Abstract
Objectives
Mucolipidoses II and III alpha/beta (ML II and ML III) are lysosomal disorders in which the essential mannose-6-phosphate recognition marker is not synthesized onto lysosomal hydrolases and other glycoproteins. The disorders are caused by mutations in GNPTAB, which encodes two of three subunits of the heterohexameric enzyme, N-acetylglucosamine-1-phosphotransferase. Clinical, biochemical, and molecular findings in 61 probands (63 patients) are presented in order to provide a broad perspective of these mucolipidoses.
Methods
GNPTAB was sequenced in all probands and/or parents. Activity of several lysosomal enzymes was measured in plasma, and GlcNac-1-phosphotransferase was assayed in leukocytes. Thirty-six patients were studied in detail, allowing extensive clinical data to be abstracted.
Results
ML II correlates with near total absence of phosphotransferase activity resulting from homozygosity or compound heterozygosity for frameshift or nonsense mutations. Craniofacial and orthopedic manifestations are evident at birth, skeletal findings become more obvious within the first year, and growth is severely impaired. Speech, ambulation, and cognitive function are impaired. ML III retains a low level of phosphotransferase activity due to at least one missense or splice site mutation. The phenotype is milder with minimal delays in milestones, the appearance of facial coarsening by early school age, and slowing of growth after age four years.
Conclusions
Fifty-one pathogenic changes in GNPTAB are presented, including 42 novel mutations. Ample clinical information improves criteria for delineation of ML II and ML III. Phenotype-genotype correlations suggested in more general terms in earlier reports on smaller groups of patients are specified and extended.
Keywords: mucolipidosis, I-cell disease, pseudoHurler polydystrophy, dysostosis multiplex, lysosomal disease
INTRODUCTION
Mucolipidosis II (ML II: MIM 252500), originally called Inclusion Cell or I-Cell disease, is an autosomal recessive lysosomal disorder, clinically evident from birth with progressive course and fatal outcome in childhood.[1–3] Mucolipidosis III alpha/beta (ML III: MIM 252600), originally described as PseudoHurler Polydystrophy, is allelic to ML II and has a closely related pathogenesis.[4,5] It is also an autosomal recessive disorder with onset in early childhood, slowly progressive course and fatal outcome from early adulthood. ML III gamma (MIM 252605) is a nonallelic mucolipidosis which has so far been observed mainly in Middle-East countries.[6,7]
The term “mucolipidosis” was introduced in 1970 by Spranger and Wiedemann to describe several conditions with features of both the mucopolysaccharidoses and the sphingolipidoses.[8] Only ML II and ML III are pathologically similar. ML I, now more descriptively called sialidosis, is due to deficiency of neuraminidase. ML IV is a neurodevelopmental disorder with retinal degeneration and is caused by mutations in the MCOLN1 gene. Activity of the lysosomal hydrolases is normal in ML IV.
ML II, ML III and ML III gamma result from the deficiency of the heterohexameric enzyme, UDP-N-acetylglucosamine: lysosomal hydrolase N-acetylglucosamine-1-phosphotransferase (IUBMB # 2.7.8.17), trivially termed UDP-GlcNAc-1-phosphotransferase (GlcNAc-1-PT or GNPT). The enzyme is composed of two alpha, two beta and two gamma polypeptides. It catalyzes the first step in the biosynthesis of the mannose-6-phosphate (M6P) recognition marker in lysosomal hydrolases and other glycoproteins in the cis Golgi cisterns.[9–11] This modification does not occur in ML II and is deficient in ML III fibroblasts. Without the M6P marker the lysosomal glycoproteins end up in the culture media of fibroblasts in vitro, where the catalytic activity is significantly increased. Concomitantly the intracellular activity of most lysosomal enzymes is considerably reduced in cultured cells.[12,13] The former finding correlates directly with the increased specific activity of lysosomal enzymes in plasma and other body fluids of patients with ML II and ML III.[13–16]
The GNPTAB gene contains 21 exons, spans about 80kb of cDNA on chromosome 12q23.3 and encodes a 1256 amino acid precursor protein that contains the catalytic domain and contributes also to substrate recognition.[6, 17–20] Proteolytic cleavage at the Lys928-Asp929 bond yields the mature alpha and beta polypeptides. Homozygous or compound heterozygous mutations in GNPTAB result in either ML II or ML III as amply proven by the reports of 37 different pathogenic mutations.[21–28] The GNPTG gene with 11 exons located at 16p13.3, encodes the gamma polypeptide with possible substrate recognition role in the GNPT hexameric enzyme complex. Mutations in the GNPTG gene cause ML III gamma.[6,7,19,29]
Here we present 51 mutations in GNPTAB, 42 of them novel, in a cohort of 63 patients (61 probands). The report includes clinical and biochemical findings in addition to the patients’ genotypes. The conclusions pertain to improving the criteria for delineation of ML II and ML III, and for distinction of rather rare patients with clinical findings intermediate between the two reference phenotypes. The results confirm, specify and extend the phenotype-genotype correlations suggested in more general terms in earlier reports on smaller groups of patients.
SUBJECTS AND METHODS
Patients and Families
This study describes the clinical, biochemical, and molecular findings in 61 ML probands (63 patients), the largest cohort reported so far. The majority of patients were from the United States, but probands were of various ethnic backgrounds and from wide ranging geographic areas including the United Kingdom (1), Canada (1), Japan (1), Australia (3), and New Zealand (2). Further diversity is represented because of foreign-born parentage (England, Venezuela, Pakistan, India, Mexico, and Guyana). GNPTAB was sequenced in 56 probands. Five probands who died before the study, are also included as their GNPTAB genotypes were determined by mutation screening in the parents.
Thirty-four probands (36 patients) were termed the “core” group of subjects, because either they were clinically evaluated by at least one of the authors or detailed review of the clinical records was possible. Of historical interest, one of the two unrelated patients in whom ML II (termed I-Cell disease at the time) was originally delineated forty years ago is included. [1,2] The core group of patients consisted of 13 probands (14 patients) with ML II, 14 probands (15 patients) with ML III and 7 probands with a phenotype called ML “intermediate.”
Twenty-seven patients were assigned to the “non-core” group of subjects as more limited clinical information accompanied the samples sent to the Greenwood Genetic Center for laboratory investigations and none was clinically evaluated by the authors.
Clinical data on the core patient group only were used to provide the basis for correlating phenotype and mutant GNPTAB genotype, but findings in the non-core group of patients were most often supportive of the conclusions regarding phenotype-genotype correlations.
Lysosomal Enzymes
Plasma activity of several lysosomal hydrolases including total β-D-N-acetylglucosaminidase (EC 3.1.2.52), β-D-glucuronidase (EC 3.2.1.31), β-D-galactosidase (EC 3.2.1.23), and α-L-fucosidase (EC 3.2.1.51) was measured according to the methods of Thomas et al.[30] Measurement of arylsulfatase A activity in plasma was performed according to Baum et al. with minor modifications.[31]
GlcNAc-1-phosphotransferase in Leukocytes
GlcNAc-1-phosphotransferase was assayed in samples of peripheral blood leukocytes according to the procedure published previously.[26] However, it was observed that GlcNAc-phosphotransferase activity was lost significantly during the storage of leukocytes from healthy controls and obligate heterozygotes. Because of this sample deterioration during storage, we used activity data only when control and patient leukocytes were collected around the same time.
GNPTAB Mutation Screening
PCR and Sequencing
Individual exons were amplified with primers previously published by Tiede et al.[21] After gel electrophoresis confirmed successful amplification the amplicons were purified by QIAquick columns or plates (QIAGEN, USA). Sequencing was performed with a standard BigDye [Applied Biosystems (ABI), Foster City, CA] protocol followed by purification using DyeEx columns or plates (QIAGEN). Products were run on an ABI3730 DNA Sequencer. Finished sequence data were compared to normal controls and published GNPTAB text sequence using Sequencher software (Gene Codes Corporation, Ann Arbor, MI).
RESULTS
Patients with ML II
The ML II core group includes 14 patients (including one twin pair), four males and 10 females. Seven patients have died, the youngest at 3 days of age and the oldest at 8 years 3 months. Four patients have been examined by one of the authors (SC, JL).
Prenatal ultrasound studies documented abnormalities in 5 of 10 pregnancies, including oligohydramnios (2), intrauterine growth retardation (2), echogenic cardiac foci (2), and short femurs (1). Two patients had preterm deliveries. Anthropometric measurements at birth were below average in most instances and seven patients were small for gestational age (Tables I and II).
Table I.
