Abstract
The mechanisms causing the rise in adrenal androgen production during the course of adrenarche remain to be defined. However, the increase in steroid release is clearly associated with a series of intra-adrenal changes in the expression of steroidogenic enzymes needed for dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) production, as well as an expansion of the adrenal zona reticularis (ZR). We and others have defined the adrenal expression pattern of key steroidogenic enzymes during adrenarche. As adrenarche proceeds, the expanding ZR expresses greater levels of cytochrome b5 (CYB5) and steroid sulfotransferase (SULT2A1) than the adjacent fasciculata. In contrast, the growing ZR is deficient in 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2). The resulting profile of steroidogenic enzymes lends itself to the production of adrenal androgens and appears to track the progression of adrenarche. This article reviews the intra-adrenal changes of the adrenal cortex associated with adrenarche.
Keywords: Adrenocortical changes, Adrenarche, Steroidogenesis
I. Overview of adrenarche
The adrenal produces large amounts of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) during fetal development, which fall rapidly after birth and remain low for the first years of life. Adrenarche can be defined as the increased production of DHEA and DHEAS that occurs between age 6 and 8 y [1–8]. The gradual increase in adrenal androgens causes the appearance of axillary and pubic hair and a transient acceleration of linear growth and bone maturation [9–15]. The increased adrenal androgen secretion in this period, however, is not associated with increased levels of ACTH or gonadotrophins, and does not require functional gonads [11, 16, 17]. Recently, candidate hormones related to body mass, such as leptin, have been suggested to play a role in adrenal growth and adrenarche [18]. While the existing evidence suggests that these hormones may modify the onset and rate of progression through adrenarche, no single factor has been proven to be the proximal signal solely responsible for the onset of adrenarche. It is clear, however, that the onset and character of adrenarche is associated with distinct changes in adrenocortical function and structure [19–25].
II. Adrenarche occurs only in humans and select nonhuman primates
Adrenarche has only been identified in the Hominoidea superfamily of Old World Monkeys, which includes humans and chimpanzees [26–30]. Therefore, this phenomenon is considered to be a relatively recent evolutionary development. Pubertal increases in DHEA are also demonstrated in rabbits and dogs, but are of a magnitude lower than in humans, and this DHEA is predominantly derived from the gonads [31]. Conversely, in the Rhesus monkey, DHEAS levels are maximal at birth and decline steadily thereafter [27]. It has been postulated, but not proven, that elevated adrenal androgen production is responsible for the earlier sexual maturation in the Rhesus monkey when compared to chimpanzees or humans [24]. On the other hand, in chimpanzees, DHEA and DHEAS levels appear to rise continuously and without delay from birth, and level off 10 to 16 years of age [26–30]. However, no age-related decline of DHEA or DHEAS has been reported in chimpanzees compared to humans [28]. In addition, there are currently no histological, immunohistochemical, or biochemical data correlating the rise in adrenal androgen synthesis in chimpanzees with maturation of the adrenal cortex that accompanies adrenarche in humans [28].
III. Morphological changes of the adrenal cortex during adrenarche
The human adrenal cortex is composed of three distinct zones: the zona glomerulosa (ZG), the zona fasciculata (ZF), and the zona reticularis (ZR) [32, 33]. These three zones all have functionally distinct roles in adrenocortical steroid hormone production [33–35]. The adult-type zonal expression pattern does not fully develop until after birth. The human fetal adrenal cortex is composed of two morphologically distinct zones: the fetal zone and neocortex, which are characteristics of human adrenal development. The fetal zone occupies a large portion of the inner cortex, while the neocortex occupies the remainder of the adrenal cortex and surrounds the fetal zone as a narrow band [36, 37]. After birth, the prominent fetal zone of the adrenal gland undergoes involution [38]. The neocortex develops into the adult adrenal, with a distinct ZG and ZF, with a paucity of cells resembling the ZR [36].
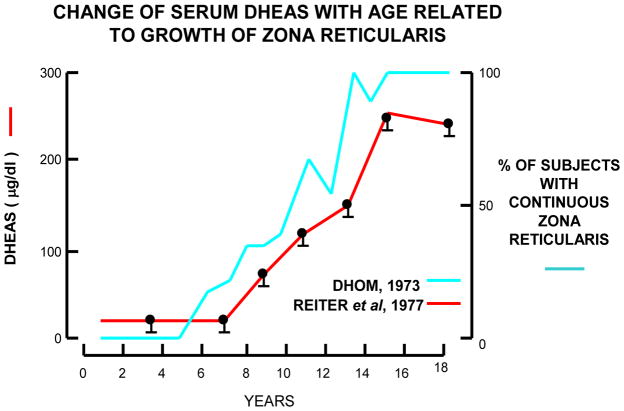
