Abstract
Background and Purpose
Plasma levels of matrix metalloproteinase-9 (MMP-9) have been proposed to be a useful biomarker for assessing pathological events in brain. Here, we examined the temporal profiles of MMP-9 in blood and brain using a rat model of acute focal cerebral ischemia.
Methods
Plasma and brain levels of MMP-2 and MMP-9 were quantified at 3, 6, 12, and 24 hours after permanent middle cerebral artery occlusion in male Sprague-Dawley rats. Infarct volumes at 24 hours were confirmed with 2,3,5-triphenyl-tetrazolium-chloride staining.
Results
In plasma, zymographic bands were detected between 70 and 95 kDa corresponding to pro-MMP-2, pro-MMP-9, and activated MMP-9. A higher 135-kDa band was also seen that is likely to be NGAL-conjugated MMP-9. After ischemia, there were no significant changes in pro-MMP-2, but plasma levels of pro-MMP-9 steadily increased over the course of 24 hours. Activated MMP-9 levels in plasma were significantly elevated only at 24 hours. Plasma NGAL-MMP-9 complexes showed a transient elevation between 3 to 6 hours, after which levels decreased back down to pre-ischemic baselines. In brain homogenates, pro-MMP-2, pro-MMP-9, and activated MMP-9 were seen but no NGAL-MMP-9 bands were detected. Compared to the contralateral hemisphere, MMP-2 and MMP-9 levels in ischemic brain progressively increased over the course of 24 hours. Overall levels of MMP-9 in plasma and brain were significantly correlated, especially at 24 hours. Plasma levels of pro-MMP-9 at 24 hours were correlated with final infarct volumes.
Conclusions
Elevated plasma levels of MMP-9 appear to be correlated with brain levels within 24 hours of acute cerebral ischemia in rats. Further investigation into clinical profiles of MMP-9 in acute stroke patients may be useful.
Keywords: cerebral ischemia, matrix metalloproteinase, stroke
Matrix metalloproteinases (MMP) have been implicated in stroke pathophysiology.1–3 MMP-2 and MMP-9 are rapidly upregulated in ischemic brain in animal models and stroke patients.3–6 Pharmacological inhibition or genetic knockdown of MMP reduces neuronal death, blood–brain barrier damage, edema, and hemorrhage.4,7–11
In this context, circulating plasma levels of MMP have been proposed to serve as useful biomarkers for stroke.12 Plasma MMP-2 and MMP-9 were positively correlated with stroke severity measured with the National Institutes of Health Stroke Scale score and ischemic lesion volumes measured on diffusion-weighted imaging scans.13,14 However, a recent experimental study in rats reported that plasma MMP-9 peaked at 4 hours after ischemia and then decreased rapidly, whereas brain MMP-9 peaked later at 24 hours and persisted until 72 hours after ischemic onset.15 Furthermore, it is now known that blood cells can contribute to MMP signatures after brain injury.16 How well plasma MMP reflects brain MMP and the progression of infarction after stroke remains to be fully dissected. In this study, we used a rat model of focal cerebral ischemia to examine the temporal profile of blood vs brain MMP and their relationship with brain infarction.