Summary of Clinical Data in Patients with ML II, ML III, and ML Intermediate
| ML II | ML III | ML Intermediate | |
|---|---|---|---|
| Number of Patients | 14 | 15 | 7 |
| Sex (M,F) | 4 M, 10 F | 5 M, 10 F | 6 M, 1 F |
| Parental consanguinity | 2/14 | 0/13 | 2/7 |
| Birth weight (g) | 2400 (2000–3100; n=12) |
3098 (2000–3800; n=14) |
2750 (1940–3400; n=7) |
| Birth length (cm) | 46.6 (42–49.5; n=10) | 50.3 (47–53; n=11) | 49.3 (43–53; n=5) |
| OFC (cm) | 32.1 (31–33; n=6) | 34.1 (30–37, n=5) | 32.8 (31.5–34, n=2) |
| Neonatal assessment: | |||
| Size: LGA | 0/12 | 1/14 | 0/5 |
| AGA | 4/12 | 13/14 | 4/5 |
| SGA | 8/12 | 0/14 | 1/5 |
| Signs: Craniofacial | 8/12 | 0/15 | 1/5 |
| Skeletal | 11/14 | 2/15 | 2/5 |
| First signs: | |||
| Birth | 14/14 | 2/14 | 3/7 |
| 1–12 months | - | 2/14 | 2/7 |
| 1–3 years | - | 6/14 | 2/7 |
| 3–5 years | - | 2/14 | - |
| >5 years | - | 2/14 | - |
| Age at Diagnosis: | |||
| Prenatal/birth | 0/14 | 0/15 | 0/7 |
| Infancy (0–1 yr) | 10/14 | 1/15 | 1/7 |
| 1–3 years | 4/14 | 1/15 | 6/7 |
| 3–5 years | - | 6/15 | - |
| >5 years | - | 7/15 | - |
| Stature: <3% | |||
| 0–5 years | 12/12 | 5/15 | 6/6 |
| 5–8 years | - | 4/15 | - |
| >8 years | - | 6/15 | - |
| Max cm (years) | 79 (age 3) | 150 (age 29) | 110 (age 13) |
| Development: | |||
| Independent ambulation | 0/12 | 15/15 | 7/7 |
| Limited ambulation | 1/12 | - | - |
| Absent speech | 1/12 | 0/14 | 0/6 |
| Limited speech | 11/12 | 0/15 | 0/6 |
| Normal speech | 0/12 | 15/15 | 6/6 |
| Cognitive deficit (IQ <70) | 12/12 | 3/11 | 1/4 |
| IQ >85 | 0/13 | 4/11 | 2/4 |
| Death: | |||
| 0–3 years | 2/7 | 0/15 | 0/7 |
| 3–5 years | 1/7 | 0/15 | 0/7 |
| 5–10 years | 4/7 | 0/15 | 0/7 |
| 10–20 years | - | 0/15 | 0/7 |
| 20–30 years | - | 1/15 | 1/7 |
Table II.
Comparison of the ML II and ML III Phenotypes
| Finding | ML II | ML III |
|---|---|---|
| Intrauterine growth | Low to normal | Normal |
| Growth, stature | Stops before age 2 years | Slow growth after age 4 years |
| Final stature* | <80 cm | >115 cm |
| Onset of signs | Birth to early infancy | Early to mid-childhood |
| Craniofacial features | From birth, pronounced gingival hypertrophy and coarse features, including round cheeks, shallow orbits, depressed nasal bridge | Gradual coarsening of facial features beginning between 5–8 years; mild gingival hypertrophy with normal tooth eruption |
| Hands, fingers | Short, broad, early clawing | Normal to long, late distal clawing |
| Unaided walking | Rarely achieved | Achieved with mild to no delay; independent ambulation variably declines as disease progresses |
| Speech | Delayed, limited expressive language | Normal |
| Cognitive function | Mild to moderate deficit, rarely severe | Borderline to normal |
| Hands radiographs | Short tubular bones, progressing to severe diaphyseal widening | Minimally short tubular bones, diaphyseal narrowing partially preserved |
| Vertebral radiographs | AP shortening, biconcave outline, irregular borders | AP elongation, mild platyspondyly, irregular borders |
| Activity of GlcNAc-1-phospho- transferase (leukocyte or fibroblasts) | <1% of control value | 1–10% of control value |
Statural measurement difficult in later part of clinical course in either clinical entity because of joint restriction
All ML II patients had craniofacial and/or skeletal abnormalities noted on the first day of life but time to diagnosis ranged from 3 weeks to 2.5 years. Cranial size remained proportional to body size. Early dysmorphic features included facial coarseness with depressed nasal bridge and shallow orbits, metopic prominence, and thickened alveolar ridges. The hypertrophied gums contributed to the notation of narrow, deeply furrowed palates in 5 newborns. Although the craniofacial features may be suggestive, true craniosynostosis is not a component of ML II. Unfortunately this descriptive label was assigned to four patients in this group, and two had craniectomies prior to the recognition of the diagnosis ML II.
Congenital abnormalities requiring orthopedic attention in infancy included hip dysplasia or dislocation (3 patients), scoliosis (1 patient), hand contracture (4 patients), bowed limbs (5 patients, including 2 with club feet). Three patients were thought to have neonatal rickets or osteogenesis imperfecta. Statural growth failed completely before or by 24 months of age.
All ML II patients had delayed and deficient neuromotor development. Some probands could never sit unsupported. Only 1/14 patients achieved unaided walking. Nearly all made vocal sounds, but verbal expression remained limited to few words, poorly and hoarsely pronounced. Cognitive development and receptive language skills fit best into the range of moderate intellectual disability, but formal psychometric testing could not be performed in most patients.
Most patients were poor feeders leading to enteral support (nasogastric or gastric tube) for 7/14 patients. All patients experienced increased morbidity of recurrent respiratory infections. Four had tracheotomies. Mitral and aortic valve thickening was the most common problem detected by echocardiography. Hypertrophy of ventricular walls was a consistent feature in the longer surviving probands. Umbilical and inguinal hernias were noted in five and two patients, respectively. Two males had hypospadias. Five patients had documented corneal clouding, the earliest noted at 5 months of age. Detailed observations on each ML II patient are available from the authors.
Patients with ML III
The ML III core group includes 15 patients (including one sib pair), five males and 10 females. As of July 1, 2008, patient ages ranged from 10 years to 45 years. Ten of the individuals have been examined by one of the authors (SC).
Eight of the 15 pregnancies had prenatal ultrasound studies. None demonstrated fetal anatomical abnormalities. One patient was delivered preterm. All were average size or above for gestational age (Tables I and II).
The time period from initial skeletal signs of disease to appropriate diagnosis varied from six months to ten years with an average of five years. Suspected diagnoses included juvenile rheumatoid arthritis, various mucopolysaccharidoses, Niemann-Pick type B, cerebral palsy, and arthrogryposis congenita. Orthopedic abnormalities were documented at birth in only two patients, both of whom had hip dysplasia. The patient with the latest onset of skeletal symptoms began complaining of joint stiffness and loss of flexibility at 7.5 years. For the others, contractures of the hands were evident between 2.5 years and 4 years of age. Decreased range of motion of the shoulders was noted by four years of age in four patients. One of the two patients appropriately diagnosed before the onset of symptoms was evaluated for lysosomal disease in infancy because routine placental pathology had revealed abnormal foamy cells. This child was entirely asymptomatic at seven months of age when the diagnosis was established. The second pre-symptomatic diagnosis was made in a two year old, four years prior to this child’s complaints of hip pain and difficulty with walking. This individual had been initially evaluated concurrently with an affected six-year-old sibling.
Early developmental milestones are known for 12 of the 15 patients in the ML III core. Mild gross motor delays were noted in seven patients, with walking the most common delayed milestone, achieved at 16 months for one and 17–24 months for the other patients in this group. Although 15/15 patients achieved independent ambulation in early childhood, all experienced progressive joint stiffness and pain. By their mid-teens, 11 patients relied on assistive devices (wheelchair, motorized scooter) for longer distances. One proband with atypical spinal cord complications lost independent ambulation at 15 years. Another proband was limited to a few painful steps by 20 years.
In contrast, the oldest patient in this cohort had bilateral hip and knee replacements and maintained independent ambulation far longer. This patient’s first hip replacement was performed at age 25 years and revised 19 years later. One of the patients with recognized neonatal hip dislocations underwent bilateral hip replacement at age 15 years. A third patient had knee replacement surgery at age 25 years. Four patients underwent spinal fusion procedures. Carpal tunnel release was performed in 10 of 15 patients, often multiple times. Two patients had inguinal hernia repair and four patients had small, uncomplicated umbilical hernias.
