Abstract
Introduction
Pulmonary nodules often require operative resection to obtain a diagnosis. However, 10–30% of operations result in a benign diagnosis. Our purpose was to determine whether negative thoracic operations are futile by describing the pathological diagnoses, determining new diagnoses and treatment changes initiated based on operative findings, and assessing morbidity, mortality and cost of the procedure.
Methods
At our academic medical center 278 thoracic operations were performed for known or suspected cancer between January 1, 2005 and April 1, 2009. We collected and summarized data pertaining to pre-operative patient and nodule characteristics, pathologic diagnosis, post-operative treatment changes resulting from surgical resection, peri-operative morbidity and mortality, and hospital charges for patients with benign pathology.
Results
Twenty-three percent (65/278) of patients who underwent surgical resection for a suspicious nodule had benign pathology. We report granulomatous disease in 57%, benign tumors in 15%, fibrosis in 12%, and autoimmune and vascular diseases in 9%. Definitive diagnosis or treatment changes occurred in 85% of cases. Surgical intervention led to a new diagnosis in 69% and treatment course changes in 68% of benign cases; medication changes in 38%, new consultation in 31%, definitive treatment in 9%, and underlying disease management in 34%. There was no intraoperative, in-hospital, or 30-day mortality. Post-operative in-hospital events occurred in 7 patients. The mean total cost was $25,515 with a mean cost per day of $7,618.
Conclusions
Patients with a benign diagnosis after surgical resection for a pulmonary nodule received a new diagnosis or had a treatment course change in 85% of the cases.
Keywords: Pulmonary Nodule, Benign Nodule, Lung Cancer, Biopsy (Lung), Thoracic Surgery, Clinical Decision Making
INTRODUCTION
Lung cancer remains the most common cause of cancer-related death worldwide.1 Computed Tomography (CT) has recently been shown to reduce lung cancer mortality2 but radiographic screening reveals pulmonary nodules in 8–51% of patients, with malignancy in 1-12% of patients.3,4 In clinical practice, 31–82% of nodules are malignant; thus, most patients require additional diagnostic tests.4 Despite advances in clinical assessment and imaging, tissue diagnosis thoracotomy is often necessary and remains the gold standard.5 At the time of surgical resection, if malignancy is revealed on frozen sections, then definitive anatomic resection with staging is usually performed. No additional surgery is usually required for those with benign disease. Therefore, in cases demonstrating a high pre-operative probability of malignancy, surgical resection provides opportunities for both diagnosis and definitive therapeutic intervention.
Recent reports have referred to invasive procedures that yield a benign diagnosis as “of limited or no clinical significance”, “negative resection”, “futile thoracotomy”, “non-therapeutic resection”, “overuse of an invasive procedure” or “unnecessary surgery”.5-8 Rates of benign disease have ranged in recent reports from 1 to 30% of surgical resection cases for suspected lung cancer, depending on patient population and pre-operative evaluation protocol.7, 9 Thus, the invasive thoracic procedure has been perceived to cause unnecessary cost, societal burden, and morbidity and mortality risk to the patient, with no therapeutic benefit.7, 8, 10-12 Most descriptive studies of patients who have undergone surgical resection of suspicious pulmonary nodules limit their analysis of benign cases to the diagnoses and costs of pre-operative evaluation,7, 10, 12-14 with no evaluation of whether this benign diagnosis affects subsequent patient management. We therefore hypothesized that surgical resection of these benign nodules presenting as suspicious nodules improves patient management. To determine what influences, if any, benign nodule resection has on patient management, we aimed to (1) describe the pathologic diagnosis of surgically resected benign pulmonary nodules, (2) determine what new diagnoses, and medication and/or treatment changes resulted from the operative findings, and (3) assess the morbidity, mortality, and cost of the procedure.