Methods and Materials
Animal Model
All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, Mass), weighing 260 to 290 grams, were subjected to permanent focal cerebral ischemia using the standard intraluminal occlusion method.7 General anesthesia was maintained with 2.0% isoflurane in 30% oxygen and 70% nitrous oxide via face mask. The rectal temperature was monitored and maintained at 37°C with heating pad. Laser Doppler flowmetry (Perimed) was used to confirm adequate ischemia. Only rats that showed at least 70% reduction of middle cerebral artery flow from baseline were included. Two separate studies were performed. In the first set of rats, we examined the temporal profile of plasma MMP by sequentially sampling blood from the left internal jugular vein just after anesthesia before ischemic surgery, and then 3, 6, 12, and 24 hours after middle cerebral artery occlusion. At the end of this study, all brains were removed for measurement of 24-hour infarction volumes. Out of 11 rats initially assigned to this first experiment, 2 rats were excluded because of filament puncture, and 1 rat was excluded because laser Doppler flowmetry-regional cerebral blood flow (LDF-CBF) values did not decline to our prespecified ischemic levels. In the second set of rats, we examined the relationship between blood and brain MMP-9 by sampling blood from the left internal jugular vein in 6 or 7 rats each, at 3, 6, and 12 hours, and 13 rats at 24 hours after ischemia. Immediately after blood withdrawal, rats were killed and brains were removed. In all cases, sampled blood was centrifuged at 4500 rpm for 15 minutes within 5 minutes, and plasma was collected, and stored at −80°C for later zymography and Western blot analysis. Out of the 45 rats initially assigned to this experiment, 4 rats were excluded because of inadequate LDF-CBF reduction for proper cerebral ischemia; 8 rats died during surgery because of technical problems, including filament puncture and hemorrhage. In all experiments, <1 mL of blood was withdrawn at a single time. All rats tolerated this procedure without problems.
Gelatin Zymography
The levels of MMP-2 and MMP-9 in plasma and ischemic brain homogenates were measured by gelatin zymogram following previously described techniques.7 Rats were deeply anesthetized and then transcardially perfused with ice-cold phosphate-buffered saline. The brains were quickly removed, divided into ipsilateral ischemic hemispheres and contralateral nonischemic hemispheres, then frozen immediately in liquid nitrogen, and stored at −80°C. Samples were homogenized in lysis buffer including protease inhibitors on ice. After centrifugation, supernatant was collected, and total protein concentrations were determined using the bicinchoninic acid protein assay method. Prepared 50 μg protein samples were loaded and separated by 10% Tris-glycine gel with 0.1% gelatin as substrate. After electrophoresis, gels were placed in 2.7% Triton X-100 for 1 hour to remove SDS, and then incubated for 21 hours at 37°C in developing buffer (50 mmol/L Tris base, 40 mmol/L HCl, 200 mmol/L NaCl, 5 mmol/L CaCl2, and 0.2% Briji 35; Invitrogen) on a rotary shaker. After incubation, gels were stained in 30% methanol, 10% acetic acid, and 0.5% wt/vol Coomassie brilliant blue for 2 hours, followed by destaining. Gelatinolytic activity was manifested as horizontal white bands on a blue background. MMP bands were identified after standard techniques using molecular weight criteria and the loading of human MMP-9 control purchased from Chemicon.17
Western Blotting Analysis
To analyze the expression patterns of MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL)-MMP-9 complexes in plasma and ischemic brains, protein concentration of the plasma and brain lysate were determined using the bicinchoninic acid protein assay method. Equal volume or amounts of proteins (plasma 6 μL, brain lysate 20 μg) were loaded onto 4% to 20% gradient gel or 8% Tris-Glycine SDS-PAGE under nonreducing conditions and transferred to polyvinylidene flouride membranes. The membrane was blocked with 10% nonfat dry milk in phosphate-buffered saline containing 0.1% Tween-20, followed by incubating with primary antibody at 4°C for 18 hours. After washing with phosphate-buffered saline containing 0.1% Tween-20, the membrane was incubated with peroxidase-conjugated secondary antibody at room temperature for 1 hour. Labeled proteins were detected by using chemical luminescence (ECL; Amersham Pharmacia Biotech). Rabbit antirat MMP-9 polyclonal antibody (Chemicon) and rabbit polyclonal NGAL (Santa Cruz Biotechnology) were used at 1:4000 and 1:2000 dilutions, respectively.