Myringotomy tube placement was performed repeatedly in seven patients for chronic or recurrent otitis media. Recurrent respiratory infections, including pneumonia, occurred in 5 of 15 patients. The patient with the most severe tracheomalacia required tracheotomy and tracheal stents. Even among patients without frequent respiratory illness, intubations for surgical procedures were often described as complicated necessitating the use of fiberoptic equipment.
Ophthalmologic findings included mild corneal clouding documented on slit lamp examination in 5 patients, juvenile glaucoma in one, and myopia in 6/15. All but one patient had at least mild cardiac valve thickening, most commonly involving the mitral and secondly the aortic valves. A single patient had aortic valve replacement at age nine years, but this was probably not entirely related to ML III. This patient’s father had required replacement of his bicuspid aortic valve. One patient was treated for congestive heart failure from mid-teens until the time of death at age 25 years.
Ten of the core ML III patients were administered the Kaufman Brief Intelligence Test, Second Edition (K-BIT). The testing results confirmed normal IQ in this group of patients, with a mean verbal IQ of 83 (range 71–100), nonverbal IQ of 83 (range 71–103), and composite IQ of 81 (range 67–96). The majority of patients required at least minimal special educational support or classroom modifications. Among the adult patients, high school diplomas were achieved by all but one. Two patients attended college, and one achieved a bachelor’s degree. Detailed observations on each ML III patient are available from the authors.
Patients with ML Intermediate
In Table I the clinical findings in 7 core group probands are summarized separately because from the outset, features of both ML II and ML III were noticed. In general these patients with intermediate phenotypes tended to have physical findings similar to, but milder than, ML II and clinical courses more reminiscent of ML III.
Radiographic Findings
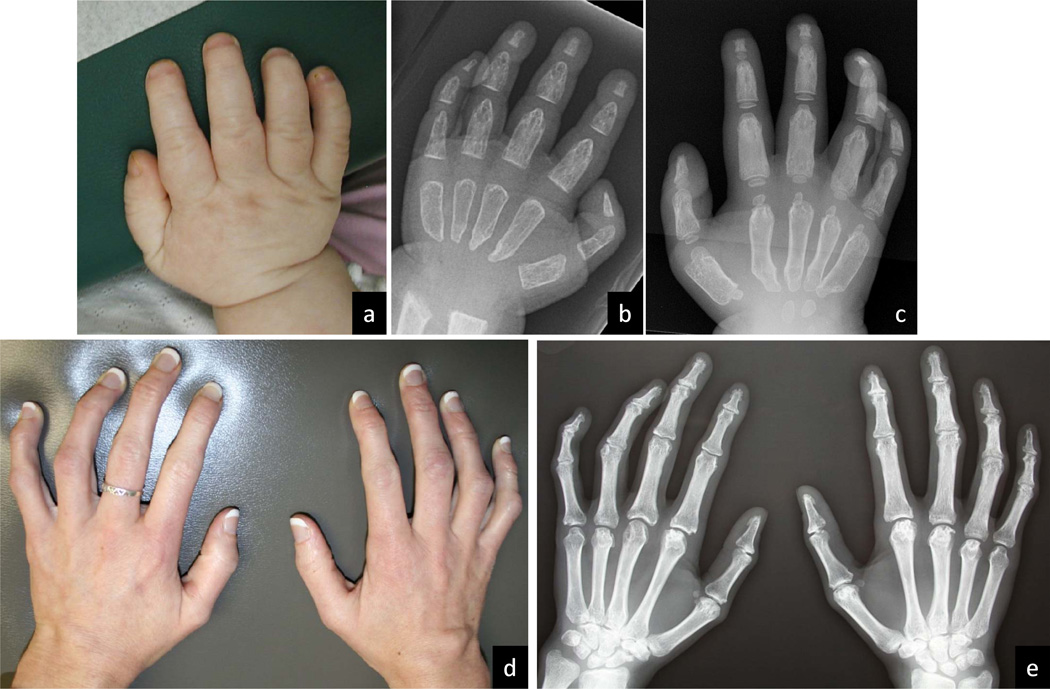
ML II and ML III belong to the larger group of metabolic disorders associated with osteochondrodystrophic changes that have been termed dysostosis multiplex.[32] The complete absence of GlcNAc-1-phosphotransferase in ML II results in earlier and more severe changes than those seen in ML III, but also in qualitative differences as is best demonstrated in hand films. The tubular hand bones are significantly shortened and the diaphyses become extremely widened in the period between infancy and age three years in the ML II patient (Figure 3). The submetaphyseal regions of the metacarpals remain narrow, resulting in a pointed configuration at both ends. In ML III, the tubular handbones are of normal or near normal length and more importantly maintain near normal diaphyseal tubulation (Figure 3). Contractures, especially of the more distal small finger joints, and the ensuing claw-like deformity, are mainly due to hardening of tendons and capsular soft connective tissue. The contractures and small joint stiffening occur much earlier and are more severe in ML II, but also occur consistently in ML III.
Figure 3.
Photographs and radiographs of hands of ML II and ML III. a–c, different ML II patients at 3 years, 15 months, and 4 10/12 years; d and e, same ML III patient at 29 years. The metacarpals and phalanges appear short and broad by 15 months (b). Irregular constriction causes proximal and distal pointing and the pinched appearance of the metacarpals (c). The hand contractures are clinically evident at a later age and remain milder than in ML II. The tubular bones of the hands (e) maintain near normal length and width.
Lysosomal Enzymes in Plasma
Plasma activity of several lysosomal hydrolases in all affected patients was significantly increased over controls. Comparison of the mean value for the patient group versus controls showed a 6.7 to 60 fold increase in plasma activity [α-L-fucosidase (x6.7), arylsulfatase A -EC 3.1.6.1- (x8.4), β-D-galactosidase (x13.7), total β-D-N-acetylglucosaminidase (x13.9), and β-D-glucuronidase (x60)]. Parents of affected patients (obligate carriers) also had higher circulating enzyme levels (1.3 – 3.5x) as compared to controls.
The mean value for each lysosomal enzyme was compared between the ML II and ML III groups using an unpaired t-test assuming unequal variance using Stata/SE 10 (Stata Corp, College Station, TX). β-D-glucuronidase and α-L-fucosidase were the only enzymes that showed statistically different (p=0.0129 and 0.0140, respectively) activities between these two patient groups.
GlcNAc-1-phosphotransferase in Leukocytes (n=9)
GlcNAc-1-phosphotransferase activity was found completely abolished in the single ML II sample, varied between 0.84% and 10% of the control sample in four ML III samples, and was 3% of the control level in the single ML intermediate sample. The specific activity of GlcNAc-1-phosphotransferase in two obligate heterozygotes was calculated to be 36.2% (ML III carrier) and 71.4% (ML II carrier) of control activity.
GNPTAB mutations
Both pathogenic GNPTAB mutations were identified and characterized in 60 of 61 probands by routine sequencing (Table III and supplemental Table 1). The single exception is an ML II patient in whom only one of the two pathogenic mutations has been fully characterized. The second change is a large insertion that may be similar in some fashion to the retrotransposition of an Alu element in GNPTAB as has been reported by Tappino et al.[28]
Table III.