METHODS AND MATERIALS
Population
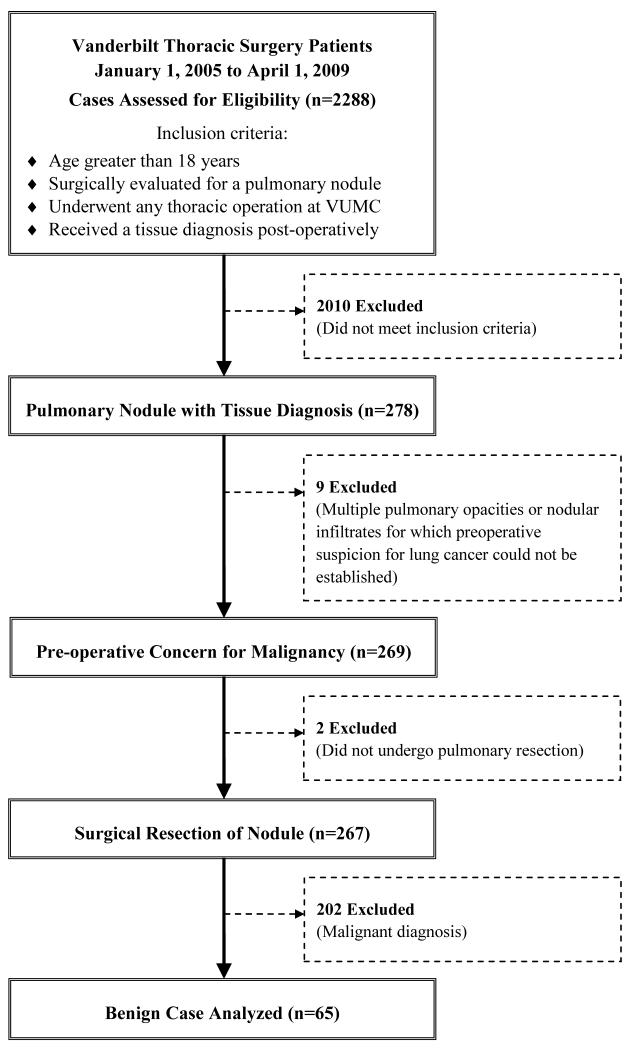
We reviewed data from 278 consecutive thoracic operations performed by the Vanderbilt Department of Thoracic Surgery for known or suspected lung cancer between January 1, 2005 and April 1, 2009. Figure 1 details the flow of analysis of cases, from the 2288 patient database to the population of interest. Pulmonary nodule was broadly defined to include radiological abnormalities of different shapes. Patients with multiple pulmonary opacities or nodular infiltrates were excluded if preoperative suspicion for lung cancer could not be established. Sixty-five cases were diagnosed with benign pathology and were included in this study. The Vanderbilt University Institutional Review Board approved this retrospective descriptive study and waived the need to obtain individual patient consent (IRB #081298).
Figure 1. Patient Selection Criteria.
Flow diagram of patient selection from all Vanderbilt Thoracic Surgery cases considered (n=2288) to the subset of benign cases analyzed (n=65) with inclusion and exclusion criteria noted.
Data sources
Patients were identified using Vanderbilt’s Thoracic Surgery Database, which contains prospective, surgeon-recorded demographic and peri-operative data for all patients that had received a thoracic surgical operation at Vanderbilt since January 1, 2005. At the time of this study, the database included 2288 patients. For all patients, complete pre-operative, operative, and post-operative notes—as well as imaging, pathology and microbiology reports—are prospectively documented by treating physicians in the electronic medical record. Hospital, clinic, and professional fees before, during, and after operation are prospectively included for all patients in Vanderbilt’s administrative database.
Data Collection, Classification, and Analysis
Specific variables for patient demographics, pre-operative nodule characteristics, operative findings, post-operative diagnoses, medication and treatment course changes, and 30-day survival were extracted from the Thoracic Surgery Database and electronic medical record. Pre-operative imaging characteristics of benign nodules (size, location, morphology, and interval growth) were recorded verbatim from the radiologist’s computed tomography (CT) review. Images were reviewed by author R.W. if there was no written description of edge or consistency. The nodule was avid if the fluorodeoxyglucose positron emission tomography (FDG-PET) report, as dictated by the radiologist, noted a standardized uptake value (SUV) exceeding 2.5. If no value was reported, FDG-PET was classified as avid if the radiologist reported a suspicion of malignancy and used the terms “cancerous”, “likely”, “possible”, or “probable.”
Pathologic diagnosis was extracted from the pathology report and categorized between granulomatous disease, benign tumors, fibrosis, autoimmune and vascular diseases, and others. The category ‘granulomatous disease’ was sub-divided into active-infectious and healed-or-nonspecific groups. Infectious etiology was indicated by the pathologist’s report of morphology on specimen stain or microbiologist’s growth report. The diagnosis was defined as active-infectious histoplasmosis if histopathology reported yeast forms compatible with Histoplasma capsulatum or microbiology reported growth of H. capsulatum in fungal culture.15 If pathology reported infectious granulomatous disease with unknown organisms and microbiology was non-revealing, ‘infectious granulomata, organism unknown’ was recorded. Granulomatous disease with no identifiable etiology was recorded as ‘healed or non-specific.’