Measurement of Infarct Volume
Eight rats were euthanized at 24 hours after focal ischemia in first experiment. Seven coronal sections per brain of 2-mm thickness were made and stained with 2,3,5-triphenyltetrazolium-chloride. Infarct volumes were quantified with standard computer-assisted image analysis technique (Image J version 1.3). To exclude possible confounding effects of brain swelling, an indirect method was used to calculate the lesion volumes.7,18
Statistical Analysis
Data from serial blood levels of MMP-9 and MMP-2 were tested with repeated measures 1-way ANOVA followed by least significant difference corrections for multiple comparisons to assess MMP levels at different time points. Brain levels of MMP were analyzed with paired t test compared with the contralateral hemisphere. Pearson and Spearman correlation coefficients were used to analyze the association between the level of plasma MMP-9 and the level of brain MMP-9 and acute infarct volume, respectively. P<0.05 was considered statistically significant. The data are expressed as mean±SEM.
Results
Temporal Profile of Plasma MMP-9
Serial plasma samples taken from rats before arterial occlusion were compared with samples taken at 3, 6, 12, and 24 hours after permanent focal cerebral ischemia. Gelatin zymography showed typical bands corresponding to pro-MMP-2 (72 kDa), pro-MMP-9 (95 kDa), and activated MMP-9 (92 kDa; Figure 1A). The presence of MMP-9 protein was confirmed with immunoblotting (Figure 1B). Besides the expected MMP-2 and MMP-9 bands, zymograms also revealed higher bands near 135 kDa (Figure 1A). Subsequent immunoblotting against MMP-9 and NGAL protein suggested that these might represent covalent complexes of NGAL and MMP-9 (Figure 1C), because both immunoreactivities (probed with antibodies against MMP-9 and against NGAL) migrated at a similar molecular weight of ≈ 135 kDa.
Figure 1.
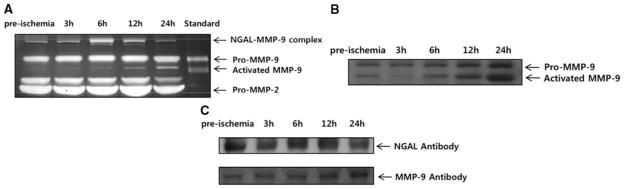
Temporal profiles of MMP-9 in plasma. A, Representative zymogram shows bands of NGAL–MMP-9 complexes, pro-MMP-9, activated MMP-9, and pro-MMP-2 before ischemia and at 3 hours, 6 hours, 12 hours, and 24 hours after permanent focal cerebral ischemia in rats. B, Western blotting against MMP-9 confirms that pro-MMP-9 and activated MMP-9 are detected in the rat plasma samples. C, Immunoblotting against NGAL and MMP-9 protein supports the existence of higher-molecular-weight bands of NGAL–MMP-9 complexes in the rat plasma samples.
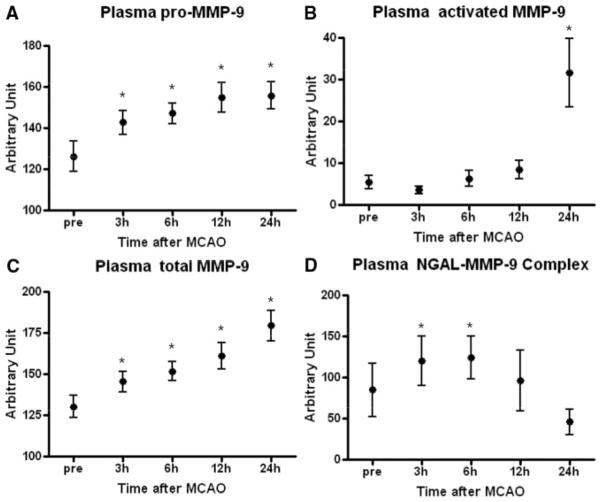
For quantitative analysis, optical density was measured in all zymographic gels. No significant changes in pro-MMP-2 were noted. However, plasma levels of pro-MMP-9 and total MMP-9 steadily increased over time (Figure 2A,C). In contrast, activated MMP-9 did not become significantly elevated until 24 hours after ischemic onset (Figure 2B). NGAL-MMP-9 levels were more variable and showed a transient response, with plasma levels peaking between 3 and 6 hours, and then declining back to baseline by 24 hours (Figure 2D).
