/3312. [DOI] [PubMed] [Google Scholar]
- 13.Levin EG, Santell L. Conversion of the active to latent plasminogen activator inhibitor from human endothelial cells. Blood. 1987;70:1090–1098. [PubMed] [Google Scholar]
- 14.Hekman CM, Loskutoff DJ. Endothelial cells produce a latent inhibitor of plasminogen activators that can be activated by denaturants. J Biol Chem. 1985;260:11581–11587. [PubMed] [Google Scholar]
- 15.Lambers JW, Cammenga M, Konig BW, Mertens K, Pannekoek H, van Mourik JA. Activation of human endothelial cell-type plasminogen activator inhibitor (PAI-1) by negatively charged phospholipids. J Biol Chem. 1987;262:17492–17496. [PubMed] [Google Scholar]
- 16.Bjorquist P, Brohlin M, Ehnebom J, Ericsson M, Kristiansen C, Pohl G, et al. Plasminogen activator inhibitor type-1 interacts exclusively with the proteinase domain of tissue plasminogen activator. Biochim Biophys Acta. 1994;1209:191–202. doi: 10.1016/0167-4838(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 17.Gils A, Pedersen KE, Skottrup P, Christensen A, Naessens D, Deinum J, et al. Biochemical importance of glycosylation of plasminogen activator inhibitor-1. Thromb Haemost. 2003;90:206–217. doi: 10.1160/TH03-01-0034. [DOI] [PubMed] [Google Scholar]
- 18.Serrano R, Barrenetxe J, Orbe J, Rodriguez JA, Gallardo N, Martinez C, et al. Tissue-specific PAI-1 gene expression and glycosylation pattern in insulin-resistant old rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1563–R1569. doi: 10.1152/ajpregu.00093.2009. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Calatrava MJ, Martinez-Larrad MT, Zabena C, Gonzalez-Sanchez JL, Fernandez-Perez C, Serrano-Rios M. The 4G/4G PAI-1 genotype is associated with elevated plasma PAI-1 levels regardless of variables of the metabolic syndrome and smoking status. A population-based study in Spanish population. Diabetes Obes Metab. 2007;9:134–135. doi: 10.1111/j.1463-1326.2006.00584.x. [DOI] [PubMed] [Google Scholar]
- 20.Bentley P, Peck G, Smeeth L, Whittaker J, Sharma P. Causal relationship of susceptibility genes to ischemic stroke: comparison to ischemic heart disease and biochemical determinants. PLoS One. 2010;5:e9136. doi: 10.1371/journal.pone.0009136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alessi MC, Juhan-Vague I. PAI-1 and the metabolic syndrome: links, causes, and consequences. Arterioscler Thromb Vasc Biol. 2006;26:2200–2207. doi: 10.1161/01.ATV.0000242905.41404.68. [DOI] [PubMed] [Google Scholar]
- 22.Dimova EY, Kietzmann T. Metabolic, hormonal and environmental regulation of plasminogen activator inhibitor-1 (PAI-1) expression: lessons from the liver. Thromb Haemost. 2008;100:992–1006. [PubMed] [Google Scholar]
- 23.Westerhausen DR, Jr, Hopkins WE, Billadello JJ. Multiple transforming growth factor-beta-inducible elements regulate expression of the plasminogen activator inhibitor type-1 gene in HEP G2 cells. J Biol Chem. 1991;266:1092–1100. [PubMed] [Google Scholar]
- 24.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown NJ, Kim KS, Chen YQ, Blevins LS, Nadeau JH, Meranze SG, et al. Synergistic effect of adrenal steroids and angiotensin II on plasminogen activator inhibitor-1 production. J Clin Endocrinol Metab. 2000;85:336–344. doi: 10.1210/jcem.85.1.6305. [DOI] [PubMed] [Google Scholar]
- 26.Fink T, Kazlauskas A, Poellinger L, Ebbesen P, Zachar V. Identification of a tightly regulated hypoxia-response element in the promoter of human plasminogen activator inhibitor-1. Blood. 2002;99:2077–2083. doi: 10.1182/blood.v99.6.2077. [DOI] [PubMed] [Google Scholar]