GNPTAB Genotypes in ML II, ML III and ML Intermediate
| A. Thirty-Six Patients (Thirty-Four Probands) in Core Group | ||||||
|---|---|---|---|---|---|---|
| ML II | ML “Intermediate” | ML III | ||||
| Genotypes | Exon | Mutant Alleles | Exon | Mutant Alleles | Exon | Mutant Alleles |
|
Nonsense/ Frameshift |
15/19 | c.3091C>T//c.3503delTC | ||||
| 10/13 | c.1123C>T//c.2693insA | |||||
| 19/19 | c.3503delTC//c.3565C>T | |||||
| 19/17 | c.3565C>T//c.3327insA | |||||
| 13/19 | c.2664C>G//c.3503delTC | |||||
|
Homozygous Frameshift |
12 | c.1581delC | 4 | c.342delCA | ||
| 11 | c.1399delG | |||||
| 19 | c.3503delTC | |||||
| 19 | c.3503delTC | |||||
|
Comp Hetero Frameshift |
7/? | c.648delAGAA/ ND | ||||
| 6/19 | c.616delCAGA//3503delTC | |||||
| 6/19 | c.616delCAGA//3503delTC | |||||
| 19/2 | c.3503delTC//c.171delA | |||||
|
Frameshift/ Splice |
19/17 | c.3443delTTTG//c.3335+6T>G | ||||
| 19/17 | c.3503delTC//c.3335+6T>G | |||||
| 17/19 | c.3335+6T>G//c.3443delTTTG | |||||
| 17/11 | c.3335+6T>G//c.1399delG | |||||
|
Frameshift/ Missense |
13/1 | c.1964delC//c.10C>A | 5/9 | c.569A>T//c.3503delTC | ||
| 1/11 | c.10C>A//c.1399delG | |||||
| 1/13 | c.10C>A//c.2190delT | |||||
| 5/7 | c.569A>T//c.750insA | |||||
|
Splice/ Nonsense |
13/2 | c.2715+2T>G//c.168T>A | ||||
| 19/17 | c.3565C>T//c.3335+6T>G | |||||
| 17/19 | c.3335+6T>G//c.3565C>T | |||||
| Splice/MS | 11/18 | c.1402T>A//c.3336-1G>A | 10/17 | c.1196C>T//c.3335+6T>G* | ||
|
Missense/ Nonsense |
10/10 | c.1123C>T//c.1196C>T | ||||
| 10/10 | c.1196C>T//c.1123C>T | |||||
| 9/5 | c.1000C>T//c.596A>T | |||||
| Homo MS | 15 | c.3053A>G | ||||
| Comp Hetero Missense | 3/12 | c.232delGTT**//c. 1514G>A | ||||
| 9/1 | c.1001G>A//c.44C>A | |||||
| B. Twenty-seven Patients in Non-Core Group | ||||||
|---|---|---|---|---|---|---|
| ML II | ML “Intermediate” | ML III | ||||
| Genotypes | Exon | Mutant Alleles | Exon | Mutant Alleles | Exon | Mutant Alleles |
|
Homozygous Nonsense |
9 | c.1090C>T | ||||
| 15 | c.3061C>T | |||||
|
Nonsense/ Frameshift |
2/19 | c.136C>T//c.3503delTC* | ||||
| 3/13 | c.1759C>T//c.1959del4* | |||||
| 11/18 | c.1399delG//c.3410T>A* | |||||
|
Homozygous Frameshift |
19 | c.3503delTC | ||||
| 13 | c.2275delA | |||||
| 4 | c.342delCA | |||||
| 19 | c.3503delTC | |||||
| 16 | c.3232delT | |||||
| 19 | c.3503delTC | |||||
|
Comp Hetero Frameshift |
11/19 | c.1399delG//c.3503delTC* | ||||
| 13/19 | c.2188delTinsAAA//c.3503delTC* | |||||
| 13/19 | c.2529insG//c.3503delTC* | |||||
| 13/19 | c.1999insT//c.3503delTC | |||||
|
Frameshift/ Splice |
4/16 | c.342delCA//c.3249+G>C* | 5/17 | c.517insA//c.3335+6T>G* | ||
| 7/19 | c.637-1G>A//c.3503delTC | 17/19 | c.3335+6T>G//c.3503delTC* | |||
| 11/17 | c.1399delG//c.3335+6T>G* | |||||
|
Frameshift/ Missense |
19/9 | c.3503delTC//c.1001G>C | 1/11 | c.10C>A//c.1399delG* | 1/19 | c.44C>A//c.3503delTC |
| 6/9 | c.625del5//c.1042A>C* | |||||
|
Splice/ Nonsense |
17/10 | c.3335+6T>G//c.1123C>T | ||||
| Homo Splice | 5 | c.571+ 3A>C | ||||
| Spl/MS | 11/14 | c.1285-2A>G//c.2867A>G* | ||||
ND indicates that an apparent structural rearrangement has not been completely delineated
Parental origin of mutations unknown; when known, paternal mutation is always on the left and maternal on the right
Three nucleotide deletion not expected to cause a frameshift; patient classified as a missense compound heterozygote
Parental origin of mutations unknown; when known, paternal mutation is always on the left and maternal on the right
Fifty-one different GNPTAB pathogenic alterations were identified, including 10 missense (MS), 11 nonsense (NS), 22 frameshift (FSh), and 7 splice site (Spl) alterations and the above-mentioned undefined structural rearrangement. Nine mutations, which had been previously reported in the literature, accounted for almost half (46.7%) of the mutant alleles in this cohort of 61 probands.[21–26,28,33] The most common was the FSh mutation, c.3503delTC, occurring in 18 ML II and four ML III patients (Table III). The next most common mutation allele was the c.3335+6T>G splice alteration found in the heterozygous state in eleven ML III probands. The 42 novel mutations, including the incompletely elucidated structural rearrangement in one ML II patient, are shown in Table III by proband and Table IV by exon. Most (38/42) of the novel mutations were detected in only one or in two alleles. Only three of the novel alterations (c.342delCA, c.1123C>T and c.1399delG) were encountered more frequently and identified in 5, 4 and 8 alleles, respectively. With the exception of two Hispanic patients in whom the frameshift c.616del ACAG was found in the heterozygous state, each recurrent mutation was detected in individuals of various ethnicities.
Table IV.
Pathogenic Mutation Summary in GNPTAB
| Exon | Alteration | Effect | Alleles | Number of Patients Homozygous |
Number of Patients Heterozygous |
|---|---|---|---|---|---|
| 1 | c.10A>C | p.K4Q | 4 | 4 | |
| 1 | c.44C>A | p.S15Y | 2 | 2 | |
| 2 | c.136C>T | p.R46X | 1 | 1 | |
| 2 | c.168T>A | p.Y56X | 1 | 1 | |
| 2 | c.171delA | frameshift | 1 | 1 | |
| 3 | c.232delGTT | p.V78del | 1 | 1 | |
| 4 | c.342delCA | frameshift | 5 | 2 | 1 |
| 5 | c.517insA | frameshift | 1 | 1 | |
| 5 | c.569A>T | p.D190V | 3 | 3 | |
| 5 | c.571+3A>C | abnormal splice | 2 | 1 | |
| 6 | c.616delCAGA | frameshift | 2 | 2 | |
| 6 | c.625delAGGGG | frameshift | 1 | 1 | |
| 7 | c.637-1G>A | abnormal splice | 1 | 1 | |
| 7 | c.648delAGAA | frameshift | 1 | 1 | |
| 7 | c.750insA | frameshift | 1 | 1 | |
| 9 | c.1000C>T | p.R334X | 1 | 1 | |
| 9 | c.1001G>A | p.R334Q | 2 | 2 | |
| 9 | c.1042A>C | p.I348L | 1 | 1 | |
| 9 | c.1090C>T | p.R364X | 2 | 1 | |
| 10 | c.1123C>T | p.R375X | 4 | 4 | |
| 10 | c.1196C>T | p.S399F | 3 | 3 | |
| 11 | c.1285-2A>G | abnormal splice | 1 | 1 | |
| 11 | c.1399del G | frameshift | 8 | 1 | 6 |
| 11 | c.1402T>A | p.C468S | 1 | 1 | |
| 12 | c.1514G>A | p.C505Y | 1 | 1 | |
| 12 | c.1581delC | frameshift | 2 | 1 | |
| 13 | c.1759C>T | p.R587X | 1 | 1 | |
| 13 | c.1959delTAGT | frameshift | 1 | 1 | |
| 13 | c.1964delC | frameshift | 1 | 1 | |
| 13 | c.1999insT | frameshift | 1 | 1 | |
| 13 | c.2188delTinsAAA | frameshift | 1 | 1 | |
| 13 | c.2189delT | frameshift | 1 | 1 | |
| 13 | c.2275delAA | frameshift | 2 | 1 | |
| 13 | c.2591insG | frameshift | 1 | 1 | |
| 13 | c.2664C>G | p.Y888X | 1 | 1 | |
| 13 | c.2693insA | frameshift | 1 | 1 | |
| 13 | c.2715+2T>G | abnormal splice | 1 | 1 | |
| 14 | c.2867A>G | p.H956R | 1 | 1 | |
| 15 | c.3053A>G | p.D1018G | 2 | 1 | |
| 15 | c.3061C>T | p.Q1021X | 2 | 1 | |
| 15 | c.3091C>T | p.R1031X | 1 | 1 | |
| 16 | c.3232delT | frameshift | 2 | 1 | |
| 16 | c.3249+1G>C | abnormal splice | 1 | 1 | |
| 17 | c.3327insA | frameshift | 1 | 1 | |
| 17 | c.3335+6T>G | abnormal splice | 11 | 11 | |
| 18 | c.3336-1G>C | abnormal splice | 1 | 1 | |
| 18 | c.3410T>A | p.L1137X | 1 | 1 | |
| 19 | c.3443delTTTG | frameshift | 2 | 2 | |
| 19 | c.3503delTC | frameshift | 27 | 5 | 17 |
| 19 | c.3565C>T | p.R1189X | 4 | 4 |
Previously reported mutations in bold
Fifteen patients were found to be homozygous for a single pathogenic gene mutation and 46 were compound heterozygotes (Table III). Seven of the homozygotes carried unique mutant alleles suggesting that parental consanguinity was likely even in cases where it was not recognized.