Figure 2.
Quantified temporal profile of plasma MMP levels via densitometric analysis of zymograms. A, Plasma pro-MMP-9 levels are significantly increased at all time points after ischemia. B, Plasma-activated MMP-9 levels are only significantly elevated at 24 hours. C, Plasma levels of total (activated plus pro) MMP-9 levels progressively increase over time. Note, however, that stable pro-MMP-9 and relatively unstable activated MMP-9 densitometry may not be comparable when summed. D, Plasma NGAL–MMP-9 complexes shows statistically significant changes at 3 and 6 hours. *P<0.05 vs values before ischemia.
Temporal Profile of Brain MMP
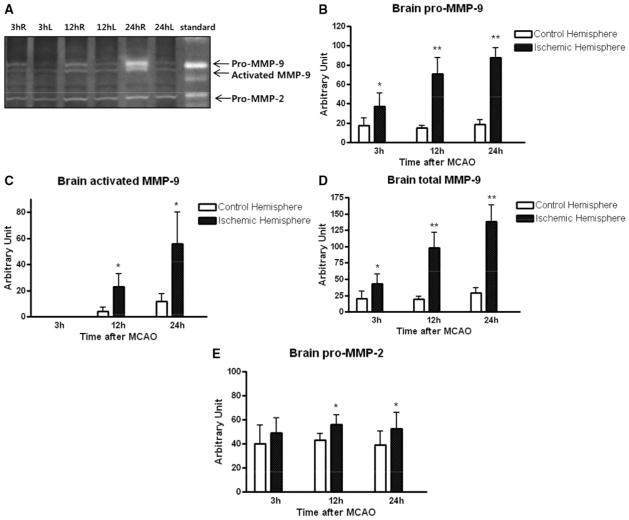
Zymography of brain homogenates showed the expected bands, corresponding to 72 kDa pro-MMP-2, 92 kDa activated MMP-9 and 95 kDa pro-MMP-9 (Figure 3A). However, no 135 kDa NGAL-MMP-9 bands were detected at any time in the rat brains (Figure 3A). Compared to the contralateral hemisphere, ischemic brain levels of pro-MMP-9, activated MMP-9, and total MMP-9 were all significantly increased over the 24-hour period after ischemic onset (Figure 3B–D). Pro-MMP-2 was also slightly elevated over time (Figure 3E).
Figure 3.
Temporal profile of MMP in brain homogenates. A, Representative zymogram shows bands of brain pro-MMP-9, activated MMP-9, and pro-MMP-2, but no NGAL-MMP-9 complexes are detected. Compared to contralateral hemisphere, MMP-9 levels are increased in ischemic ipsilateral tissue over time. B, Quantitative densitometry shows that brain pro-MMP-9 is elevated at all time points. C, Activated MMP-9 is increased at 12 and 24 hours. D, Total (activated plus pro) MMP-9 is increased at all time points. E, Brain MMP-2 appears to be increased at 12 and 24 hours. *P<0.05 and **P<0.01 between ipsilateral and contralateral levels.