While there is strong evidence that it is the ZR that produces DHEA and DHEAS, we know very little about the factors that cause the zone to start its expansion during the late infant years [39, 40]. As opposed to adrenal androgens, the production of mineralo- and glucocorticoids is primarily independent of sexual maturation during the infant or pubertal years [41, 42]. On the other hand, during adrenarche, the increase in adrenal androgen production is closely associated with alterations in the ZR [19, 22, 43, 44]. Dhom also characterized the emergence of the ZR in children before and during adrenarche, reporting a highly significant correlation between adrenal weight and age as well as between adrenal weight and body surface [22]. The adrenal cortex of the infant consists of a clearly demarked, continuous, and lipoid-rich ZG and a spongiocytic ZF that extends down to the medullary capsule; with no apparent ZR [22]. The focal islands of the adrenal reticularis morphologically appear in children around age 3 y [18]. Focal development of the ZR further increases at age 5 y, and continuous ZR is first detectable in some individuals at age 6 y and in almost all by age 13 y [22]. The thickness of the ZR increases progressively until age 13 y. With a fully developed ZR as is seen in young adults, the ratio between the thickness of the ZR to that of the ZG and the ZF is about 1:1 [22]. On the other hand, the ZG, which in adrenals of infants is always present as a continuous zone, become discontinuous during the pre-pubertal development [22]. However, in these studies, the ZR was identified only by morophological observations, and no immunohistochemical analysis related to the ZR was done. Figure 1 shows the ZR at age 3 y is focally clear although this appearance is not continuous in all the areas of this adrenal cortex. The immunohistochemical analysis for steroidogenic enzyme expression is important to determine the exact areas of the ZR and ZF [43]. By the age of 12 y, the thickness of the ZR is similar to that of the ZF. Microscopically, nuclei and cytoplasm are larger in the ZR cells than those of the ZF cells [22]. The thickness of the ZR is also reported to correspond to the increased production of DHEA and DHEAS (Figure 2) [22, 45]. Because little is know about the factors that regulate ZR steroid production, we and others have focused on defining the phenotype that constitutes a ZR cell. Adrenal cell proliferation and continuous remodeling occur mostly in the outer part of the adrenal cortex, in the ZG and ZF, and cell death mostly occurs in the ZR [46, 47]. ZR cells are likely to originate mostly by re-differentiation of ZF cells rather than by cell division within the zone [39]. Therefore, it is likely that ZR cells have a unique phenotype compared to ZG and ZF cells. With use of microarray analysis, Wang et al. showed the ZF and ZR cells differ in their patterns of gene expression [48]. For example, CD74 was more highly expressed in ZR cells and 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) was more highly expressed in ZF cells [48]. The role of differential HSD3B2 expression will be discussed below, but the role of CD74 expression is not known. CD74 is known to be a major histocompatability complex (MHC) class II invariant chain [48]. It has been postulated that maturation of the adult adrenal gland during adrenarche and the capacity for androgen production in the ZR cells may be related to the expression of MHC class II expression on these cells via potential immune-adrenal interactions [48–52]. However, it remains unclear and speculative as to how these factors contribute to the regulation of the ZR and adrenarche. On the other hand, several studies have shown that steroidogenic enzyme expression in the adrenal cortex is quite relevant to adrenarche [43, 53–64]. Therefore, we have focused this review on the changes seen in adrenal expression patterns of steroidogenic enzymes and their associations with adrenarche.
Figure 1.
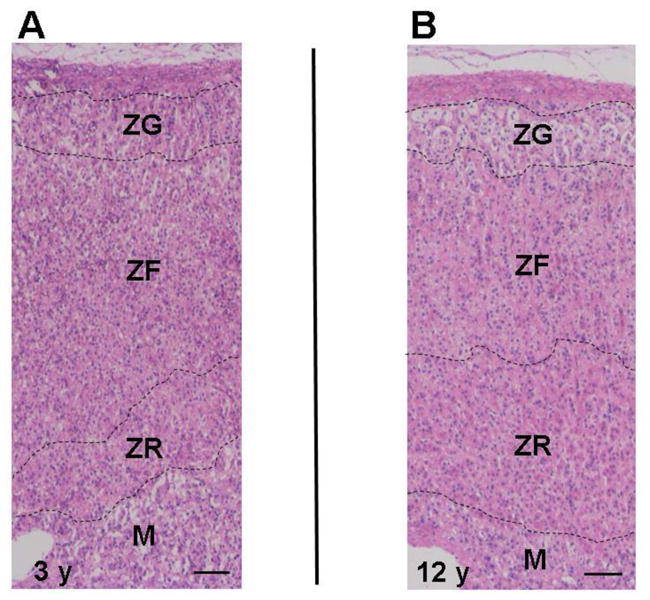
Representative hematoxylin and eosin stained adrenal, showing the zona glomerulosa (ZG), the zona fasciculata (ZF), the zona reticularis (ZR), and medulla (M) at age 3 y (A) and 12 y (B).
Figure 2.
Correlation between the development of the adrenal and the increase in plasma DHEAS level. This figure is based on the original publications by Dhom and Reiter [6, 12].
IV. Alterations in Adrenal Steroidogenic Enzymes at Adrenarche
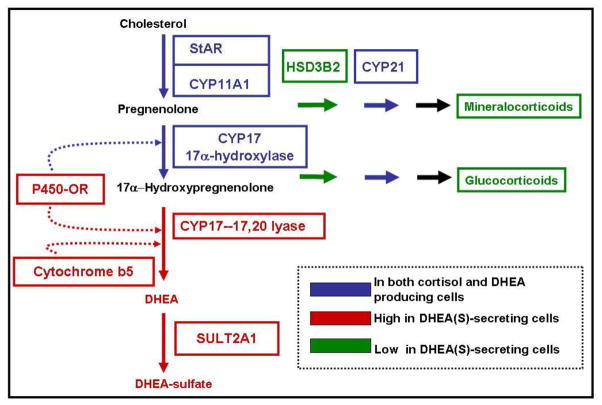
Clarifying the variations in steroidogenesis in the adrenal glands requires an understanding of the zone specific production of steroids (Figure 3). Although some enzymes and cofactor proteins are common to all adrenal zones, the specific classes of steroid produced within the zones are determined predominantly by zone-specific expression of characteristic steroidogenic enzymes. Although only two steroid-metabolizing enzymes are required for the synthesis of DHEA from cholesterol, changes in other steroid-metabolizing enzymes and cofactors underlie the changes in adrenal androgen production biosynthesis during adrenarche. Figure 3 illustrates the key differences in expression of steroid-metabolizing enzymes that facilitates the production of adrenal androgens.