For each benign case, medical records were reviewed to collect data on new diagnosis, specialty service consultations, reliance for underlying disease management, and changes in treatment due to operative findings or post-operative diagnosis. New diagnosis was classified as positive if pathology, microbiology, or provider gave a new diagnosis at post-operative follow-up with documentation in the medical record. Medication change was defined as the post-operative initiation of a new therapeutic medication or discontinuation of a medication no longer indicated, recommended by the surgeon or surgeon’s consultant, and documented by the thoracic surgeon in the post-operative note. The management of the primary diagnosis being contingent on nodule pathology was determined from the peri-operative referral and thoracic surgery notes. These influences of operation on treatment course were considered independently and not mutually exclusive.
Post-operative hospital course event variables were captured prospectively from Vanderbilt’s Thoracic Surgery Database and extracted directly for all 65 patients with benign nodules. Events included in-hospital mortality, wound infection, ventilation requirement exceeding 48-hours, re-intubation, air leak exceeding 4-days, pneumothorax, post-operative bleed, atrial fibrillation, change in status, and hospital discharge with chest tube. Length-of-stay data were extracted from Vanderbilt’s administrative database and 30-day mortality from the medical record. Cost of care for each inpatient’s operative stay was based on hospital fees retrieved by administrative chart abstraction and estimated using the medical center’s internal activity-based cost accounting system (TSI/Eclipsys) for base year 2009. We applied our institution specific inflation factor to total inpatient costs based upon discharge date of the patient. In those cases where costs were not available, a cost-to-charge ratio of 0.6 was applied to total inpatient charges prior to adjusting costs to the base year of 2009. Descriptive statistics were performed on de-identified data using STATA (version 9, College Station, TX).
RESULTS
Surgical resection was performed in 278 patients with suspicious lesions. Pathologic diagnosis was benign in 65 cases (23%) and the mean age was 52 ± 13.6 years (Table 1). Of this group, 64% were current or former smokers (average 38.5 pack-years) and pre-operative history of extra-thoracic cancer was present in 17 patients. Pre-operative CT imaging was performed on all 65 patients and mean nodule size was 20.8 ± 12.6 mm (range 4–63 mm) (Table 2). CT imaging data regarding nodule size distribution, edge characteristics, interval growth, and location are reported in Table 2. Of the benign nodules, 42 were imaged using FDG-PET, and 26 of these were FDG-PET avid (62%). Pre-operative fine needle aspiration (FNA) was performed in 15 cases. Patients underwent sublobar resection in 54 cases (83%), lobectomy in 9 (14%), and thoracotomy with nodule biopsy or excision in 2 (3%). The thoracic operation was limited to video-assisted thoracic surgery (VATS) in 43 cases (66%). Pathologic evaluation revealed granulomatous disease in 37 of the 65 benign nodules (Table 3); these were active infectious granulomata in 27, 15 of which corresponded to active histoplasmosis. Benign tumors were identified in 10 cases (15%), fibrosis in 8 (12%), and autoimmune or vascular diseases in 6 (9%).
Table 1.
Characteristics of Patients with benign nodules
| Number (%) or Mean (SD) (n=65) |
|
|---|---|
| Age (years) | 52 (13.6) |
| Gender: | |
| Male | 32 (49%) |
| Female | 33 (51%) |
| BMI | 29 (5.7) |
| Smoking History: | |
| Never smoked | 23 (35%) |
| Current smoker | 7 (11%) |
| Former smoker | 35 (54%) |
| Pack-years a | 38.5 (26.3) |
| Pre-op hemoptysis | 4 (6%) |
| Pre-op cancer diagnosis (extra-thoracic) | 17 (26%) |
| Zubrod Performance Score b | 0.48 (0.62) |
| Pre-op ASA: | |
| Class I, healthy | 9 (14%) |
| Class II, mild systemic disease | 24 (37%) |
| Class III, severe systemic disease, function-limiting | 30 (46%) |
| Class IV, severe systemic disease, life-threatening | 2 (3%) |
| FEV1 c | |
| Pre-op predicted (%) | 82 (23) |
| Pre-op actual (L/Sec) | 2.7 (0.93) |
| Thoracic operation: | |
| Sublobar resection (wedge or segmentectomy) | 54 (83%) |
| Lobectomy | 9 (14%) |
| Other d | 2 (3%) |
| VATS e | 43 (66%) |
ASA = American Society of Anesthesiologists; BMI = body mass index; FEV1 = forced expiratory volume in 1 second; SD = standard deviation; VATS = video-assisted thoracoscopic surgery
Mean over all ever-smokers
SD with 2 missing
SD with 15 missing
1 thoracotomy with multiple core needle biopsies and 1 thoracotomy with excision of a nodal mass within the left upper lobe
VATS with 42 wedge resections and 1 lobectomy
Table 2.