- 27.Kluft C, Jie AF, Rijken DC, Verheijen JH. Daytime fluctuations in blood of tissue-type plasminogen activator (tPA) and its fast-acting inhibitor (PAI-1) Thromb Haemost. 1988;59:329–332. [PubMed] [Google Scholar]
- 28.Birdane A, Haznedaroglu IC, Bavbek N, Kosar A, Buyukasik Y, Ozcebe O, et al. The plasma levels of prostanoids and plasminogen activator inhibitor-1 in primary and secondary thrombocytosis. Clin Appl Thromb Hemost. 2005;11:197–201. doi: 10.1177/107602960501100209. [DOI] [PubMed] [Google Scholar]
- 29.Brogren H, Wallmark K, Deinum J, Karlsson L, Jern S. Platelets retain high levels of active plasminogen activator inhibitor 1. PloS One. 2011;6:e26762. doi: 10.1371/journal.pone.0026762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brogren H, Karlsson L, Andersson M, Wang L, Erlinge D, Jern S. Platelets synthesize large amounts of active plasminogen activator inhibitor 1. Blood. 2004;104:3943–3948. doi: 10.1182/blood-2004-04-1439. [DOI] [PubMed] [Google Scholar]
- 31.Brogren H, Sihlbom C, Wallmark K, Lonn M, Deinum J, Karlsson L, et al. Heterogeneous glycosylation patterns of human PAI-1 may reveal its cellular origin. Thromb Res. 2008;122:271–281. doi: 10.1016/j.thromres.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Declerck PJ, Moreau H, Jespersen J, Gram J, Kluft C. Multicenter evaluation of commercially available methods for the immunological determination of plasminogen activator inhibitor-1 (PAI-1) Thromb Haemost. 1993;70:858–863. [PubMed] [Google Scholar]
- 33.Gram J, Declerck PJ, Sidelmann J, Jespersen J, Kluft C. Multicentre evaluation of commercial kit methods: plasminogen activator inhibitor activity. Thromb Haemost. 1993;70:852–857. [PubMed] [Google Scholar]
- 34.Gravanis I, Tsirka SE. Tissue plasminogen activator and glial function. Glia. 2005;49:177–183. doi: 10.1002/glia.20115. [DOI] [PubMed] [Google Scholar]
- 35.Hultman K, Blomstrand F, Nilsson M, Wilhelmsson U, Malmgren K, Pekny M, et al. Expression of plasminogen activator inhibitor-1 and protease nexin-1 in human astrocytes: response to injury-related factors. J Neurosci Res. 2010;88:2441–2449. doi: 10.1002/jnr.22412. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Rosell A, Lo EH. Targeting extracellular matrix proteolysis for hemorrhagic complications of tPA stroke therapy. CNS Neurol Disord Drug Targets. 2008;7:235–242. doi: 10.2174/187152708784936635. [DOI] [PubMed] [Google Scholar]
- 37.Benchenane K, Lopez-Atalaya JP, Fernandez-Monreal M, Touzani O, Vivien D. Equivocal roles of tissue-type plasminogen activator in stroke-induced injury. Trends Neurosci. 2004;27:155–160. doi: 10.1016/j.tins.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Nagai N, Suzuki Y, Van Hoef B, Lijnen HR, Collen D. Effects of plasminogen activator inhibitor-1 on ischemic brain injury in permanent and thrombotic middle cerebral artery occlusion models in mice. J Thromb Haemost. 2005;3:1379–1384. doi: 10.1111/j.1538-7836.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- 39.Yang D, Nemkul N, Shereen A, Jone A, Dunn RS, Lawrence DA, et al. Therapeutic administration of plasminogen activator inhibitor-1 prevents hypoxic–ischemic brain injury in newborns. J Neurosci. 2009;29:8669–8674. doi: 10.1523/JNEUROSCI.1117-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- 41.Dohgu S, Takata F, Matsumoto J, Oda M, Harada E, Watanabe T, et al. Autocrine and paracrine up-regulation of blood–brain barrier function by plasminogen activator inhibitor-1. Microvasc Res. 2011;81:103–107. doi: 10.1016/j.mvr.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Soeda S, Koyanagi S, Kuramoto Y, Kimura M, Oda M, Kozako T, et al. Anti-apoptotic roles of plasminogen activator inhibitor-1 as a neurotrophic factor in the central nervous system. Thromb Haemost. 2008;100:1014–1020. doi: 10.1160/th08-04-0259. [DOI] [PubMed] [Google Scholar]