Figure 3.
Steroid pathways for the production of adrenocortical steroids. Production relies on the coordinated expression of steroidogenic acute regulatory protein (StAR), cytochrome P450scc (CYP11A1), cytochrome P450c17; 17α-hydroxylase/17,20-lyase (CYP17), and DHEA sulfotransferase (SULT2A1). Also positively impacting DHEAS biosynthesis is cytochrome b5 (CYB5), which can enhance the 17,20-lyase activity of CYP17. Negatively impacting the production of DHEAS is 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) and 21-hydroxylase cytochrome P450 (CYP21). This figure is modified from a previous report [5].
Cytochrome P450scc; side-chain cleavage enzyme (CYP11A1)
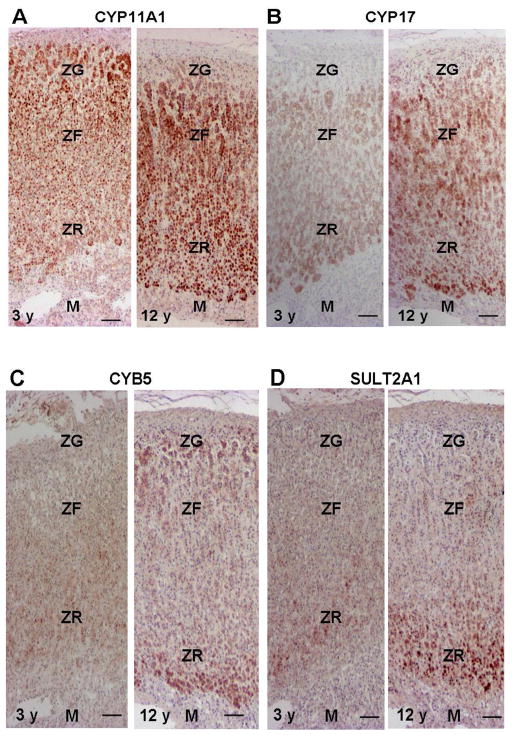
The mitochondrial enzyme, CYP11A1, performs the first committed step common to all cells that synthesize steroid hormones: the conversion of cholesterol to pregnenolone (Figure 3). The acute regulation of this reaction is mediated via cholesterol access, which is controlled by the steroidogenic acute regulatory protein (StAR). The chronic maintenance of this enzyme is mediated by ACTH [53]. The expression of CYP11A1 is expressed in all zones of the adrenal and there does not appear to be changes in expression within the ZR during the procession of adrenarche (Figure 4) [43]. However, as CYP11A1 with StAR are the quantitative regulators of steroidogenesis, alterations in cholesterol flux into the mitochondria or the activity of CYP11A1 may contribute to the genesis of premature and/or exaggerated adrenarche.
Figure 4. Immunohistochemistry for CYP11A1 (A), CYP17 (B), CYB5 (C), and SULT2A1 (D) in the adrenal at age 3 and 12 y.
(A) Immunoreactivity of CYP11A1 is detected in the zona glomerulosa (ZG), the zona fasciculata (ZF), and the zona reticularis (ZR), but not in the medulla (M) of both adrenals.
(B) Immunoreactivity of CYP17 is weakly detected in the ZF and ZG, whereas it is not detected in the ZG or M in the adrenal at age 3 y. Immunoreactivity of CYP17 is strongly detected in the ZF and ZR in the adrenal at age 12 y.
(C) Immunoreactivity of CYB5 is weakly detected in the cytoplasm of pre-adrenarche adrenocortical cells in the ZG, the ZF, and the ZR at age 3 y. CYB 5 immunoreactivity is strongly detected in the ZR at 12 y.
(D) Immunoreactivity of SULT2A1 is weakly detected in the ZF and ZR but not in the ZG or M in the adrenal at age 3 y. SULT2A1 immunoreactivity is strongly detected in the ZR and weakly positive in the ZF, while it is not detected in the ZG or M. Bar = 100 μm.
Cytochrome P450c17; 17α-hydroxylase/17,20-lyase (CYP17)
CYP17 catalyzes the 17α-hydroxylation of both pregnenolone and progesterone and subsequently the 17,20-lyase reaction on their 17-hydroxy derivatives. In human adrenal glands, the CYP17 enzyme is found in both the ZF and ZR of the cortex [43, 54]. The 17α-hydroxylase activity is necessary for production of the glucocorticoid, cortisol, in human adrenal ZF, and both 17α-hydroxylase and 17,20-lyase activities are needed for adrenal androgen production in human adrenal ZR. Therefore, CYP17 is recognized as one of the principal qualitative regulators of steroidogenesis [55]. We previously reported in the adrenal glands from pre-adrenarcheal children that the expression level of CYP17 protein was weak in both the ZF and ZR [43]. The immuno-intensity of CYP17 increased both in the ZF and ZR after age 5 y and reached a plateau level at age 13 y (Figure 4) [43]. Therefore, the regulation of CYP17 expression does not appear to be a cause of adrenarche. However, adequate amounts of CYP17 are certainly required for maximal DHEA production so that CYP17 is an essential component of the adrenarche process.