Benign Pulmonary Nodule Characteristics
| Number (%) or Mean (SD) (n=65) |
|
|---|---|
| Pre-op CT performed | 65 (100%) |
| Mean nodule a size on CT (mm) | 20.8 (12.6) |
| Size distribution: | |
| <10 mm | 18 (28%) |
| 11–20 mm | 20 (31%) |
| 20–30 mm | 10 (15%) |
| >30 mm | 17 (26%) |
| CT Edge: | |
| Smooth | 27 (42%) |
| Lobulated | 14 (22%) |
| Spiculated | 22 (34%) |
| Not described | 2 |
| CT Consistency: | |
| Cavitary | 7 (11%) |
| Semi-solid | 3 (5%) |
| Solid | 53 (82%) |
| Ground glass | 1 (1.5%) |
| Not Described | 1 |
| Nodule growth reported by CT | 22 (34%) |
| CT Location: | |
| Left upper lobe | 16 (25%) |
| Left lower lobe | 6 (9%) |
| Right upper lobe | 19 (29%) |
| Right middle lobe | 8 (12%) |
| Right lower lobe | 6 (9%) |
| Not reported | 10 (15%) |
| Pre-op FDG-PET performed | 42 (65%) |
| FDG-PET Avid b | 26/42 (62%) |
| Pre-op FNA Biopsy performed | 15 (23%) |
CT = computed tomography; FDG-PET = fluorodeoxyglucose-positron emission tomography; FNA = fine needle aspiration; SD = standard deviation; SUV = standardized uptake value
The majority of lesions were ≤3cm; there were 17 ‘masses’ (>3cm). The term nodule is used throughout the manuscript for consistency acknowledging that a small percentage were actually masses.
FDG-PET Avid defined as SUV > 2.5 or mild, moderate, or intense uptake, or radiology report indicated suspicion of cancer.
Table 3.
Pathology of Benign Nodules
| Number (%) (n=65) |
FDG-PET Avid a (n=26) |
FDG-PET Non-avid (n=16) |
|
|---|---|---|---|
| Granulomatous disease: | 37 (57%) | 15 | 9 |
| Active infectious: | 27 (42%) | ||
| Histoplasmosis | 15 (23%) | 5 | 3 |
| Atypical Mycobacteria | 2 (3%) | - | 1 |
| Blastomycosis | 2 (3%) | 1 | - |
| Cryptococcus | 2 (3%) | 1 | - |
| Mycobacterium tuberculosis | 1 (1.5%) | 1 | - |
| Aspergillus | 1 (1.5%) | - | - |
| Actinomyces | 1 (1.5%) | 1 | - |
| Nocardia | 1 (1.5%) | - | 1 |
| Mucor | 1 (1.5%) | - | - |
| Organism unknown | 1 (1.5%) | 5 | 4 |
| Healed or non-specific | 10 (15%) | - | - |
| Benign Tumors: | 10 (15%) | 2 | 5 |
| Hamartoma | 6 (9%) | 1 | 3 |
| Assorted benign tumors b | 4 (6%) | 1 c | 2 d |
| Fibrosis | 8 (12%) | 3 | 2 |
| Autoimmune and Vasculitides: | 6 (9%) | 3 | - |
| Wegener Granulomatosis | 3 (5%) | 2 | - |
| Pneumonitis | 1 (1.5%) | 1 | - |
| Rheumatoid | 1 (1.5%) | - | - |
| Churg-Strauss vasculitis | 1 (1.5%) | - | - |
| Other e | 4 (6%) | 3 | - |
FDG-PET Avid defined as SUV > 2.5 or mild, moderate, or intense uptake, or radiology report indicated suspicion of cancer
Assorted Benign Tumors: Lipoma, Premalignancy was specified in the pathologist’s report for (1) inflammatory myofibroblastic, (2) atypical lymphoproliferative, and (3) neuroendocrine cell hyperplasia.