- 43.Hultman K, Tjarnlund-Wolf A, Odeberg J, Eriksson P, Jern C. Allele-specific transcription of the PAI-1 gene in human astrocytes. Thromb Haemost. 2010;104:998–1008. doi: 10.1160/TH10-04-0243. [DOI] [PubMed] [Google Scholar]
- 44.Aso Y. Plasminogen activator inhibitor (PAI)-1 in vascular inflammation and thrombosis. Front Biosci. 2007;12:2957–2966. doi: 10.2741/2285. [DOI] [PubMed] [Google Scholar]
- 45.Dunn EJ, Grant PJ. Type 2 diabetes: an atherothrombotic syndrome. Curr Mol Med. 2005;5:323–332. doi: 10.2174/1566524053766059. [DOI] [PubMed] [Google Scholar]
- 46.Arenillas JF, Alvarez-Sabin J, Molina CA, Chacon P, Fernandez-Cadenas I, Ribo M, et al. Progression of symptomatic intracranial large artery atherosclerosis is associated with a proinflammatory state and impaired fibrinolysis. Stroke. 2008;39:1456–1463. doi: 10.1161/STROKEAHA.107.498600. [DOI] [PubMed] [Google Scholar]
- 47.Fay WP, Garg N, Sunkar M. Vascular functions of the plasminogen activation system. Arterioscler Thromb Vasc Biol. 2007;27:1231–1237. doi: 10.1161/ATVBAHA.107.140046. [DOI] [PubMed] [Google Scholar]
- 48.Schneider DJ, Hayes M, Wadsworth M, Taatjes H, Rincon M, Taatjes DJ, et al. Attenuation of neointimal vascular smooth muscle cellularity in atheroma by plasminogen activator inhibitor type 1 (PAI-1) J Hisochem Cytochem. 2004;52:1091–1099. doi: 10.1369/jhc.4A6260.2004. [DOI] [PubMed] [Google Scholar]
- 49.Lijnen HR. Role of fibrinolysis in obesity and thrombosis. Thromb Res. 2009;123(suppl 4):S46–S49. doi: 10.1016/S0049-3848(09)70143-4. [DOI] [PubMed] [Google Scholar]
- 50.Trost S, Pratley R, Sobel B. Impaired fibrinolysis and risk for cardiovascular disease in the metabolic syndrome and type 2 diabetes. Curr Diab Rep. 2006;6:47–54. doi: 10.1007/s11892-006-0052-5. [DOI] [PubMed] [Google Scholar]
- 51.Festa A, D’Agostino R, Jr, Tracy RP, Haffner SM. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the Insulin Resistance Atherosclerosis study. Diabetes. 2002;51:1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 52.Jankun J, Al-Senaidy A, Skrzypczak-Jankun E. Can inactivators of plasminogen activator inhibitor alleviate the burden. Int J Mol Med. 2012;29:3–11. doi: 10.3892/ijmm.2011.810. [DOI] [PubMed] [Google Scholar]
- 53.Sobel BE, Hardison RM, Genuth S, Brooks MM, McBane RD, III, Schneider DJ, et al. Profibrinolytic, antithrombotic, and anti-inflammatory effects of an insulin-sensitizing strategy in patients in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation. 2011;124:695–703. doi: 10.1161/CIRCULATIONAHA.110.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Part 2, short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–838. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 55.Vaughan DE. Angiotensin and vascular fibrinolytic balance. Am J Hypertens. 2002;15:3S–8S. doi: 10.1016/s0895-7061(01)02273-7. [DOI] [PubMed] [Google Scholar]
- 56.Buch MH, Prendergast BD, Storey RF. Antiplatelet therapy and vascular disease: an update. Ther Adv Cardiovasc Dis. 2010;4:249–275. doi: 10.1177/1753944710375780. [DOI] [PubMed] [Google Scholar]