DISCUSSION
Phenotype–Genotype Correlations
The majority of patients (29/36) in the core group clearly exhibited the ML II or ML III phenotype (Table II). The ML II phenotype correlates directly with those GNPTAB genotypes that are homozygous or compound heterozygous for two alleles expected to produce no or nearly no gene product (Table III). That these “null” or “amorph” alleles are associated with absence or near absence of GlcNAc-1-phosphotransferase activity, was proven strictly only in one ML II patient in our study, but shown in a number of similar patients by others.[21,25,26]
In the core group, 12/13 probands with ML II were either homozygous for FSh mutations inducing a premature stop-codon (four patients) or compound heterozygous either for two different FSh mutations (three patients) or for a FSh and NS mutation (five patients). The same clinical consequence was obvious in the remaining patient whose genotype was a compound heterozygote with a FSh mutation and a thoroughly rearranged mutant allele. The direct correlation observed is corroborated by similar genotypes in 15/19 ML II patients in the non-core group (NS//FSh–three patients; FSh homozygotes–six patients; FSh//compound heterozygotes– four patients; NS homozygotes–two patients).
The null alleles in this cohort of ML II patients have affected nine different exons, ranging from exon 2 to exon 19. Hence, mutation type rather than intragenic location has determined the phenotypic outcome. Premature truncation of either the alpha or beta subunit in GlcNAc-1-phosphotransferase is likely to abolish the enzyme activity. This is supported by the homogeneous clinical effect of the null alleles wherever located in the gene.
The presence of at least one hypomorph (MS or Spl) allele in the GNPTAB mutant genotype appears to protect against ML II and results in the clinically milder ML III in all instances; i.e., 15/15 patients in the core group: (FSh//Spl – four patients; FSh//MS – one patient; Spl//NS – four patients; MS//NS – three patients; MS//MS – two patients; Spl//MS – one patient) and in 7/7 subjects in the non-core ML III group (FSh//Spl – three patients; FSh//MS – one patient; Spl//NS – one patient; Spl//Spl – one patient; Spl//MS – one patient) (Table III). Spl and MS mutations are termed hypomorph alleles because instead of total inactivation, they often result in significantly reduced enzyme activity due to decreased amount of and/or ineffective enzyme protein. That the mutant genotype in ML III patients may allow some residual GLcNAc-1-phosphotransferase activity was confirmed in the leukocytes of four ML III patients with enzyme activities ranging between 0.84% and an estimated 10%.
Although the clinical variability among patients with ML III is more extensive than in ML II, all ML III patients in the genotypic category with one null and one hypomorph allele, irrespective of the locations within either the alpha or beta subunit, are phenotypically well within the criteria defining this form of mucolipidosis (Table II). Comparison of the phenotypes in two patients with identical FSh/Spl genotypes, but with the FSh mutation of different parental origins, did not reveal any objective difference. A similar conclusion was reached by comparing the phenotypes in two patients with the identical MS//NS genotype, while taking differences in age well into account.
Our data also indicate that patients with ML III and GNPTAB genotypes composed of two hypomorph alleles are not consistently clinically milder than patients with one null and only one hypomorph allele. For example, one such MS/Spl patient with only minor facial changes and mild dysostosis multiplex has since adolescence developed severe thoracolumbar scoliosis. Another MS/MS patient, now in her forties, had a late onset and slowly evolving ML III phenotype that however required bilateral hip replacement at age 25 years. Painful and crippling hip disease is, at least from adolescence, a major and consistent feature of ML III. A third patient with a milder than average ML III phenotype, had compound heterozygosity for a MS mutation in GNPTAB exon 12 and a deletion of three consecutive nucleotides in exon 3 resulting only in the loss of valine 77 or 78.
A minority (7/36) of patients were found to have an intermediate phenotype, even when taking into account the inherent clinical variability within either reference phenotype. Findings in the seven patients so classified are given separately in Table I. They tended to have earlier appearance of craniofacial and skeletal signs than ML III patients (first signs before age three years), but experienced a clinical course in terms of developmental progress, ambulation, speech, and survival quite similar to ML III patients. With one exception, these patients have at least one MS mutation, either coupled with a FSh (four patients), a Spl (one patient), or another MS mutation (one patient) (Table III). The exception is a patient who is homozygous for a FSh mutation in exon 4. This specific two base pair deletion in exon 4 has subsequently been identified in additional patients, also homozygous. The milder than expected phenotype is remarkably consistent. All other patients with two FSh alleles have fallen into the ML II phenotype.
The mutation spectrum reported here is consistent with the 37 mutations found in eight previous studies.[21–28,34,35] The previously reported alterations are rather evenly spread across the GNPTAB gene, with frameshift causing mutations being the most common (16/37=43%) as in our study (22/51=43%). The mutation encountered most frequently, both in the literature data and in this study (c.3503delTC), has been reported as the single causal mutation in the GNPTAB gene in a French-Canadian founder population.[33] In a recent report, Otomo et al analyzed GNPTAB in 40 Japanese patients.[36] The most common mutation in that population (c. 3565C>T, R1189X) was detected in four of our probands- two US Caucasians, one Hispanic, and one New Zealander. Of the 14 mutations reported as novel by Otomo et al, we report 4 similar mutations at the same or within one base pair, with similar effect, including one missense. We suspect mutations will become less ethnic specific as GNPTAB analysis is transitioned into the diagnostic setting, with the possible exception of the exon 2 duplication reported in 6 Japanese patients.[36]
The issue of clinical significance must be considered in the 10 cases with missense mutations. To address their significance we have run each missense alteration through the basic prediction software programs PolyPhen-Polymorphism Phenotyping (http://genetics.bwh.harvard.edu/pph/) and SIFT-Sorting Intolerant from Tolerant (http://blocks.fhcrc.org/sift/SIFT_BLink_submit.html). Four of the alterations (c.1402T>A→p.C468S, c.1514G>A→p.C505Y, c.2867A>G→p.H956R and c.3053A>G→p.D1018G) were classified as probably damaging and not tolerated which is consistent with a high likelihood of pathogenicity. Five alterations (c.10C>A→p.K4Q, c.44C>A→p.S15Y, c.569A>T→p.D190V, c.1001G>A→p.R334Q and c.1196C>T→p.S399F) were predicted to be possibly damaging and poorly tolerated by both PolyPhen and SIFT. Alterations p.K4Q, p.D190V and p.S399F have been previously reported in ML patients.[25,26] Only the c.1042A>C→p.I348L alteration was predicted to be a benign and tolerated change. Here it was detected in a non-core patient with ML II in combination with another clearly pathogenic frameshift alteration. Based on the fact that it does not block phenotypic expression of the latter (Table III), p.I348L has been accepted as being detrimental.
There is ample clinical, radiographic and pathology-based evidence that ML II and ML III are systemic disorders mainly of connective tissue.[32,37–39] In ML II the intensive egress of structurally abnormal “lysosomal” glycoproteins into the extracellular matrix (ECM) is correlated not only with early cessation of endochondral ossification and linear growth but also with apparently highly overactive intramembranous ossification leading to extreme diaphyseal widening in the metacarpals (Figure 3) probably caused by abnormal signaling effects triggered in the damaged connective tissue. The transient phenomenon of periosteal cloaking around the diaphyses of long bones in infants and the periarticular punctuate calcifications in some neonates and even in third trimester fetuses with ML II [38,40], may be the result of this ECM generated mechanism. Similar findings in the hand bones of patients with geleophysic dysplasia with ADAMTSL2 mutations have been ascribed to dysregulation of TGF-β signaling.[41] Dysregulated signaling of TGF-β is triggered by either abnormal fibrillin-1, the major constituent of extracellular microfibrils, or by mutant TGF-β receptors in Marfan syndrome and Loeys-Dietz syndrome respectively.[42] Apparently the progressive features in the ECM are non-specific and triggered by various abnormalities in connective tissue as they are observed also in patients with some of the glycosaminoglycan or oligosaccharide storage disorders.
Murine models of ML II and III do not have cytoplasmic inclusions in fibroblasts or mesenchymal cells and do not have prominent skeletal and connective tissue abnormalities.[43] Feline ML II is spontaneously occurring, demonstrates inclusion bodies in cultured fibroblasts, and shows skeletal and joint abnormalities making the feline a better but still not ideal model that needs further study.[44] Studies of animal models of MPS VI suggest that proinflammatory cytokines alter expression of several matrix metalloproteinases, enzymes involved in ECM degradation.[45] In ML III, a variable fraction of lysosomal glycoproteins still acquires the M6P recognition marker and is correctly routed to and functional within lysosomes. It is apparently sufficient to maintain some statural growth and to postpone clinical expression of the abnormal ECM signaling. Diaphyseal widening in the small hand bones, if present at all, is much milder in ML III. The abnormal signaling is however the likely cause of progressive stiffening of weak connective tissue, of the osteopenic osteochondrodysplasia in the hips and spine overt in the longer ML II survivors and in ML III patients.