Inflammatory myofibroblastic
Atypical lymphoproliferative and Lipoma
Other: Bronchiolitis obliterans organizing pneumonia, Broncholith with post-obstructive pneumonia, Reactive lymph nodes, Infarct with organizing thrombus
Operative findings led to a new diagnosis in 45 of the 65 patients in the benign group (69%) and changed the treatment course for 44 patients (68%) in one or more ways (Table 4). New diagnosis led to a medication change for 24 patients and new consultations in 20 benign cases. Development of a treatment plan for the management of an underlying disease awaited nodule diagnosis in 22 cases; including 17 patients with extra-thoracic cancer and 1 with a prior lung cancer resection for whom recurrence was subsequently no longer suspected. Resection was therapeutic for 1 patient with an enlarging hamartoma encroaching on the pulmonary artery, 2 patients with an obstructed airway causing recurrent infection, and 3 patients with pre-malignancy. Premalignancy was specified in the pathologist’s report for (1) inflammatory myofibroblastic, (2) atypical lymphoproliferative, and (3) neuroendocrine cell hyperplasia. In 10 cases (15%), neither a new diagnosis nor a change in management resulted from operative findings. These 10 cases included 5 granulomata not otherwise specified, 2 fibrotic nodules, reactive lymph node, rheumatoid nodule, and hamartoma. Thus, nodule resection resulted in a new diagnosis, medication change, new consultation, underlying disease management, or definitive therapy in 55 of 65 total cases (85%).
Table 4.
Influences of Operation & Post-operative Diagnosis on Patient Treatment Courses
| Number (%) (n=65) |
|
|---|---|
| New diagnosis a | 45 (69%) |
| Treatment course changed by results | 44 (68%) |
| New consultations for new diagnosis | 20 (31%) |
| Total number of new consultations | 25 |
| Consult Service: | |
| Infectious disease | 15 |
| Pulmonology | 4 |
| Rheumatology | 3 |
| Hematology | 1 |
| Other (nephrology and transplant) | 2 |
| Change in medication regimen b | 24 (37%) |
| Antifungal initiated | 11 (17%) |
| Immunosuppressant initiated | 4 (6%) |
| Chemotherapy initiated | 3 (5%) |
| Immunosuppressant held or discontinued | 2 (3%) |
| Antibiotic initiated | 2 (3%) |
| Other c | 2 (3%) |
| Management of underlying disease contingent on diagnosis or procedure |
22 (34%) |
| Treatment of extra-thoracic cancer d | 17 (26%) |
| Transplant management | 4 (6%) |
| Rule out metastatic lung cancer | 1 (1.5%) |
| Resection was therapeutic e | 6 (9%) |
| Total patients with new diagnosis or treatment course change |
55 (85%) |
| Total patients with neither new diagnosis nor treatment course change f |
10 (15%) |
Definitive diagnosis on pathology or microbiology or new diagnosis given by provider at follow-up
Defined by post-operative initiation of a new therapeutic medication or discontinuation of medication no longer indicated
Other changes included initiation of somatostatin-analog and antituberculosis therapies
Extra-thoracic cancers were most commonly breast, squamous cell of head and neck, and lymphomatous
Resection was therapeutic for 1 patient with enlarging hamartoma encroaching on pulmonary artery, 2 patients with an obstructive nidus for recurrent infection, and 3 with pre-malignancy
Cases without new diagnosis or treatment course change included 5 granulomata not otherwise specified, 2 fibrotic nodules, reactive lymph node, rheumatoid nodule, and hamartoma
The mean hospital stay for patients in the benign group was 3.7 days (excluding one outlier of 44 days). There was no intraoperative, in-hospital, or 30-day death (Table 5). Post-operative in-hospital events occurred in 7 patients (11%) and included pleural air leaks that exceeded 4 days in 2 cases (3%), re-intubation in 1, post-operative bleeding in 1, and atrial fibrillation in 1 patient. There were no cases of wound infection, requirement of ventilation for more than 48-hours, or change in patient status. The mean total cost was $25,518 (median = $19,894), with a cost per day of $6,891.
Table 5.