- 57.Shulga O, Bornstein N. Antiplatelets in secondary stroke prevention. Front Neurol. 2011;2:36. doi: 10.3389/fneur.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakata T, Kario K. Antiplatelet therapy effectively reduces plasma plasminogen activator inhibitor-1 levels. Atherosclerosis. 2011;214:490–491. doi: 10.1016/j.atherosclerosis.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 59.Montaner J. Blood biomarkers to guide stroke thrombolysis. Front Biosci (Elite Ed) 2009;1:p200–p208. doi: 10.2741/E19. [DOI] [PubMed] [Google Scholar]
- 60.Fernandez-Cadenas I, Del Rio-Espinola A, Rubiera M, Mendioroz M, Domingues-Montanari S, Cuadrado E, et al. PAI-1 4G/5G polymorphism is associated with brain vessel reocclusion after successful fibrinolytic therapy in ischemic stroke patients. Int J Neurosci. 2010;120:245–251. doi: 10.3109/00207451003597169. [DOI] [PubMed] [Google Scholar]
- 61.Diedler J, Ahmed N, Sykora M, Uyttenboogaart M, Overgaard K, Luijckx GJ, et al. Safety of intravenous thrombolysis for acute ischemic stroke in patients receiving antiplatelet therapy at stroke onset. Stroke. 2010;41:288–294. doi: 10.1161/STROKEAHA.109.559724. [DOI] [PubMed] [Google Scholar]
- 62.Whiteley W. Identifying blood biomarkers to improve the diagnosis of stroke. J R Coll Physicians Edinb. 2011;41:152–154. doi: 10.4997/JRCPE.2011.207. [DOI] [PubMed] [Google Scholar]
- 63.Vaughan DE. PAI-1 antagonists: the promise and the peril. Trans Am Clin Climatol Assoc. 2011;122:312–325. [PMC free article] [PubMed] [Google Scholar]
- 64.Lip GY, Felmeden DC, Dwivedi G. Antiplatelet agents and anticoagulants for hypertension. Cochrane Database Syst Rev. 2011;12:CD003186. doi: 10.1002/14651858.CD003186.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Konya H, Hasegawa Y, Hamaguchi T, Satani K, Umehara A, Katsuno T, et al. Effects of gliclazide on platelet aggregation and the plasminogen activator inhibitor type 1 level in patients with type 2 diabetes mellitus. Metabolism. 2010;59:1294–1299. doi: 10.1016/j.metabol.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Walter T, Szabo S, Suselbeck T, Borggrefe M, Lang S, Swoboda S, et al. Effect of atorvastatin on haemostasis, fibrinolysis and inflammation in normocholesterolaemic patients with coronary artery disease: a post hoc analysis of data from a prospective, randomized, double-blind study. Clin Drug Invest. 2010;30:453–460. doi: 10.2165/11536270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 67.Vaughan DE, De Taeye BM, Eren M. PAI-1 antagonists: predictable indications and unconventional applications. Curr Drug Targets. 2007;8:962–970. doi: 10.2174/138945007781662364. [DOI] [PubMed] [Google Scholar]
- 68.Wu Q, Zhao Z. Inhibition of PAI-1: a new anti-thrombotic approach. Curr Drug Targets Cardiovasc Haematol Disord. 2002;2:27–42. doi: 10.2174/1568006023337727. [DOI] [PubMed] [Google Scholar]
- 69.Izuhara Y, Yamaoka N, Kodama H, Dan T, Takizawa S, Hirayama N, et al. A novel inhibitor of plasminogen activator inhibitor-1 provides antithrombotic benefits devoid of bleeding effect in nonhuman primates. J Cereb Blood Flow Metab. 2010;30:904–912. doi: 10.1038/jcbfm.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hennan JK, Morgan GA, Swillo RE, Antrilli TM, Mugford C, Vlasuk GP, et al. Effect of tiplaxtinin (PAI-039), an orally bioavailable PAI-1 antagonist, in a rat model of thrombosis. J Thromb Haemost. 2008;6:1558–1564. doi: 10.1111/j.1538-7836.2008.03063.x. [DOI] [PubMed] [Google Scholar]
- 71.Cabral KP, Ansell J, Hylek EM. Future directions of stroke prevention in atrial fibrillation: the potential impact of novel anticoagulants and stroke risk stratification. J Thromb Haemost. 2011;9:441–449. doi: 10.1111/j.1538-7836.2010.04179.x. [DOI] [PubMed] [Google Scholar]