Correlations Between Brain MMP-9, Plasma MMP-9, and Final Infarction
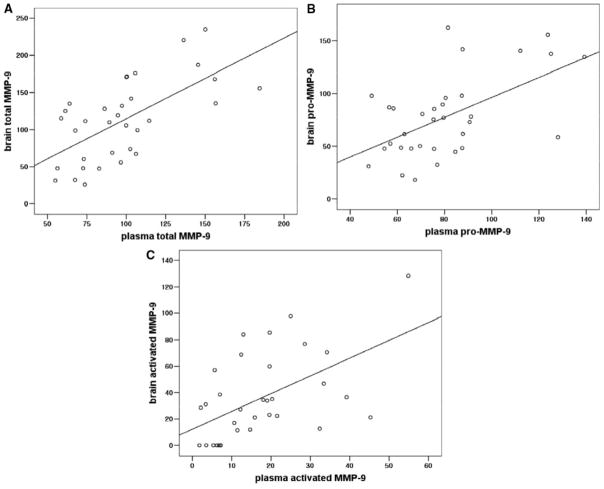
An overall analysis including all time points showed that blood and brain MMP-9 (total, pro, and activated forms) were significantly correlated (r=0.643, P<0.001 in total MMP-9; r=0.561, P=0.001 in pro-MMP-9; r=0.540, P=0.001 in activated MMP-9; Figure 4A-C). Furthermore, a dependence on time was observed. Subsequent analysis showed that the strongest correlation between plasma and brain MMP-9 was found at 24 hours (r=0.560, P=0.046 for total MMP-9; r=0.585, P=0.036 for pro-MMP-9). Interestingly, no correlation was found for activated MMP-9 at 24 hours. Individual correlations for all time points are presented in the Table.
Figure 4.
Overall correlation between plasma and brain MMP-9 regardless of time. A, Total MMP-9: r=0.643, P<0.001. B, Pro-MMP-9: r=0.561, P=0.001. C, Activated MMP-9: r=0.540, P=0.001.
Table.
Correlations in Plasma MMP, Brain MMP, and Infarct Volume
| Versus Brain MMP Levels
|
Versus 24-Hour Infarct Volume
|
|||
|---|---|---|---|---|
| Plasma MMP | R | P | R | P |
| 3 hours | ||||
| Pro-MMP-2 | −0.241 | 0.603 | 0.119 | 0.779 |
| Pro-MMP-9 | 0.337 | 0.459 | 0.667 | 0.071 |
| Activated MMP-9 | NA | NA | 0.238 | 0.570 |
| NGAL–MMP-9 | NA | NA | 0.500 | 0.207 |
| 6 hours | ||||
| Pro-MMP-2 | −0.152 | 0.773 | 0.667 | 0.071 |
| Pro-MMP-9 | 0.033 | 0.951 | 0.667 | 0.071 |
| Activated MMP-9 | 0.371 | 0.468 | 0.476 | 0.233 |
| NGAL–MMP-9 | NA | NA | 0.524 | 0.183 |
| 12 hours | ||||
| Pro-MMP-2 | −0.190 | 0.684 | 0.262 | 0.531 |
| Pro-MMP-9 | 0.263 | 0.569 | 0.643 | 0.086 |
| Activated MMP-9 | −0.695 | 0.083 | −0.071 | 0.867 |
| NGAL–MMP-9 | NA | NA | 0.405 | 0.302 |
| 24 hours | ||||
| Pro-MMP-2 | 0.447 | 0.126 | −0.262 | 0.531 |
| Pro-MMP-9 | 0.585 | 0.036 | 0.762 | 0.028 |
| Activated MMP-9 | 0.195 | 0.522 | 0.048 | 0.911 |
| NGAL–MMP-9 | NA | NA | 0.095 | 0.823 |
NA indicates not applicable.
The mean infarct volume at 24 hours after focal cerebral ischemia was 499±157 mm3. No correlation was found between plasma pro-MMP-2 and infarct volume at any time point (see Table). However, a pattern of correlations was detected between plasma pro-MMP-9 and final infarct volumes (Table). This reached statistical significance for 24-hour plasma pro-MMP-9 and 24-hour final infarct volumes (r=0.762, P=0.028). Overall, however, these relationships between plasma MMP and brain infarct volumes were limited by the small numbers available for this portion of the study.