Hospital Events and Costs
| Hospital course | Number (%) or Mean (Median) (n=65) |
|---|---|
| Intraoperative deaths | 0 |
| In-hospital mortality | 0 |
| 30-day mortality | 0 |
| Patients with a post-operative event | 7 (11%) |
| Lobectomy patients with long term respiratory difficulties a |
3 |
| Events: | |
| Discharge with chest tube | 4 (6%) |
| Air leak > 4 days | 2 (3%) |
| Re-intubation | 1 (1%) |
| Post-op bleeding | 1 (1%) |
| Atrial fibrillation | 1 (1%) |
| Pneumothorax | 0 |
| Ventilation > 48 hours | 0 |
| Wound infection | 0 |
| Change in status | 0 |
| Length of stay, days b | 3.7 (3.1) |
| Costs b,c | |
| Mean total cost (median) | $25,518 ($19,894) |
| Cost per day | $6,891 |
SD = standard deviation
Subset analysis of the 9 lobectomy cases for post discharge sequelae revealed 2 patients with mild exercise limitations and one patient who was discharged on temporary home oxygen.
One outlier of 44 days was excluded from length of stay, mean total cost, and cost per day. When included, the mean length of stay was 4.3, mean total cost was $28,006, median total cost was $20,344 and cost per day was $6,478.
Cost is determined from charge data, by factoring in cost-to-charge conversion (0.6) and an institutional specific inflation factor to make costs comparable in the base year, 2009.
DISCUSSION
Futile thoracotomies pose unnecessary risk and burden on patients and society. However, we demonstrate in this investigation that only 10 of 65 thoracic operations (15%) for pulmonary nodules yielding a benign diagnosis are futile. Surgical wedge resection or lobectomy led to a new diagnosis in 69% of benign cases and changed treatment in 68% of cases. In one third of cases (34%), findings directed management of an underlying disease, including concurrent extra-thoracic cancer in 26% of our benign group and transplant management in 6% (4 cases). In 6 cases (9%), resection resulted in definitive treatment. In 55 of 65 cases (85%), patients with benign diagnosis after surgical resection directly benefitted from the surgical intervention.
A prevalence of granulomatous disease within our patient population likely accounts for the relatively high benign rate in our study. The proportion of patients with granulomatous disease in this series (57%) is near the higher end of the previously reported (40–65%) ranges. 5, 7, 16 Gould et al.17, in a meta-analysis of 10 PET imaging studies published between 1993 and 1998, found the most common benign diagnosis to be granulomatous disease (40%), with active granulomatous infection reported in only 15% of benign cases. In our study, active infection accounted for 42%; this increased prevalence deriving from a high percentage of H. capsulatum-containing granulomata (23%) within our cohort. While Gould’s meta-analysis combined studies from a variety of geographical regions, our patient population draws from a tertiary academic medical center located in Nashville, TN. H. capsulatum is endemic within our region, and 57–100% of the population has reported exposure.18-20
We report several cases of infectious nodules that, in hindsight, may be appropriately managed by surgical resection, although we do not assume this to be the optimal approach. Nonetheless, when antibiotic therapy is given empirically for indeterminate nodules, medical management alone has shown to provide limited benefit.5, 21 Premalignancy is another example of benign nodule pathology potentially managed with resection. Further analysis of various combined medical and surgical management approaches for infectious and premalignant nodules is needed to propose guidelines for best management of indeterminate pulmonary nodules.
Our morbidity, mortality, and cost results are particularly relevant for the development of evidence-based comparative effectiveness guidelines in the management of patients with suspicious pulmonary nodules found both in clinical practice and in CT screening programs. Prior models have calculated life expectancy of patients undergoing resection of a benign nodule resection as their normal life expectancy minus the morbidity of surgery.22, 23 The more recent model of Gould et al.24 adjusted life expectancy for quality of life using age- and sex-specific utilities and estimated health care costs for patients with benign nodules, including age-specific, annual health care expenditures. However, the assumption that outcomes of benign diagnoses correspond to the normal population has been challenged recently as oversimplification and underestimation of morbidity, mortality, and costs associated with benign diagnoses.14 Barnett et al. suggested that evaluation alone of infectious nodules incurs cost and morbidity comparable to that of malignancy. We have extended their work by specifically identifying the costs and morbidity associated with ruling out malignancy in a high-risk population and adding the value of the clinical information resulting from the pathology or microbiology reports. Since our data do not include the treatment cost of the benign diagnoses, the results represent a conservative estimate. The expenses of both ruling out malignancy and treating benign diseases must be factored into future management models for patients with indeterminate nodules in endemic areas with histoplasmosis. This data will also be applicable for determining the cost-effectiveness of CT screening programs in these geographic regions.