Cytochrome b5 (CYB5)
An important feature of adrenarche is the increased conversion of 17α-hydroxypregnenolone to DHEA that results from an increase in 17,20-lyase activity of CYP17. The relative activity of 17α-hydroxylase vs. 17,20-lyase activity appears to be regulated by multiple post-translational events, all of which could be influenced at adrenarche. CYB5 is regarded as an important regulator of CYP17 function and particularly its 17,20 lyase activity. CYB5 protein is most evident in the ZR in the human adrenal gland [56]. It has been suggested that human CYB5 acts as an allosteric effector that interacts primarily with the CYP17/oxidoreductase complex to stimulate 17,20-lyase activity [57]. Thus, the ZR-specific expression of CYB5 appears to be a key factor in promoting adrenal androgen production. We have reported that CYB5 immunoreactivity is weakly detected in the cytoplasm of pre-adrenarche adrenocortical cells in the ZG, the ZF, and the ZR (Figure 4) [43]. The immunoreactivity of CYB5 becomes more marked in the ZR after age 5 y and reaches a plateau at 13 y (Figure 4) [43]. However, immunoreactivity of CYB5 in the ZG and ZF is low in all the cases examined, and no significant age-related changes are detected [43]. Therefore, these findings suggest that the electron transfer system plays an important role in the increment of 17,20-lyase activity of CYP17 in the ZR in the developing adrenal cortex.
DHEA sulfotransferase (SULT2A1)
In addition to CYP11A1 and CYP17, steroid sulfotransferase (SULT2A1) carries out the terminal reaction in the synthesis of DHEAS (Figure 3). Like adrenal androgen production, the expression of SULT2A1 within the adrenal is most notable in primates [28]. SULT2A1 has a broad substrate specificity, which includes metabolism of pregnenolone, 17α-hydroxypregnenolone, and DHEA to their respective sulfated products [58–60]. SULT2A1 is obligatory for the synthesis of the sulfated form of DHEA, which is, by mass, the principal steroid produced by the human adult adrenal and the most abundant circulating steroid in the adult human. SULT2A1 is predominantly expressed in the cytoplasm of adrenocortical cells in the ZR, and its substrates include pregnenolone, 17α hydroxypregnenolone, and DHEA. To determine whether the SULT2A1 enzyme is specifically associated with cells that secrete DHEAS, immunohistochemistry has been performed to localize SULT2A1 in adrenals throughout the postnatal period from infancy to old age [43]. Expression of SULT2A1 remains low through early childhood until the onset of adrenarche; however, some cells express SULT2A1 even in the pre-adrenarche years (Figure 4). Recent detailed studies of steroid levels during the course of adrenarche support the concept that, even in the pre-adrenarche years, there is a gradual increase in production of the adrenal androgens [61]. As the adrenal reticularis begins to grow, adrenal expression of SULT2A1 increases (Figure 4). ZR expression of SULT2A1 continues into adulthood and is maintained into the later years of life. These data show that the increase in DHEAS production occurring at adrenarche is associated with accelerated expression of SULT2A1 in the adrenal reticularis, which would increase the conversion of nascent DHEA into DHEAS. There is very little known about the mechanisms regulating the human SULT2A1 expression during adrenarche. However, we have recently confirmed several of the key transcription factors that regulate the transcription of the SULT2A1 gene [65, 66]. Therefore, in the future, it will be important to define the potential role of these transcription factors in the regulation of the adrenal reticularis and the process of adrenarche.
3β-hydroxysteroid dehydrogenase type 2 (HSD3B2)
HSD3B2 catalyzes the conversion from pregnenolone, 17α-hydroxypregnenolone, and DHEA to progesterone, 17α-hydroxyprogesterone, and androstendione, respectively (Figure 3). HSD3B2 normally acts to decrease DHEAS production through competition with CYP17, and decreased HSD3B2 activity may play an important role in adrenarche [24, 42, 62]. Gell et al. demonstrated that relative immunoreactivity of HSD3B2 was significantly weaker in the ZR than in the ZF in children age 8 y and older [24]. In addition, Dardis et al. reported a decrease in mRNA levels of HSD3B2 in adrenals from children age 8 y and older compared to children less than 8 y [62]. Using immunoreactivity of HSD3B2, we have demonstrated a marked decrease in the ZR after age 8 y with little alteration in the adjacent ZG and the ZF (Figure 5) [24, 43]. However, as seen in Figure 5, some cells of the small 3 y adrenal ZR have lower levels of HSD3B2 than in the adjacent ZR. This supports the concept put forth by Palmert and colleagues that even in the early years the initial stages of adrenal androgen production may have already been initiated [63].
Figure 5. Immunohistochemistry for HSD3B2 (A) and CYP21 (B) in the adrenal at age 3 and 12 y.
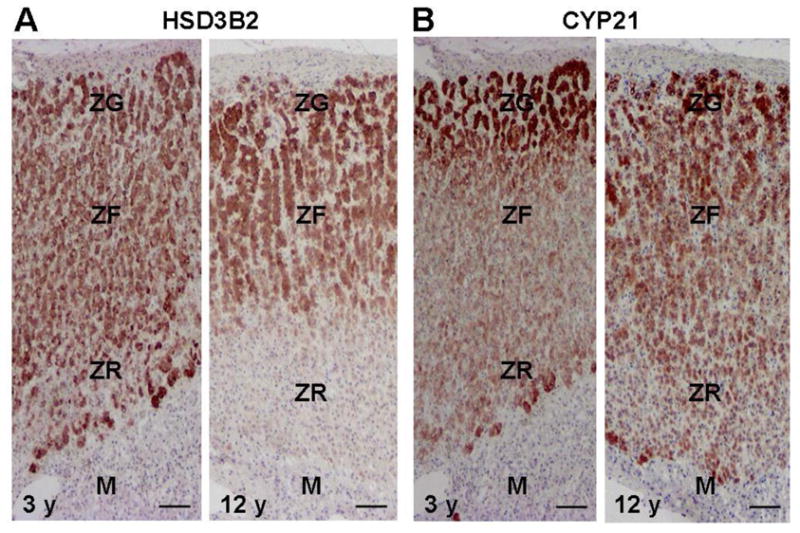
(A) Immunoreactivity of HSD3B2 is detectable in the zona glomerulosa (ZG), the zona fasciculata (ZF), and zona reticularis (ZR), whereas it is not detected in the medulla (M) in the adrenal at age 3 y. In the 12 h adrenal, immunoreactivity of HSD3B2 is marked in the ZG and ZF, but is almost negative in ZR and M.