In conclusion, the clinical work has confirmed the diagnosis of either ML II or ML III in 29/36 probands in the core group and in the large majority of the non-core patients. In only seven probands both clinical features and course of the disorder were found to straddle the boundaries of variability set for delineation of the two reference phenotypes. Their disorder was termed ML intermediate.
ML II, recognizable at birth, often causes intrauterine growth impairment and sometimes the prenatal “Pacman” dysplasia.[40] The main postnatal manifestations of ML II include gradual coarsening of neonatally evident craniofacial features, early cessation of statural growth and neuromotor development, dysostosis multiplex and major morbidity by hardening of soft connective tissue about the joints and in the cardiac valves. Fatal outcome occurs often before or in early childhood. ML III with clinical onset rarely detectable before three years of age, progresses slowly with gradual coarsening of the facial features, growth deficiency, dysostosis multiplex, restriction of movement in all joints before or from adolescence, painful gait impairment by prominent hip disease. Cognitive handicap remains minor or absent even in the adult, often wheelchair-bound patient with variable though significantly reduced life expectancy.
The phenotypic spectrum shown to be more dichotomous than continuously variable has been directly correlated with the GNPTAB mutant genotypes detected. ML II patients were found to be either homozygotes or compound heterozygous for NS or FSh mutant alleles expected to produce no or nearly no functional GlcNAc 1-phosphotransferase and, hence, complete failure to sort lysosomal enzymes devoid of the necessary M6P recognition marker to the lysosomal compartment. ML III represents the clinical outcome of GNPTAB homozygous or compound heterozygous mutant genotypes comprising at least one hypomorph (MS or Spl) allele and resulting in up to 10% of residual GlcNAc 1-phosphotransferase activity. The milder more chronic course underscores the important role of GlcNAc 1-phosphotransferase in physical growth and in quality and maintenance of connective tissue.
The intermediate phenotypes found in only few subjects in the patient cohort, confirm the overall direct genotype-phenotype correlation. More detailed clinical studies in progress indicate that clinical subgroups may be recognizable within the group of intermediate ML patients and provide information on the effect of MS mutations in specific locations of the GNPTAB gene.
The diagnosis of ML II or ML III is established in the metabolic laboratory for individuals with characteristic physical and radiographic features. Urine screens support the diagnosis. Urinary excretion of glycosaminoglycans is normal while oligosaccharide excretion may be excessive. Assay of multiple acid hydrolases in plasma demonstrates 5–20 fold increased activity compared to controls. Specific activities of lysosomal hydrolases in peripheral leukocytes are normal. GNPTAB mutation screening provides solid confirmation of the diagnosis of either ML II or ML III and informs valuably about the patient’s prognosis. The assay of GlcNAc 1-phosphotransferase activity is not routinely available and the finding of highly hyperactive acid hydrolases in plasma does not differentiate ML II from ML III, further contributing to the utility of molecular analysis in these diseases.[34,35] In addition, early knowledge of the GNPTAB genotype should favor gathering clinical information more prospectively and may influence the development of successful therapies.
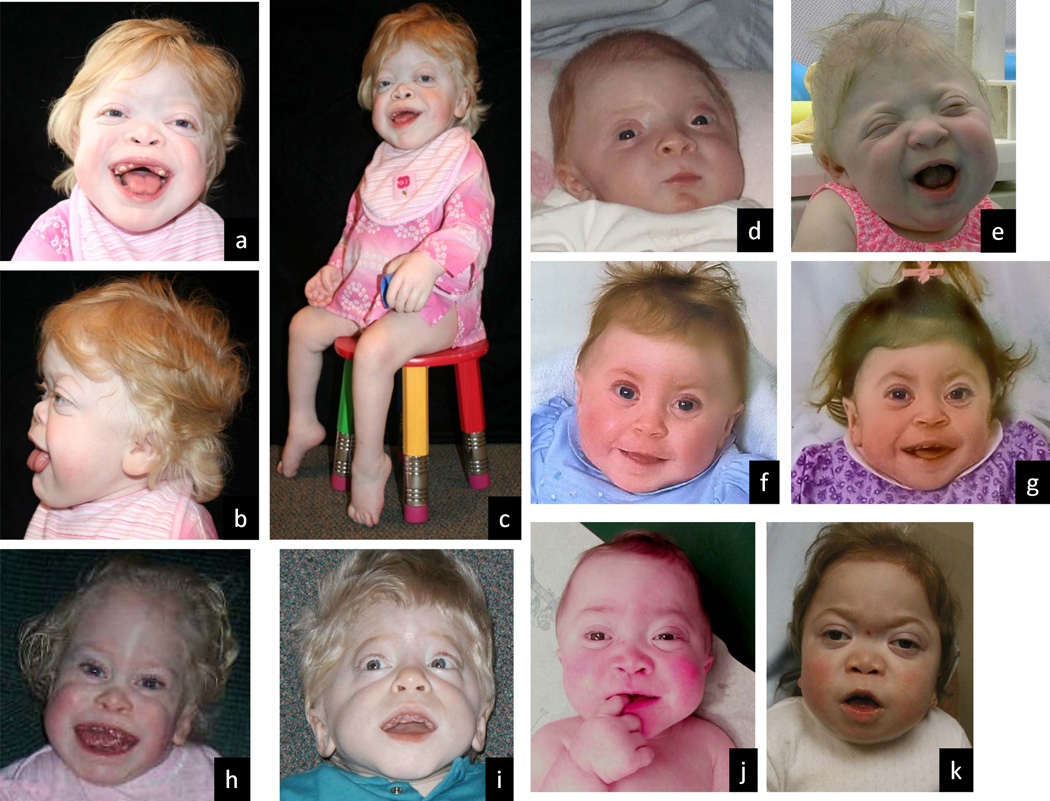
Figure 1.
Manifestations in ML II. a–c, same patient at 5 10/12 years; d and e, same patient at ages 3 months and 20 months; f and g, same patient at 1 and 2 years; h and i, different patients at 3 years; j and k, same patient at 13 months and 3 years. Coarsening of facial features is apparent from infancy and evolves in the first years of life. The common features include flat facies with depressed nasal bridge, prominent mouth, and gingival hypertrophy. The shallow orbits, thick skin and full cheeks contribute to the appearance of deep infraorbital creases and prominent cutaneous veins and capillaries on the face. Metopic prominence that gives the impression of craniosynostosis is depicted in b, h and i. Short, broad “claw” hands and contractures of knees and ankles are seen in c.
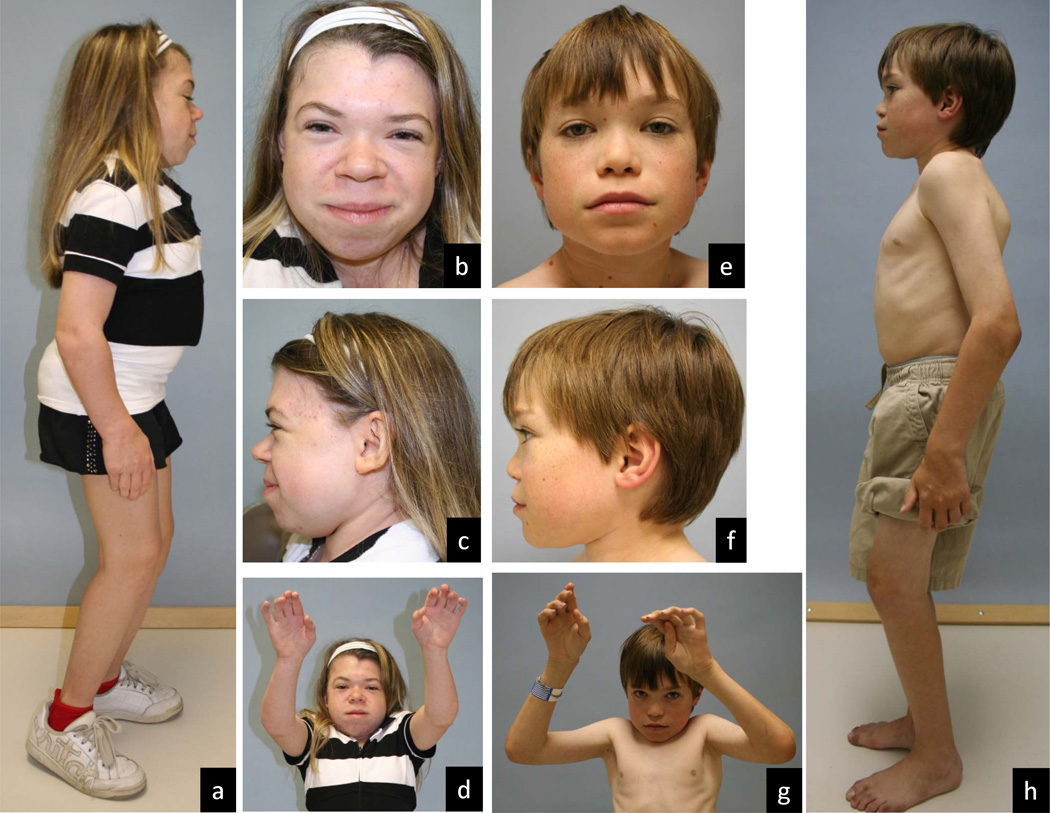
Figure 2.