Several limitations of our descriptive study must be noted. The study population was specifically defined to include only those thoracotomies deemed “futile” for their benign diagnosis and those benign nodules suspicious for malignancy. “Futile thoracotomies” referring to incomplete resection of high-stage non-small cell lung cancer25 were not included. Similarly, indications for thoracic intervention other than ruling-out lung cancer, such as diagnostic wedge biopsies for diffuse infiltrates or multiple nodules were excluded. Finally, patients with benign diagnoses may have received more aggressive management than diagnostically-similar non-operative candidates because the former received multi-specialty care in a tertiary center. Therefore, our findings may not be generalizable to a broader population and should be applied within similar context. Our outcome results are also limited and potentially biased by retrospective data capture in only those patients who received an operation, so analysis did not include those followed with serial imaging. However, even if over-classification occurred, this could not explain the magnitude of our results.
Additional research is needed to elucidate reliable non-invasive alternatives to diagnostic operative resection that can identify benign diagnoses pre-operatively. Increased sensitivity for common benign diagnoses may help decision-making regarding pre-operative probability of malignancy and guide appropriate treatment of benign nodules. In particular, advances in pre-operative diagnostic discrimination between infectious granulomata and autoimmune nodules may significantly reduce the number of cases currently indicated for diagnostic operation by influencing physicians’ willingness to try anti-fungal or immunosuppressive medications when the risk involved in this therapy approaches that of surgery. A prospective study including patients managed both medically and surgically is needed to determine the best treatment options for these patients.
We conclude that patients who receive a benign diagnosis after surgical evaluation for a pulmonary nodule may benefit from this intervention with minimal surgical morbidity and mortality. In addition, obtaining this new information from surgical resection has considerable costs, and further studies are needed to determine the most cost-effective strategies for management of such patients.
ACKNOWLEDGMENTS
The authors would like to acknowledge Aileen McAinsh, Ph.D. for her editorial assistance.
This research was supported by: Vanderbilt Physician Scientist Development Award (E.L.G.), SPECS in lung cancer U01 CA114771 (P.P.M.) the lung SPORE CA90949 (P.P.M.), Research Electronic Data Capture (REDCap) 1 UL1 RR024975 from NCRR/NIH, and a Merit award from the Department of Veterans Affairs (P.P.M.).
This work was performed at Vanderbilt University Medical Center, Nashville, TN
ABBREVIATIONS
- ASA
American Society of Anesthesiologists
- BMI
body mass index
- CT
computed tomography
- FDG-PET
fluorodeoxyglucose-positron emission tomography
- FEV1
forced expiratory volume in 1 second
- FNA
fine needle aspiration
- IRB
Institutional Review Board
- NSCLC
non-small cell lung cancer
- SD
standard deviation
- SUV
standardized uptake value
- VATS
video assisted thoracoscopic surgery
REFERENCES
- 1. [Accessed Jan 1 2010];Cancer. 2009 Feb; Available at http://www.who.int/mediacentre/factsheets/fs297/en/
- 2.Marshall E. Cancer screening. The promise and pitfalls of a cancer breakthrough. Science. 2010;330:900–901. doi: 10.1126/science.330.6006.900-b. [DOI] [PubMed] [Google Scholar]
- 3.Bastarrika G, Garcia-Velloso MJ, Lozano MD, et al. Early Lung Cancer Detection using Spiral Computed Tomography and Positron Emission Tomography. Am J Respir Crit Care Med. 2005 doi: 10.1164/rccm.200411-1479OC. [DOI] [PubMed] [Google Scholar]
- 4.Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:94S–107S. doi: 10.1378/chest.07-1352. [DOI] [PubMed] [Google Scholar]
- 5.Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:108S–130S. doi: 10.1378/chest.07-1353. [DOI] [PubMed] [Google Scholar]
- 6.Libby DM, Smith JP, Altorki NK, et al. Managing the small pulmonary nodule discovered by CT. Chest. 2004;125:1522–1529. doi: 10.1378/chest.125.4.1522. [DOI] [PubMed] [Google Scholar]