Discussion
By degrading neurovascular substrates, MMP promote blood–brain barrier leakage that may underlie cerebral edema and hemorrhage.1,5,6 By disrupting cell–cell and cell–matrix signaling, MMP may trigger brain cell dysfunction and death.19,20 Furthermore, it is now recognized that tissue plasminogen activator can upregulate MMP, perhaps mediating some of the hemorrhagic complications of delayed thrombolysis.21,22 Taken together, the data suggest that MMP, especially the gelatinases MMP-2 and MMP-9, play important roles in the pathophysiology of cerebral ischemia and hemorrhage. Hence, measuring MMP and finding ways to target these proteases may lead to useful diagnostic and therapeutic approaches for stroke.9
In experimental stroke models, MMP in brain are easy to identify and quantify for specific hypothesis testing. In recent years, emerging data suggest that similar MMP signatures may also be detected in clinical stroke patients. The majority of these findings have come from measurements of circulating MMP in plasma. Levels of MMP-9 within the acute (<24–48 hours) periods of ischemic strokes were found to be correlated with worsened NIH stroke scores, larger infarct volumes, and increased risk of hemorrhagic conversion.13,14,21,23,24 However, all of these clinical studies were unable to demonstrate a clear temporal progression of these MMP signals over time. Levels at very early 3- to 6-hour time points were not significantly different from those at later 24- to 48-hour time points. Together with the heterogenous characteristics of human stroke (comorbidities and various risk factors in patients vs controls, variable infarct size, location, and severity), this raises the question as to whether MMP in blood can truly serve as causative biomarkers of stroke pathophysiology or if they are only nonspecific markers of tissue injury.
Surprisingly, there has only been 1 previous study that tried to compare blood vs brain levels of MMP in cerebral ischemia. In a rat model of focal cerebral ischemia, Koh et al15 found that plasma levels of MMP-9 peaked very early at 4 hours, then declined back to baseline by 24 hours. In contrast, they found that brain levels of MMP-9 progressively increased and peaked at 24 hours. These data would suggest that there is no good correlation between plasma and brain MMP-9 responses after cerebral ischemia. Our findings here are different. In our rat focal ischemia model, plasma levels of pro-MMP-9 progressively increased and remained elevated at 24 hours, in parallel with brain levels. Plasma levels of activated MMP-9 showed a late peak only at 24 hours, whereas in ischemic brain, activated MMP-9 increased at 12 and 24 hours. Overall, total MMP-9 levels in plasma and brain were highly correlated. There may be several potential reasons for the discrepancy between our data and the Koh et al15 study. First, we used a model of permanent ischemia, whereas Koh et al15 used a model of transient ischemia. It is conceivable that reperfusion may lead to differential MMP responses in blood vs brain. Second, it was not clear how pro vs activated MMP-9 forms were quantified in the previous study. In our experiments, we found significant differences between pro and activated MMP-9 bands in blood vs brain. For some reason, activated MMP-9 bands were not markedly elevated in blood until 24 hours, long after these corresponding levels were increased in brain tissue. Further studies will be required to examine the reason for these intriguing differences. However, it should be noted that activated forms of MMP-9 are much less stable than inactive proforms of this enzyme and consequently more difficult to visualize on zymography analysis. In this regard, one might speculate that all else being equal, proforms of MMP-9 may be easier to interpret when considering biomarkers for clinical stroke. Nevertheless, how blood MMP levels relate to actual brain infarction remains to be unequivocally resolved, especially for active MMP-9 levels. In brain, these steadily increase over time, whereas in plasma, only 24-hour samples show significantly elevated active MMP-9 forms. It is possible that these profiles may be related to different sources of MMP-9 in periphery vs brain parenchyma. A recent report showed that after transient focal cerebral ischemia in rats, 24-hour MRI lesion volumes correlated with brain MMP-9 levels but not plasma MMP-9.11 Further studies are required to separate how much of plasma MMP levels come leaked from damaged brain vs how much might come form activated blood cells and nonlocal endothelium.