Manifestations in ML III. a–d, same female patient at 17 years; e–h, same male patient at 9 years. Coarsening of facial features is mild and gradual in ML III and more apparent in profile, with full cheeks, low nasal bridge and prominent mouth. Stiffness in all large and small joints is slowly progressive but disabling hip pain is the major contributor to morbidity. Contractures of the hips and knees cause the squatting appearance of the standing posture. Limited range of motion in the shoulders may be the presenting symptom. Hand configuration is near normal although contractures are evident.
ACKNOWLEDGEMENTS
We thank the patients and their families who participated in this study; the physicians and counselors who referred patients and provided records; David Sillence, Paul Orchard, Virginia Proud, Omar Abdul-Rahman, Thaddeus Kurczynski, Andrea Atherton, Stephen Romansky; Jonathan Rollins for statistical analysis; and the clinical geneticists, counselors, and nurses who evaluated the families at the Greenwood Genetic Center. Support for patient travel and evaluation was provided, in part, by ISMRD, the International Advocate for Glycoprotein Storage Diseases; the National MPS Society; and the Genetics Endowment of South Carolina.
Footnotes
Competing Interests: None Declared
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Journal of Medical Genetics and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://jmg.bmj.com/iforalicence.pdf).
REFERENCES
- 1.Leroy JG, DeMars RI. Mutant enzymatic and cytological phenotypes in cultured human fibroblasts. Science. 1967;157:804–806. doi: 10.1126/science.157.3790.804. [DOI] [PubMed] [Google Scholar]
- 2.Leroy JG, DeMars RI, Opitz JM. I-cell disease. Birth Defects Orig Art Ser. 1969;V(4):174–185. [Google Scholar]
- 3.Cathey SS, Kudo M, Tiede S, Raas-Rothschild A, Braulke T, Beck M, Taylor HA, Canfield WM, Leroy JG, Neufeld EF, McKusick VA. Molecular order in mucolipidosis II and III nomenclature. Am J Med Genet. 2008;146A:512–513. doi: 10.1002/ajmg.a.32193. [DOI] [PubMed] [Google Scholar]
- 4.Maroteaux P, Lamy M. La pseudopolydystrophie de Hurler. Presse Méd. 1966;74:2889–2892. [PubMed] [Google Scholar]
- 5.Taylor HA, Thomas GH, Miller CS, Kelly TE, Siggers D. Mucolipidosis 3 (pseudo-Hurler polydystrophy): cytological and ultrastructural observations in cultured fibroblast cells. Clin Genet. 1973;4:388–397. doi: 10.1111/j.1399-0004.1973.tb01165.x. [DOI] [PubMed] [Google Scholar]
- 6.Raas-Rothschild A, Zeigler M, Cormier-Daire V, Bao M, Genin E, Salomon R, Brewer K, Mandal H, Toth S, Roe B, Munnich A, Canfield WM. Molecular basis of variant pseudo-Hurler polydystrophy (mucolipidosis IIIC) J Clin Invest. 105:563–564. doi: 10.1172/JCI5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raas-Rothschild A, Bargal R, Goldman O, Ben-Asher E, Groener JE, Toutain A, Stemmer E, Ben-Neriah Z, Flusser H, Beemer FA, Penttinen M, Olender T, Rein AJ, Bach G, Zeigler M. Genomic organisation of the UDP-N-acetylglucosamine-1-phosphotransferase gamma subunit (GNPTAG) and its mutations in mucolipidosis III. J Med Genet. 2004;41:e52. doi: 10.1136/jmg.2003.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spranger JW, Wiedemann HR. The genetic mucolipidoses. Diagnostic and differnential diagnosis. Humangenetik. 1970;9:113–139. doi: 10.1007/BF00278928. [DOI] [PubMed] [Google Scholar]
- 9.Hasilik A, Waheed A, von Figura K. Enzymatic phosphorylation of lysosomal enzymes in the presence of UDP-N-acetylglucosamine. Absence of the activity in I-cell fibroblasts. Biochem Biophys Res Commun. 1981;98:761–767. doi: 10.1016/0006-291x(81)91177-3. [DOI] [PubMed] [Google Scholar]
- 10.Reitman ML, Varki A, Kornfeld S. Fibroblasts from patients with I-cell disease and pseudo-Hurler polydystrophy are deficient in uridine 5’-diphosphate N-acetylglucosamine: glycoprotein N-acetylglucosaminylphosphotransferase activity. J Clin Invest. 1981;67:1574–1579. doi: 10.1172/JCI110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pohlmann R, Waheed A, Hasilik A, von Figura K. Synthesis of phosphorylated recognition marker in lysosomal enzymen is located in the cis part of the Golgi apparatus. J Biol Chem. 1982;257:5323–5325. [PubMed] [Google Scholar]
- 12.Wiesmann UN, Lightbody J, Vasella F, Herschkowitz N. Multiple lysosomal enzyme efficiency due to enzyme leakage? New Engl J Med. 1971;284:109–110. doi: 10.1056/NEJM197101142840221. [DOI] [PubMed] [Google Scholar]
- 13.Leroy JG, Ho MW, MacBrinn MC, Zielke K, Jacob J, O’Brien JS. I-cell disease: biochemical studies. Pediatr Res. 1972;6:752–757. doi: 10.1203/00006450-197210000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Wiesmann UN, Vasella F, Herschkowitz N. “I-cell disease”: Leakage of lysosomal enzymes into extracellular fluids. New Engl J Med. 1971;285:1090–1091. doi: 10.1056/NEJM197111042851922. [DOI] [PubMed] [Google Scholar]
- 15.Kornfeld S, Sly WS. I-cell disease and pseudo-Hurler polydystrophy: disorders of lysosomal enzyme phosphorylation and localization. In: Scriver CR, Beaud Al, Sly WS, Valle D, et al., editors. The Metabolic and Molecular Bases of Inherited Disease. 8 ed. vol 3. New York, NY: McGraw-Hill; 2001. pp. 3469–3482. [Google Scholar]
- 16.Leroy JG. Oligosaccharidoses, disorders allied to the oligosaccharides. In: Rimoin DL, Connor JM, Pyeritz RE, Korf BR, editors. Emery and Rimoin’s Principles and Practice of Medical Genetics. 5 ed. Philadelpha, PA: Churchill Livingstone Elsevier; 2007. pp. 2413–2448. [Google Scholar]
- 17.Bao M, Booth JL, Elmendorf BJ, Canfield WM. Bovine UDP-N-acetylglucosamine: lysosomal enzymze N-acetylglucosamine-1-phosphotransferase I. Purification and subunit structure. J Biol Chem. 1996;271:31437–31445. doi: 10.1074/jbc.271.49.31437. [DOI] [PubMed] [Google Scholar]
- 18.Bao M, Elmendorf BJ, Booth JL, Drake RR, Canfield WM. Bovine UDP-N-acetylglucosamine: lysosomal enzyme N-acetylglucosamine-1-phosphotransferase II. Enzymatic characterization and identification of the catalytic subunit. J Biol Chem. 1996;271:31446–31451. doi: 10.1074/jbc.271.49.31446. [DOI] [PubMed] [Google Scholar]
- 19.Canfield WLM, Bao M, Pan J, D’Souza A, Brewer K, Pan H, Roe B, Raas-Rothschild A. Mucolipidosis II and mucolipidosis III are caused by mutations in the GlcNAc-phosphotransferase α/β gene on chromosome 12p. Am J Hum Genet Suppl. 1998;63:A15. [Google Scholar]
- 20.Kudo M, Bao M, D’Sousa A, Ying F, Pan H, Roe BA, Canfield WM. The α- and β-subunits of the human UDP-N-acetylglucosamine: lysosomal enzyme N-acetylglucosamine-1-phosphotransferase are encoded by a single cDNA. J Biol Chem. 2005;280:36141–36149. doi: 10.1074/jbc.M509008200. [DOI] [PubMed] [Google Scholar]
- 21.Tiede S, Storch S, Lübke T, Henrissat B, Bargal R, Raas-Rothschild A, Braulke T. Mucolipidosis II is caused by mutations in GNPTA encoding the α/β GlcNAc-1-phosphotransferase. Nat Med. 2005;11:1109–1112. doi: 10.1038/nm1305. [DOI] [PubMed] [Google Scholar]