(B) Immunoreactivity of CYP21 is detectable in the ZG, ZF, and ZR but not in the M of the adrenals at ages 3 y and 12 y. Bar = 100 μm.
21-hydroxylase cytochrome P450 (CYP21)
CYP21 produces deoxycorticosterone (DOC) and deoxycortisol in the adrenal ZG and ZF, respectively. Although CYP21 is not directly involved in the production of DHEA, its presence metabolizes and diverts steroid products of HSD3B2 towards the formation of mineralocorticoids and glucocorticoids (Figure 3). Deficient 21-hydroxylase activity leads to increased C19 steroid production and the clinical manifestations of androgen excess [64]. Therefore, alterations in zonal CYP21 expression may influence the increased adrenal DHEA and DHEAS production seen at adrenarche. However, we previously demonstrated that the ZR continues to express CYP21 as children undergo adrenarche, and there does not appear to be a significant difference in CYP21 expression among the ZG, ZF, and ZR (Figure 5) [24]. This suggests that adrenarche does not rely on alterations in CYP21 expression within the ZR.
17β-hydroxysteroid dehydrogenase type 5 (HSD17B5)
As described above, large amounts of DHEA and DHEAS are produced in the adrenal ZR but only small amounts of the more active androgen, testosterone. Testosterone is mainly produced in the testis through the action of 17β-hydroxysteroid dehydrogenase type 3 (HSD17B3) which efficiently converts androstenedione to testosterone [67]. On the other hand, HSD17B5 (also called AKR1C3) also converts androstenedione to testosterone [67]. HSD17B5 is more widely expressed than HSD17B3 in human tissues, including the ovaries and the adrenals [68, 69]. Using quantitative PCR and immunohistochemistry, we demonstrated that HSD17B5 is expressed at higher levels in the ZR than the ZF [70]. However, it awaits further examination to demonstrate a role for reticularis HSD17B5 in the production of testosterone in the human adrenals. The potential that HSD17B5 expression in the ZR is increased at adrenarche also requires examination.
IV. Summary and future direction
Adrenarche is an enigmatic phenomenon that occurs only in human beings and some Old World monkeys. The proximal signal for adrenarche remains unknown, although some biochemical clues to the process of adrenarche are being elucidated. It remains clear that adrenarche is associated with intra-adrenal changes in morphology and in the expression of steroidogenic enzymes and members of the electron transport system. These changes create a steroidogenic phenotype within the ZR cells that facilitates the production of adrenal androgens at adrenarche. These alterations are maintained in the adult, further suggesting that differences in enzymatic activity that occur at adrenarche are the key to adrenal DHEA production throughout life.
References
- 1.de Peretti E, Forest MG. Unconjugated dehydroepiandrosterone plasma levels in normal subjects from birth to adolescence in human: the use of a sensitive radioimmunoassay. J Clin Endocrinol Metab. 1976;43:982–91. doi: 10.1210/jcem-43-5-982. [DOI] [PubMed] [Google Scholar]
- 2.Parker LN. Adrenarche. Endocrinol Metab Clin North Am. 1991;20:71–83. [PubMed] [Google Scholar]
- 3.Hopper BR, Yen SS. Circulating concentrations of dehydroepiandrosterone and dehydroepiandrosterone sulfate during puberty. J Clin Endocrinol Metab. 1975;40:458–61. doi: 10.1210/jcem-40-3-458. [DOI] [PubMed] [Google Scholar]
- 4.Cutler GB, Jr, Loriaux DL. Andrenarche and its relationship to the onset of puberty. Fed Proc. 1980;39:2384–90. [PubMed] [Google Scholar]
- 5.Ibáñez L, Dimartino-Nardi J, Potau N, Saenger P. Premature adrenarche--normal variant or forerunner of adult disease? Endocr Rev. 2000;21:671–96. doi: 10.1210/edrv.21.6.0416. [DOI] [PubMed] [Google Scholar]
- 6.Papadimas J. Adrenarche. Ann N Y Acad Sci. 1997;816:57–9. doi: 10.1111/j.1749-6632.1997.tb52129.x. [DOI] [PubMed] [Google Scholar]
- 7.Zemel BS, Katz SH. The contribution of adrenal and gonadal androgens to the growth in height of adolescent males. Am J Phys Anthropol. 1986;71:459–66. doi: 10.1002/ajpa.1330710409. [DOI] [PubMed] [Google Scholar]
- 8.Largo RH. Catch-up growth during adolescence. Horm Res. 1993;39 (Suppl 3):41–8. doi: 10.1159/000182783. [DOI] [PubMed] [Google Scholar]
- 9.Voutilainen R, Perheentupa J, Apter D. Benign premature adrenarche: clinical features and serum steroid levels. Acta Paediatr Scand. 1983;72:707–11. doi: 10.1111/j.1651-2227.1983.tb09798.x. [DOI] [PubMed] [Google Scholar]