Thus far, our data are not inconsistent with the possibility that plasma levels of pro and activated MMP-9 forms may partially derive from ischemic brain parenchyma and activated cerebral endothelium. However, it is increasingly recognized that systemic blood cells may also be highly reactive after stroke.25 Neutrophils can be an especially potent source of MMP-9 and in the human circulation this leukocyte type is certainly the most abundant one.16 Recent studies in experimental models of cerebral ischemia showed that neutrophils can secrete MMP-9 into ischemic brain areas.26,27 In the present study, we attempted to look for higher molecular weight zymographic bands of NGAL-conjugated MMP-9 as a surrogate marker for neutrophil-derived MMP-9.28 In ischemic brain homogenates, we were unable to detect covalent NGAL–MMP-9 complexes at the examined time points. But in plasma, NGAL–MMP-9 forms were clearly detected, and these signals transiently peak at ≈3 to 6 hours and then decreased back to baseline levels. In most clinical studies, MMP-9 assays are performed using enzyme-linked immunosorbent assays, which may not be able to distinguish between the various pro, activated, and NGAL-conjugated MMP-9 isoforms. How neutrophil-derived MMP-9 contributes to clinical plasma signals warrants further investigation.
Conclusion
Overall, our study suggests that in rat focal cerebral ischemia, plasma and brain levels of MMP-9 may be correlated. However, there are several important caveats to be considered. First, our data remain comparative and correlative, and we cannot truly assess causality. We can only say that our plasma MMP measurements seem interesting as potential biomarkers. Second, we cannot determine cell source. Although we were able to detect NGAL–MMP-9, which most likely comes from activated neutrophils, the rest of our zymographic data are noncell-specific. Third, we cannot say anything about specific mechanisms of brain injury. We only compared MMP levels with overall tissue infarction. Further studies are required to examine how plasma MMP may or may not truly track the evolution of blood–brain barrier damage and other specific mechanisms of pathophysiology within the neurovascular unit. Fourth, our numbers and time points are relatively limited. We detected a significant correlation between plasma and brain pro-MMP-9 at 24 hours, and between 24-hour plasma pro-MMP-9 and 24-hour infarct volumes. But it is possible that higher numbers may have increased our power to pull out correlations at different time points. Besides, we have no long-term follow-up. Delayed changes in MMP-2 will be missed in our study. And we cannot be sure how our so-called biomarkers may or may not correlate with actual functional outcomes after stroke. Fifth, our study only examined MMP profiles in permanent ischemia. How these MMP biomarkers respond to reperfusion will have to be carefully investigated, especially in the context of thrombolytic therapy. Finally, our analysis is focused on MMP-9. Future studies using protein arrays may be needed to examine the network response of all MMP in cerebral ischemia. For biomarker purposes, a broader signature of multiple MMP may generate more power than reliance on a single protease alone.
In conclusion, our study showed that in a rat model of focal cerebral ischemia, plasma and brain levels of MMP-9 were correlated with each other, both plasma and brain MMP-9 progressively increased over the first 24 hours after ischemia, and 24-hour plasma pro-MMP-9 levels are correlated with 24-hour brain infarction. But, it is important to emphasize that mechanisms and causality remain unproven, so that more careful investigation of clinical MMP profiles in stroke patients would be useful.
Acknowledgments
Sources of Funding
This study was supported in part by NIH grants R37-NS37074, R01-NS48422, R01-NS56458, P01-NS55104, and P50-NS51343, and grant 2006-34 from the Medical Research Institute, Pusan National University.
Footnotes
Disclosures
None.
