Abstract
PURPOSE
To determine in a longitudinal study whether there is correlation between videokeratography and clinical signs of keratoconus that might be useful to practicing clinicians.
SETTING
Cornea-Genetic Eye Institute, Cedars-Sinai Medical Center, Los Angeles, California, USA.
METHODS
Eyes grouped as keratoconus, early keratoconus, keratoconus suspect, or normal based on clinical signs and videokeratography were examined at baseline and followed for 1 to 8 years. Differences in quantitative videokeratography indices and the progression rate were evaluated. The quantitative indices were central keratometry (K), the inferior–superior (I–S) value, and the keratoconus percentage index (KISA). Discriminant analysis was used to estimate the classification rate using the indices.
RESULTS
There were significant differences at baseline between the normal, keratoconus-suspect, and early keratoconus groups in all indices; the respective means were central K: 44.17 D, 45.13 D, and 45.97 D; I–S: 0.57, 1.20, and 4.44; log(KISA): 2.49, 2.94, and 5.71 (all P<.001 after adjusting for covariates). Over a median follow-up of 4.1 years, approximately 28% in the keratoconus-suspect group progressed to early keratoconus or keratoconus and 75% in the early keratoconus group progressed to keratoconus. Using all 3 indices and age, 86.9% in the normal group, 75.3% in the early keratoconus group, and 44.6% in the keratoconus-suspect group could be classified, yielding a total classification rate of 68.9%.
CONCLUSIONS
Cross-sectional and longitudinal data showed significant differences between groups in the 3 indices. Use of this classification scheme might form a basis for detecting subclinical keratoconus.
Keratoconus is a clinical term used to describe a condition in which the cornea assumes a conical shape as a result of noninflammatory thinning and protrusion. The estimated prevalence of keratoconus is approximately 50 to 230 per 100 000 in the general population.1 It is a major cause of cornea transplantation in Western developed countries.
Computer-assisted videophotokeratoscopy is a sensitive means for detecting subtle changes in topography on the anterior corneal surface and allows detailed qualitative and quantitative analysis of corneal shape.2,3 Because Placido disk–based computer videokeratoscopes have the combined features of a keratometer and a photokeratoscope, recording curvature changes in the central cornea and the paracentral cornea, they are well suited for detecting subtle topographic changes in early keratoconus and for documenting serial changes in corneal curvature over time.4–6 Thus, corneal topography using videokeratography is at present the most commonly used diagnostic modality for diagnosing keratoconus when obvious clinical signs of the disease are absent.7
Ectasia after laser in situ keratomileusis (LASIK) is one of the most dreaded complications for the refractive surgeon.8 In most cases, it results in unhappy patients, many of whom require subsequent rigid contact lens fitting, intrastromal corneal ring implantation or, ultimately, cornea transplantation. Ectasia is also becoming an increasing cause of litigation against practicing ophthalmologists.9
Reports in the literature suggest that a major cause of post-LASIK ectasia is when patients with undiagnosed early keratoconus or suspected keratoconus have LASIK. Although ophthalmologists now routinely screen for these entities using videokeratography before refractive surgery, many still find it difficult to identify subtle topographic abnormalities that might progress to keratoconus.1
One problem in making a diagnosis of early keratoconus or suspected keratoconus is that there is no clear definition of these entities in the literature. To assist the clinician in the decision-making process and to avoid inadvertently operating on patients with suspected or early keratoconus, we devised a classification scheme based on clinical signs and then analyzed the videokeratography data from our database of keratoconus patients and their family members. We studied the progression of keratoconus in their eyes to determine whether there was a videokeratographic correlation with clinical signs that might prove useful to the practicing clinician.
SUBJECTS AND METHODS
Subjects
This prospective study was part of a longitudinal videokeratography and genetic study at Cedars-Sinai Medical Center, Los Angeles, California. The eyes recruited to the study were clinically normal or had suspected keratoconus, early keratoconus, or moderate to advanced keratoconus. Institutional review board/ethics committee approval was obtained before the study began.
All eyes had clinical evaluation and videokeratography. The clinical examination included slitlamp biomicroscopy, retinoscopy, and fundus evaluation. The slitlamp biomicroscope was used to determine whether there was stromal corneal thinning, Vogt striae, or a Fleischer ring. Retinoscopy was performed with a fully dilated pupil (20 minutes after instillation of phenylephrine 2.5% and cyclopentolate 1% drops) to determine the presence or absence of retroillumination signs of keratoconus (eg, oil droplet sign, scissoring of retinoscopy reflex). Videokeratography evaluation was also performed in each eye. Based on clinical and videokeratography evaluation, each eye was placed into 1 of 4 groups according to the following classification scheme:
Keratoconus—stromal corneal thinning by slitlamp evaluation accompanied by 1 or more of the following clinical signs: Vogt striae, iron ring, Munson sign, scissoring on retinoscopy
Early keratoconus—no slitlamp findings of keratoconus; scissoring on retinoscopy only and an asymmetric bowtie (AB) with a skewed radial axes (SRAX) (ie, AB/SRAX) pattern on videokeratography
Keratoconus suspect—no slitlamp findings, no scissoring on retinoscopy, and AB/SRAX pattern on videokeratography only
Normal—no clinical signs of keratoconus, no scissoring on retinoscopy, and no AB/SRAX pattern on videokeratography.