- 7.Smith MA, Battafarano RJ, Meyers BF, et al. Prevalence of Benign Disease in Patients Undergoing Resection for Suspected Lung Cancer. The Annals of Thoracic Surgery. 2006;81:1824–1829. doi: 10.1016/j.athoracsur.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Black WC, Baron JA. CT screening for lung cancer: spiraling into confusion? Jama. 2007;297:995–997. doi: 10.1001/jama.297.9.995. [DOI] [PubMed] [Google Scholar]
- 9.Henschke CI, Naidich DP, Yankelevitz DF, et al. Early lung cancer action project: initial findings on repeat screenings. Cancer. 2001;92:153–159. doi: 10.1002/1097-0142(20010701)92:1<153::aid-cncr1303>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Wilson DO, Weissfeld JL, Fuhrman CR, et al. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med. 2008;178:956–961. doi: 10.1164/rccm.200802-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early lung cancer action project: a summary of the findings on baseline screening. Oncologist. 2001;6:147–152. doi: 10.1634/theoncologist.6-2-147. [DOI] [PubMed] [Google Scholar]
- 12.Crestanello JA, Allen MS, Jett JR, et al. Thoracic surgical operations in patients enrolled in a computed tomographic screening trial. J Thorac Cardiovasc Surg. 2004;128:254–259. doi: 10.1016/j.jtcvs.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Davies B, Ghosh S, Hopkinson D, et al. Solitary pulmonary nodules: pathological outcome of 150 consecutively resected lesions. Interact Cardiovasc Thorac Surg. 2005;4:18–20. doi: 10.1510/icvts.2004.091843. [DOI] [PubMed] [Google Scholar]
- 14.Barnett PG, Ananth L, Gould MK. Cost and outcomes of patients with solitary pulmonary nodules managed with PET scans. Chest. 2010;137:53–59. doi: 10.1378/chest.08-0529. [DOI] [PubMed] [Google Scholar]
- 15.Baddley JW, Sankara IR, Rodriquez JM, et al. Histoplasmosis in HIV-infected patients in a southern regional medical center: poor prognosis in the era of highly active antiretroviral therapy. Diagn Microbiol Infect Dis. 2008;62:151–156. doi: 10.1016/j.diagmicrobio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Mack MJ, Hazelrigg SR, Landreneau RJ, et al. Thoracoscopy for the diagnosis of the indeterminate solitary pulmonary nodule. Ann Thorac Surg. 1993;56:825–830. doi: 10.1016/0003-4975(93)90339-j. discussion 830-822. [DOI] [PubMed] [Google Scholar]
- 17.Gould MK, Maclean CC, Kuschner WG, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. Jama. 2001;285:914–924. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- 18.Cano MV, Hajjeh RA. The epidemiology of histoplasmosis: a review. Semin Respir Infect. 2001;16:109–118. doi: 10.1053/srin.2001.24241. [DOI] [PubMed] [Google Scholar]
- 19.Ajello L. A comparative study of the pulmonary mycoses of Canada and the United States. Public Health Rep. 1969;84:869–877. [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards LB, Acquaviva FA, Livesay VT. Further observations on histoplasmin sensitivity in the United States. Am J Epidemiol. 1973;98:315–325. doi: 10.1093/oxfordjournals.aje.a121561. [DOI] [PubMed] [Google Scholar]
- 21.Khokhar S, Mironov S, Seshan VE, et al. Antibiotic use in the management of pulmonary nodules. Chest. 2010;137:369–375. doi: 10.1378/chest.09-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gambhir SS, Shepherd JE, Shah BD, et al. Analytical decision model for the cost-effective management of solitary pulmonary nodules. J Clin Oncol. 1998;16:2113–2125. doi: 10.1200/JCO.1998.16.6.2113. [DOI] [PubMed] [Google Scholar]
- 23.Tsushima Y, Endo K. Analysis models to assess cost effectiveness of the four strategies for the work-up of solitary pulmonary nodules. Med Sci Monit. 2004;10:MT65–72. [PubMed] [Google Scholar]
- 24.Gould MK, Sanders GD, Barnett PG, et al. Cost-effectiveness of alternative management strategies for patients with solitary pulmonary nodules. Ann Intern Med. 2003;138:724–735. doi: 10.7326/0003-4819-138-9-200305060-00009. [DOI] [PubMed] [Google Scholar]
- 25.Grannis FW., Jr. Is primary resection of stage IIIA lung cancer futile? Ann Thorac Surg. 2008;86:353–354. doi: 10.1016/j.athoracsur.2008.01.025. author reply 354. [DOI] [PubMed] [Google Scholar]