- 10.Korth-Schutz S, Levine LS, New MI. Serum androgens in normal prepubertal and pubertal children and in children with precocious adrenarche. J Clin Endocrinol Metab. 1976;42:117–24. doi: 10.1210/jcem-42-1-117. [DOI] [PubMed] [Google Scholar]
- 11.Ilondo MM, Vanderschueren-Lodeweyckx M, Vlietinck R, Pizarro M, Malvaux P, Eggermont E, Eeckels R. Plasma androgens in children and adolescents. Part II. A longitudinal study in patients with hypopituitarism. Horm Res. 1982;16:78–95. doi: 10.1159/000179487. [DOI] [PubMed] [Google Scholar]
- 12.Sizonenko PC, Paunier L. Hormonal changes in puberty III: Correlation of plasma dehydroepiandrosterone, testosterone, FSH, and LH with stages of puberty and bone age in normal boys and girls and in patients with Addison’s disease or hypogonadism or with premature or late adrenarche. J Clin Endocrinol Metab. 1975;41:894–904. doi: 10.1210/jcem-41-5-894. [DOI] [PubMed] [Google Scholar]
- 13.Katz SH, Hediger ML, Zemel BS, Parks JS. Adrenal androgens, body fat and advanced skeletal age in puberty: new evidence for the relations of adrenarche and gonadarche in males. Hum Biol. 1985;57:401–13. [PubMed] [Google Scholar]
- 14.Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99:505–12. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 15.Sklar CA, Kaplan SL, Grumbach MM. Evidence for dissociation between adrenarche and gonadarche: studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotropin deficiency, and constitutionally delayed growth and adolescence. J Clin Endocrinol Metab. 1980;51:548–56. doi: 10.1210/jcem-51-3-548. [DOI] [PubMed] [Google Scholar]
- 16.Genazzani AR, Facchinetti F, Petraglia F, Pintor C, Bagnoli F, Puggioni R, Corda R. Correlations between plasma levels of opioid peptides and adrenal androgens in prepuberty and puberty. J Steroid Biochem. 1983;19:891–5. doi: 10.1016/0022-4731(83)90030-4. [DOI] [PubMed] [Google Scholar]
- 17.Genazzani AR, Facchinetti F, Pintor C, Puggioni R, Parrini D, Petraglia F, Bagnoli F, Corda R. Proopiocortin-related peptide plasma levels throughout prepuberty and puberty. J Clin Endocrinol Metab. 1983;57:56–61. doi: 10.1210/jcem-57-1-56. [DOI] [PubMed] [Google Scholar]
- 18.Biason-Lauber A, Zachmann M, Schoenle EJ. Effect of leptin on CYP17 enzymatic activities in human adrenal cells: new insight in the onset of adrenarche. Endocrinology. 2000;141:1446–54. doi: 10.1210/endo.141.4.7402. [DOI] [PubMed] [Google Scholar]
- 19.Parker LN, Lifrak ET, Ramadan MB, Lai MK. Aging and the human zona reticularis. Arch Androl. 1983;10:17–20. doi: 10.3109/01485018308990164. [DOI] [PubMed] [Google Scholar]
- 20.Auchus RJ, Rainey WE. Adrenarche - physiology, biochemistry and human disease. Clin Endocrinol (Oxf) 2004;60:288–96. doi: 10.1046/j.1365-2265.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- 21.Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004;22:337–47. doi: 10.1055/s-2004-861550. [DOI] [PubMed] [Google Scholar]
- 22.Dhom G. The prepuberal and puberal growth of the adrenal (adrenarche) Beitr Pathol. 1973;150:357–377. doi: 10.1016/s0005-8165(73)80086-1. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen AD, Conley AJ. Adrenal androgens in humans and nonhuman primates: production, zonation and regulation. Endocr Dev. 2008;13:33–54. doi: 10.1159/000134765. [DOI] [PubMed] [Google Scholar]
- 24.Gell JS, Carr BR, Sasano H, Atkins B, Margraf L, Mason JI, Rainey WE. Adrenarche results from development of a 3beta-hydroxysteroid dehydrogenase-deficient adrenal reticularis. J Clin Endocrinol Metab. 1998;83:3695–701. doi: 10.1210/jcem.83.10.5070. [DOI] [PubMed] [Google Scholar]
- 25.Gell JS, Atkins B, Margraf L, Mason JI, Sasano H, Rainey WE, Carr BR. Adrenarche is associated with decreased 3 beta-hydroxysteroid dehydrogenase expression in the adrenal reticularis. Endocr Res. 1996;22:723–8. doi: 10.1080/07435809609043768. [DOI] [PubMed] [Google Scholar]
- 26.Cutler GB, Jr, Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL. Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology. 1978;103:2112–8. doi: 10.1210/endo-103-6-2112. [DOI] [PubMed] [Google Scholar]
- 27.Smail PJ, Faiman C, Hobson WC, Fuller GB, Winter JS. Further studies on adrenarche in nonhuman primates. Endocrinology. 1982;111:844–8. doi: 10.1210/endo-111-3-844. [DOI] [PubMed] [Google Scholar]
- 28.Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med. 2004;22:311–26. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- 29.Winter JS, Faiman C, Hobson WC, Reyes FI. The endocrine basis of sexual development in the chimpanzee. J Reprod Fertil Suppl. 1980;(Suppl 28):131–8. [PubMed] [Google Scholar]