To identify the potential indices associated with early detection of keratoconus, only 3 groups (normal, keratoconus suspect, and early keratoconus) were used in the analysis. Most eyes in these groups had clinical and videokeratographic evaluations annually.
Videokeratography Measurements
Videokeratography was performed in both eyes of each subject using the Topographic Modeling System (TMS-1, software version 1.61, Computed Anatomy, Inc.) at baseline and at all follow-up visits. At least 4 pictures were taken of each eye to ensure the reproducibility of video images. The best videokeratograph of the 4 was selected based on the quality of the keratoscopic mires by visual inspection. Each videokeratograph from each eye was put into 1 of 10 categories based on subjective judgment by 3 observers, who agreed on the same pattern in 90% of videokeratographs studied. This classification scheme has been reported in detail.10,11 The other 10% of videokeratographs were assigned according to a pattern agreed to by at least 2 of the 3 observers. The categories were as follows: round, oval, irregular, inferior steepening (IS), superior steepening (SS), symmetric bow tie (SB), AB with SS, AB with IS, SB with SRAX, and AB/SRAX.11,12 Because of the moderate sample size, the 10 patterns were placed into 1 of 3 groups as follows: Group 1, symmetric patterns including round, oval, and SB; Group 2, all asymmetric patterns except AB/SRAX; Group 3, only the AB/SRAX pattern (extremely rare in normal eyes11,12).
Three quantitative indices—central keratometry (K), inferior– superior (I–S), and the keratoconus percentage index (KISA)—were generated for each eye using the videokeratograph device. The indices have been described in detail.12
The central K value was calculated by averaging the dioptric power points on rings 2, 3, and 4 of the videokeratographs.
The I–S value, which is the amount of steepening of the inferior cornea compared with that of the superior cornea, was calculated by subtracting the superior value from the inferior value. The inferior value was calculated by averaging 5 data points along the inferior cornea 3.0 mm from the center of the cornea at 30-degree intervals (ie, at 210, 240, 270, 300, and 330 degrees). The superior value was derived from averaging 5 points on the superior cornea 3.0 mm from the center of the cornea (ie, at 30, 60, 90, 120, and 150 degrees).
The KISA index was derived from the following 4 indices: central K; I–S; the astigmatism index (AST), which quantifies the degree of the regular corneal astigmatism (simulated K1 − simulated K2); and the SRAX index, an expression of irregular astigmatism occurring in keratoconus. The algorithm for calculating the KISA index was as follows:
KISA = (Central K) × (I−S) × (AST) × (SRAX) × 100/300
These indices have been described in detail.12
Statistical Analysis
All data, including demographic information, clinical examination, and videokeratographic measures, were entered into a relational database. Statistical analyses were performed using SAS software (version 8.0, SAS Institute, Inc.). For quantitative traits, comparisons between 2 groups were tested by the Student t test when variables had a normal distribution or by the nonparametric Wilcoxon rank test when the variables deviated from normal distribution. Logarithm transformation was used for the KISA index to obtain normal distribution for the statistical analysis, with the transformed value expressed as log(KISA). The chi-square test was used to compare proportions of qualitative traits between groups. The rate of progression of keratoconus was evaluated by the proportion of the numbers of patients developed in the group, ie, the number of patients developed divided by the total number of subjects in the group, during the follow-up.
To adjust for the family structure and relatedness of 2 eyes in 1 person to compare the difference in videokeratography indices, mixed-effect linear models were used to account for intrasubject and intrafamily correlations. Because there is a relationship between subjects within families and 2 eyes in the same person, some standard methods (eg, analysis of variance, which assumes that within-subjects errors are not correlated or each individual is independent) were not realistic in terms of this study. The mixed linear model is a flexible approach to modeling within-subject correlations (ie, relatedness of 2 eyes in 1 person and repeated measures of same person during the follow-up) and between-subject correlation (eg, related subjects in 1 family). Thus, for longitudinal data with repeated measures, the mixed model could be used to estimate whether subjects developed keratoconus differently by videokeratography indices over time. Using each quantitative index as a dependent variable, the progression of each index in the 3 groups (normal, suspect, and early keratoconus) during the follow-up period was tested. Group variables, centered age during the follow-up (time), and the interaction between them were used as the fixed effects in a mixed model. Centered age was defined as the patient’s age minus 30 because keratoconus is more likely to progress in those who are younger than 30 during a given follow-up. The interaction term of group and centered age during follow-up (time) in the mixed model represented the difference in progression between the 3 groups. Sex, race, and family history of keratoconus were considered possible covariates.