References
- 1.Mun-Bryce S, Rosenberg GA. Matrix metalloproteinases in cerebrovascular disease. J Cereb Blood Flow Metab. 1998;18:1163–1172. doi: 10.1097/00004647-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Yong VW. Metalloproteinases: Mediators of pathology and regeneration in the CNS. Nat Rev Neurosci. 2005;6:931–944. doi: 10.1038/nrn1807. [DOI] [PubMed] [Google Scholar]
- 3.Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke. 2007;38:748–752. doi: 10.1161/01.STR.0000253500.32979.d1. [DOI] [PubMed] [Google Scholar]
- 4.Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: Inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and timps are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 6.Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: Effects of gene knockout and enzyme inhibition with bb-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Koistinaho M, Malm TM, Kettunen MI, Goldsteins G, Starckx S, Kauppinen RA, Opdenakker G, Koistinaho J. Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease-9-deficient mice. J Cereb Blood Flow Metab. 2005;25:460–467. doi: 10.1038/sj.jcbfm.9600040. [DOI] [PubMed] [Google Scholar]
- 9.Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6:480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 11.Nagel S, Su Y, Horstmann S, Heiland S, Gardner H, Koziol J, Martinez-Torres FJ, Wagner S. Minocycline and hypothermia for reperfusion injury after focal cerebral ischemia in the rat: Effects on bbb breakdown and mmp expression in the acute and subacute phase. Brain Res. 2008;1188:198–206. doi: 10.1016/j.brainres.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 12.Whiteley W, Tseng MC, Sandercock P. Blood biomarkers in the diagnosis of ischemic stroke. A systematic review Stroke. 2008;39:2902–2909. doi: 10.1161/STROKEAHA.107.511261. [DOI] [PubMed] [Google Scholar]
- 13.Montaner J, Alvarez-Sabin J, Molina C, Angles A, Abilleira S, Arenillas J, Gonzalez MA, Monasterio J. Matrix metalloproteinase expression after human cardioembolic stroke: Temporal profile and relation to neurological impairment. Stroke. 2001;32:1759–1766. doi: 10.1161/01.str.32.8.1759. [DOI] [PubMed] [Google Scholar]
- 14.Montaner J, Rovira A, Molina CA, Arenillas JF, Ribo M, Chacon P, Monasterio J, Alvarez-Sabin J. Plasmatic level of neuroinflammatory markers predict the extent of diffusion-weighted image lesions in hyperacute stroke. J Cereb Blood Flow Metab. 2003;23:1403–1407. doi: 10.1097/01.WCB.0000100044.07481.97. [DOI] [PubMed] [Google Scholar]
- 15.Koh SH, Chang DI, Kim HT, Kim J, Kim MH, Kim KS, Bae I, Kim H, Kim DW, Kim SH. Effect of 3-aminobenzamide, parp inhibitor, on matrix metalloproteinase-9 level in plasma and brain of ischemic stroke model. Toxicology. 2005;214:131–139. doi: 10.1016/j.tox.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, Proost P, Van Damme J. Gelatinase b functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69:851–859. [PubMed] [Google Scholar]
- 17.Arai K, Lee SR, Lo EH. Essential role for erk mitogen-activated protein kinase in matrix metalloproteinase-9 regulation in rat cortical astrocytes. Glia. 2003;43:254–264. doi: 10.1002/glia.10255. [DOI] [PubMed] [Google Scholar]
- 18.Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- 19.Lee SR, Lo EH. Induction of caspase-mediated cell death by matrix metalloproteinases in cerebral endothelial cells after hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2004;24:720–727. doi: 10.1097/01.WCB.0000122747.72175.47. [DOI] [PubMed] [Google Scholar]
- 20.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: Signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 21.Ning M, Furie KL, Koroshetz WJ, Lee H, Barron M, Lederer M, Wang X, Zhu M, Sorensen AG, Lo EH, Kelly PJ. Association between tpa therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006;66:1550–1555. doi: 10.1212/01.wnl.0000216133.98416.b4. [DOI] [PubMed] [Google Scholar]
- 22.Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: Mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33:2711–2717. doi: 10.1161/01.str.0000033932.34467.97. [DOI] [PubMed] [Google Scholar]
- 23.Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, Davalos A. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–46. [PubMed] [Google Scholar]
- 24.Horstmann S, Kalb P, Koziol J, Gardner H, Wagner S. Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: Influence of different therapies. Stroke. 2003;34:2165–2170. doi: 10.1161/01.STR.0000088062.86084.F2. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amantea D, Corasaniti MT, Mercuri NB, Bernardi G, Bagetta G. Brain regional and cellular localization of gelatinase activity in rat that have undergone transient middle cerebral artery occlusion. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. Mmp-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type iv collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- 28.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]