- 30.Copeland KC, Eichberg JW, Parker CR, Jr, Bartke A. Puberty in the chimpanzee: somatomedin-C and its relationship to somatic growth and steroid hormone concentrations. J Clin Endocrinol Metab. 1985;60:1154–60. doi: 10.1210/jcem-60-6-1154. [DOI] [PubMed] [Google Scholar]
- 31.Schiebinger RJ, Albertson BD, Barnes KM, Cutler GB, Jr, Loriaux DL. Developmental changes in rabbit and dog adrenal function: a possible homologue of adrenarche in the dog. Am J Physiol. 1981;240:E694–99. doi: 10.1152/ajpendo.1981.240.6.E694. [DOI] [PubMed] [Google Scholar]
- 32.Lack EE, Kozakewich HPW. Embryology, developmental anatomy, and selected aspects of non-neoplastic pathology. In: Lack EE, editor. Pathology of the Adrenal Glands. Churchill Livingstone; New York: 1990. pp. 1–74. [Google Scholar]
- 33.Neville AM, O’Hare MJ. The Human Adrenal Cortex. Springer-Verlag; New York: 1982. Functional activity of the adrenal cortex; pp. 68–98. [Google Scholar]
- 34.Cutler GB, Jr, Schiebinger RJ, Albertson BD, Cassorla FG, Chrousos GP, Comite F, Booth JD, Levine J, Hobson WC, Loriaux DL. Control of the Onset of Puberty. Williams& Wilkins; Baltimore: 1990. The adrenarche (human and animal) pp. 506–533. [Google Scholar]
- 35.Griffiths K, Grant JK, Symington T. A biochemical investigation of the functional zonation of the adrenal cortex in man. J Clin Endocrinol Metab. 1963;23:776–85. doi: 10.1210/jcem-23-8-776. [DOI] [PubMed] [Google Scholar]
- 36.Narasaka T, Suzuki T, Moriya T, Sasano H. Temporal and spatial distribution of corticosteroidogenic enzymes immunoreactivity in developing human adrenal. Mol Cell Endocrinol. 2001;174:111–20. doi: 10.1016/s0303-7207(00)00445-7. [DOI] [PubMed] [Google Scholar]
- 37.Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18:378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- 38.Bech K, Tygstrup I, Nerup J. The involution of the foetal adrenal cortex. A light microscopic study. Acta Pathol Microbiol Scand. 1969;76:391–400. doi: 10.1111/j.1699-0463.1969.tb03270.x. [DOI] [PubMed] [Google Scholar]
- 39.Hyatt PJ, Bhatt K, Tait JF. Steroid biosynthesis by zona fasciculata and zona reticularis cells purified from the mammalian adrenal cortex. J Steroid Biochem. 1983;19:953–9. doi: 10.1016/0022-4731(83)90039-0. [DOI] [PubMed] [Google Scholar]
- 40.Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3 beta-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab. 1996;81:3558–65. doi: 10.1210/jcem.81.10.8855801. [DOI] [PubMed] [Google Scholar]
- 41.Kenny FM, Preeyasombat C, Migeon CJ. Cortisol production rate. II. Normal infants, children, and adults. Pediatrics. 1966;37:34–42. [PubMed] [Google Scholar]
- 42.Kowarski A, Katz H, Migeon CJ. Plasma aldosterone concentration in normal subjects from infancy to adulthood. J Clin Endocrinol Metab. 1974;38:489–91. doi: 10.1210/jcem-38-3-489. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 2000;53:739–47. doi: 10.1046/j.1365-2265.2000.01144.x. [DOI] [PubMed] [Google Scholar]
- 44.Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13:234–9. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- 45.Reiter EO, Fuldauer VG, Root AW. Secretion of the adrenal androgens dehydroepiandrosterone sulfate during normal infancy, childhood, and adolescence, in sick children and in children with endocrinologic abnormalities. J Pediatr. 1977;90:766–70. doi: 10.1016/s0022-3476(77)81244-4. [DOI] [PubMed] [Google Scholar]
- 46.Hornsby PJ. Adrenal Cortex. Butterworth; London: 1985. The regulation of adrenocortical function by control of growth and structure; pp. 1–31. [Google Scholar]
- 47.Hornsby PJ. Aging of the human adrenal cortex. Ageing Res Rev. 2002;1:229–42. doi: 10.1016/s1568-1637(01)00007-1. [DOI] [PubMed] [Google Scholar]
- 48.Wang W, Yang L, Suwa T, Casson PR, Hornsby PJ. Differentially expressed genes in zona reticularis cells of the human adrenal cortex. Mol Cell Endocrinol. 2001;28;173:127–34. doi: 10.1016/s0303-7207(00)00404-4. [DOI] [PubMed] [Google Scholar]
- 49.Chen CC, Parker CR., Jr Adrenal androgens and the immune system. Semin Reprod Med. 2004;22:369–77. doi: 10.1055/s-2004-861553. [DOI] [PubMed] [Google Scholar]
- 50.Marx C, Bornstein SR, Wolkersdörfer GW, Peter M, Sippell WG, Scherbaum WA. Relevance of major histocompatibility complex class II expression as a hallmark for the cellular differentiation in the human adrenal cortex. J Clin Endocrinol Metab. 1997;82:3136–40. doi: 10.1210/jcem.82.9.4194. [DOI] [PubMed] [Google Scholar]