- 22.Tiede S, Muscol N, Reutter G, Cantz M, Ullrich K, Braulke T. Missense mutations in N-acetylglucosamine-1-phosphotransferase α/β subunit gene in a patient with mucolipidosis III and a mild clinical phenotype. Am J Med Genet. 2005;137A:235–240. doi: 10.1002/ajmg.a.30868. [DOI] [PubMed] [Google Scholar]
- 23.Paik KH, Song SM, Ki CS, Yu H-W, Kim JS, Min KH, Chang SH, Yoo EJ, Lee IJ, Kwan EK, Han SJ, Jin D-K. Identification of mutations in the GNPTA (MGC4170) gene coding for GlcNAc-phosphotransferase α/β subunits in Korean patients with mucolipidosis type II and type IIIA. Hum Mutat. 2005;26:308–314. doi: 10.1002/humu.20205. [DOI] [PubMed] [Google Scholar]
- 24.Steet RA, Hullin R, Kudo M, Martinelli M, Bosshard NU, Schaffner T, Kornfeld S, Steinmann B. A splicing methadon in the α/β GlcNAc-1-phosphotransferase gene results in an adult onset form of Mucolipidosis III associated with sensory neuropathy and cardiomyopathy. Am J Med Genet A. 2005;132:369–375. doi: 10.1002/ajmg.a.30498. [DOI] [PubMed] [Google Scholar]
- 25.Bargal R, Zeigler M, Abu-Libdeh B, Zuri V, Mandel H, Ben neriah Z, Stewart F, Elcioglu N, Hindi T, Le Merrer M, Bach G, Raas-Rothschild A. When mucolipidosis III meets mucolipidosis II: GNPTA gene mutations in 24 patients. Mol Genet Metab. 2006;88:359–363. doi: 10.1016/j.ymgme.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Kudo M, Brem MS, Canfield WM. Mucolipidosis II (I-cell disease) and mucolipidosis IIIA (classical pseudo-Hurler polydystrophy) are caused by mutations in the GlcNac-phosphotransferase a/b-subunits precursor gene. Am J Hum Genet. 2006;78:451–463. doi: 10.1086/500849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam C-W, Yan MS-C, Lau K-C, Tong S-F. DNA-based diagnosis of mucolipidosis type IIIA and mucopolysaccharidosis type VI in a chinese family: a chance of 1 in 7.6 trillion. Clin Chim Acta. 2007;376:250–252. doi: 10.1016/j.cca.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Tappino B, Regis S, Corsolini F, Filocamo M. An Alu insertion in compound heterozygosity with a microduplication in GNPTAB gene underlies Mucolipidosis II. Mol Genet Metab. 2008;93:129–133. doi: 10.1016/j.ymgme.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Tiede S, Cantz M, Raas-Rothschild A, Muschol N, Bürger F, Ulrich K, Braulke T. A novel mutation in UDP-N-acetylglucosamine-1-phosphotransferase gamma subunit (GNPTAG) in two siblings with mucolipidosis Type III alters a used glycosylation site. Hum Mutat. 2004;24:535. doi: 10.1002/humu.9293. [DOI] [PubMed] [Google Scholar]
- 30.Thomas G, Taylor H, Reynolds L, Miller C. Mucolipidosis 3 (Pseudo-Hurler polydystrophy): multiple lysosomal enzyme abnormalities in serum and cultured fibroblast cells. Pediatr Res. 1973;7:751–756. doi: 10.1203/00006450-197309000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Baum H, Dodgson K, Spencer B. The assay of arylsulphatases A and B in human urine. Clinica Chimica Acta. 1959;4:453–455. doi: 10.1016/0009-8981(59)90119-6. [DOI] [PubMed] [Google Scholar]
- 32.Spranger JW, Brill PW, Poznanski A. Bone Dysplasias: Atlas of Genetic Disorders of Skeletal Development. 2 ed. New York, NY: Oxford Univ Press; 2002. pp. 295–299. [Google Scholar]
- 33.Plante M, Claveau S, Lepage P, Lavoie E-M, Brunet S, Roquis D, Morin C, Vezina H, Laprise C. Mucolipidosis II: a single causal mutation in the N-acetylglucosamine-1-phosphotransferase gene (GNPTAB) in a French Canadian founder population. Clin Genet. 2008;73:236–244. doi: 10.1111/j.1399-0004.2007.00954.x. [DOI] [PubMed] [Google Scholar]
- 34.Leroy JG, Cathey S, Friez M. GeneReviews at Gene Tests: Medical Genetics information resource [database online] Seattle, WA: University of Washington Seattle; 1997–2008. Mucolipidosis II. http://www.genetests.org. [Google Scholar]
- 35.Leroy JG, Cathey S, Friez M. GeneReviews at Gene Tests: Medical Genetics information resource [database online] Seattle, WA: Univ of Washington Seattle; 1997–2008. Mucolipidosis III Alpha/Beta. http://www.genetests.org. [Google Scholar]
- 36.Otomo T, Muramatsu T, Yorifuji T, Okuyama T, Nakabayashi H, Fukao T, Ohura T, Yoshino M, Tanaka A, Okamoto N, Inui K, Ozono K, Sakai N. Mucoliposis II and III alpha/beta: mutation ananlysis of 40 Japanese patients showed genotype-phenotype correlation. J Hum Genet. 2009;54:145–151. doi: 10.1038/jhg.2009.3. [DOI] [PubMed] [Google Scholar]
- 37.Martin JJ, Leroy JG, Farriaux J-P, Fontaine G, Desnick RJ, Cabello A. I-cell disease (Mucolipidosis II). A report on its pathology. Acta Neuropathol (Berlin) 1975;33:285–305. doi: 10.1007/BF00686161. [DOI] [PubMed] [Google Scholar]
- 38.Martin JJ, Leroy JG, Van Eygen M, Ceuterick C. I-cell disease. A further report on its pathology. Acta Neuropathol (Berlin) 1984;64:234–242. doi: 10.1007/BF00688114. [DOI] [PubMed] [Google Scholar]
- 39.Pazzaglia UE, Beluffi G, Bianchi E, Castello A, Coci A, Marchi A. Study of the bone pathology in early mucolipidosis II (I-cell disease) Eur J Pediatr. 1989;148:553–557. doi: 10.1007/BF00441557. [DOI] [PubMed] [Google Scholar]
- 40.Saul RA, Proud V, Taylor HA, Leroy JG, Spranger J. Prenatal mucolipidosis II (I-cell disease) can present as Pacman dysplasia. Am J Med Genet. 2005;135A:328–323. doi: 10.1002/ajmg.a.30716. [DOI] [PubMed] [Google Scholar]
- 41.Le Goff C, Morice-Picard F, Dagoneau N, Wang LW, Perrot C, Crow YJ, Bauer F, Flori E, Prost-Squarcioni C, Krakow D, Ge G, Greenspan DS, Bonnet D, Le Merrer M, Munnich A, Apte SS, Cormier-Daire V. ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-β bioavailability regulation. Nat Genet. 2008;40:1119–1123. doi: 10.1038/ng.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramirez F, Dietz HC. Marfan syndrome: from molecular pathogenesis to clinical treatment. Curr Opin Genet Devel. 2007;17:252–258. doi: 10.1016/j.gde.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Vogel P, Payne BJ, Read R, Lee WS, Gelfman CM, Kornfeld S. Comparative pathology of murine mucolipidosis types II and IIIc. Vet Pathol. 2009;46:313–324. doi: 10.1354/vp.46-2-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazrier H, Van Hoeven M, Wang P, Knox VW, Aguirre GD, Holt E, Viemelt SP, Sleeper MM, Hubler M, Haskins ME, Giger U. Inheritance, biochemical abnormalities, and clinical features of feline mucolipidosis II: The first animal model of human I-cell disease. J Hered. 2003;94:363–373. doi: 10.1093/jhered/esg080. [DOI] [PubMed] [Google Scholar]
- 45.Simonaro CM, D’Angelo M, He X, Eliyahu E, Shtraizent N, Haskins M, Schuchman EH. Mechanism of glycosaminoglycan-mediated bone and joint disease. Am J Pathol. 2008;172:112–122. doi: 10.2353/ajpath.2008.070564. [DOI] [PMC free article] [PubMed] [Google Scholar]