- 51.Khoury EL, Greenspan JS, Greenspan FS. Adrenocortical cells of the zona reticularis normally express HLA-DR antigenic determinants. Am J Pathol. 1987;127:580–91. [PMC free article] [PubMed] [Google Scholar]
- 52.Hirokawa K, Utsuyama M, Kasai M, Kurashima C. Aging and immunity. Acta Pathol Jpn. 1992;42:537–48. doi: 10.1111/j.1440-1827.1992.tb03103.x. [DOI] [PubMed] [Google Scholar]
- 53.Stocco DM. The steroidogenic acute regulatory (StAR) protein two years later. An update Endocrine. 1997;6:99–109. doi: 10.1007/BF02738952. [DOI] [PubMed] [Google Scholar]
- 54.Sasano H, Mason JI, Sasano N. Immunohistochemical study of cytochrome P-45017 alpha in human adrenocortical disorders. Hum Pathol. 1989;20:113–17. doi: 10.1016/0046-8177(89)90174-3. [DOI] [PubMed] [Google Scholar]
- 55.Miller WL. Early steps in androgen biosynthesis: from cholesterol to DHEA. Baillieres Clin Endocrinol Metab. 1998;12:67–81. doi: 10.1016/s0950-351x(98)80461-8. [DOI] [PubMed] [Google Scholar]
- 56.Yanase T, Sasano H, Yubisui T, Sakai Y, Takayanagi R, Nawata H. Immunohistochemical study of cytochrome b5 in human adrenal gland and in adrenocortical adenomas from patients with Cushing’s syndrome. Endocr J. 1998;45:89–95. doi: 10.1507/endocrj.45.89. [DOI] [PubMed] [Google Scholar]
- 57.Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–65. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- 58.Strott CA. Steroid sulfotransferases. Endocr Rev. 1996;17:670–97. doi: 10.1210/edrv-17-6-670. [DOI] [PubMed] [Google Scholar]
- 59.Luu-The V, Bernier F, Dufort I. Steroid sulfotransferases. J Endocrinol. 1996;150:S87–S97. [PubMed] [Google Scholar]
- 60.Weinshilboum RM, Otterness DM, Aksoy IA, Wood TC, Her C, Raftogianis RB. Sulfation and sulfotransferases 1: Sulfotransferase molecular biology: cDNAs and genes. FASEB J. 1997;11:3–14. [PubMed] [Google Scholar]
- 61.Warne GL, Carter JN, Faiman C, Reyes FI, Winter JS. Hormonal changes in girls with precocious adrenarche: a possible role for estradiol or prolactin. J Pediatr. 1978;92:743–47. doi: 10.1016/s0022-3476(78)80141-3. [DOI] [PubMed] [Google Scholar]
- 62.Dardis A, Saraco N, Rivarola MA, Belgorosky A. Decrease in the expression of the 3beta-hydroxysteroid dehydrogenase gene in human adrenal tissue during prepuberty and early puberty: implications for the mechanism of adrenarche. Pediatr Res. 1999;45:384–88. doi: 10.1203/00006450-199903000-00016. [DOI] [PubMed] [Google Scholar]
- 63.Palmert MR, Hayden DL, Mansfield MJ, Crigler JF, Jr, Crowley WF, Jr, Chandler DW, Boepple PA. The longitudinal study of adrenal maturation during gonadal suppression: evidence that adrenarche is a gradual process. J Clin Endocrinol Metab. 2001;86:4536–42. doi: 10.1210/jcem.86.9.7863. [DOI] [PubMed] [Google Scholar]
- 64.White PC, New MI, Dupont B. Congenital adrenal hyperplasia. N Engl J Med. 1987;316:1519–24. 1580–86. doi: 10.1056/NEJM198706113162406. [DOI] [PubMed] [Google Scholar]
- 65.Saner KJ, Suzuki T, Sasano H, Pizzey J, Ho C, Strauss JF, 3rd, Carr BR, Rainey WE. Steroid sulfotransferase 2A1 gene transcription is regulated by steroidogenic factor 1 and GATA-6 in the human adrenal. Mol Endocrinol. 2005;19:184–97. doi: 10.1210/me.2003-0332. [DOI] [PubMed] [Google Scholar]
- 66.Seely J, Amigh KS, Suzuki T, Mayhew B, Sasano H, Giguere V, Laganière J, Carr BR, Rainey WE. Transcriptional regulation of dehydroepiandrosterone sulfotransferase (SULT2A1) by estrogen-related receptor alpha. Endocrinology. 2005;146:3605–13. doi: 10.1210/en.2004-1619. [DOI] [PubMed] [Google Scholar]
- 67.Labrie F, Luu-The V, Bélanger A, Lin SX, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–96. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- 68.Petry CJ, Ong KK, Wingate DL, de Zegher F, Ibáñez L, Dunger DB. Lack of association between common polymorphisms in the 17beta-hydroxysteroid dehydrogenase type V gene (HSD17B5) and precocious pubarche. J Steroid Biochem Mol Biol. 2007;105:176–80. doi: 10.1016/j.jsbmb.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 69.Qin KN, Rosenfield RL. Expression of 17 beta-hydroxysteroid dehydrogenase type 5 in human ovary: a pilot study. J Soc Gynecol Invest. 2000;7:61–4. doi: 10.1016/s1071-5576(99)00067-2. [DOI] [PubMed] [Google Scholar]
- 70.Nakamura Y, Hornsby PJ, Casson PR, Morimoto R, Satoh F, Sasano H, Rainey WE. Gene profiling of the reticularis and fasciculata zones of human adult adrenals. The Endocrine Society’s 90th Annual Meeting; San Francisco, CA. 2008. [Google Scholar]