To estimate the correct classification rate for the quantitative variables, discriminant analysis was used to classify eyes into 3 groups. First, each quantitative variable was evaluated separately in the model. Then, all 3 indices and age were entered into the model and selected by the backward method to obtain the best subset of predicted variables. Any associated index at the 0.05 significance level was included as a final variable to estimate the correct classification rate.
Using central K, I–S, log(KISA), and age as predictive variables, the canonical variables were estimated. Canonical variables are the linear combinations of the quantitative variables that summarize between-class variation. Thus, each eye had 2 estimated canonical correlations based on predictive variables, and these correlations were used to more directly differentiate between the 3 groups.
RESULTS
Distribution of Eyes at Baseline
The study recruited 1795 subjects (3464 eyes) at baseline. Of the eyes, 2138 were clinically normal, 277 had suspected keratoconus, 86 had early keratoconus, and 963 had moderate to advanced keratoconus. Of the 2501 eyes in the 3 groups used in the analysis (normal, keratoconus suspect, early keratoconus), 1627 were followed longitudinally from 1993 to 2004, with a minimum of 2 visits, and 22 were followed for less than 1 year. Table 1 shows the demographic characteristics in the 3 groups. The mean age in the keratoconus-suspect group was statistically significantly higher than in the early keratoconus group (P < .05). There were statistically significantly more men in the normal group and the keratoconus-suspect group than in the early keratoconus group (P < .01). The proportion of white patients was statistically significantly higher in the keratoconus-suspect group than in the other 2 groups (P < .01).
Table 1.
Distribution of demographic characters of eyes at baseline.
| Group | |||
|---|---|---|---|
| Variable | Normal (n = 2138) |
KC Suspect (n = 277) |
Early KC (n = 86) |
| Mean age (y) ± SD | 35.6 ± 18.6 | 38.4 ± 17.9 | 32.2 ± 12.1 |
| Proportions | |||
| Sex (M:F) | 58:42 | 58:42 | 40:60 |
| Race (white: Hispanic:others) | 46:38:16 | 62:26:12 | 47:35:18 |
KC = keratoconus
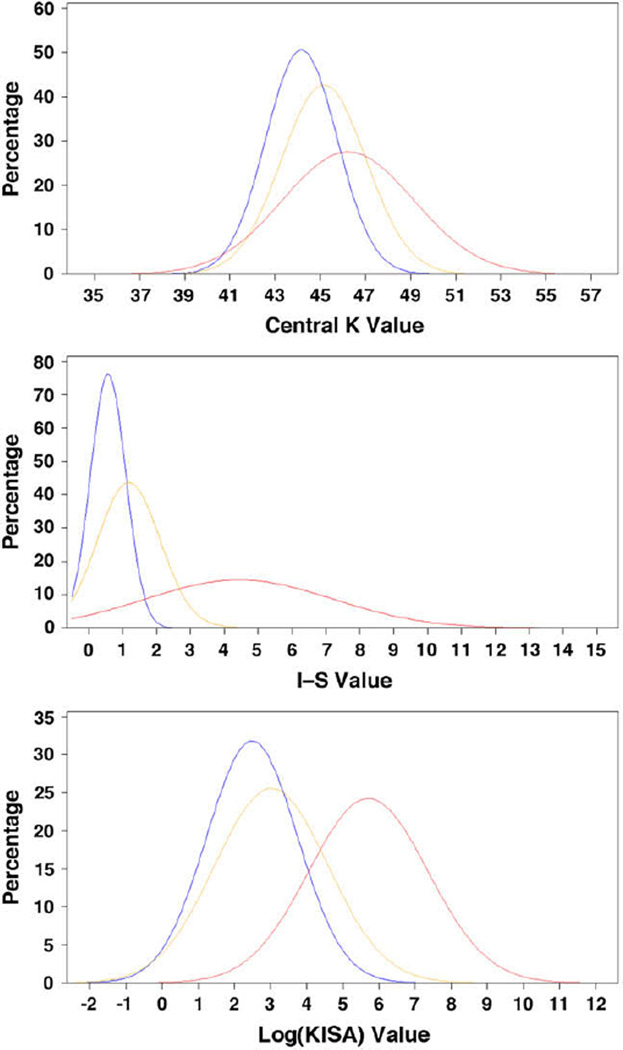
Table 2 shows the distribution of central K, I–S, and KISA values in the 3 groups at baseline. There were statistically significant differences between the 3 groups, with an increasing trend from normal eyes to early keratoconus eyes for all 3 indices. After adjusting for age, sex, race, family history of keratoconus, family structure, and relatedness of 2 eyes by the mixed model, there were still significant differences between the 3 groups for each index (all P < .001). Figure 1 shows the estimated normality curve for central K, I–S, and KISA. For each index, although the values were statistically significantly different between groups, there was a degree of overlap, especially between the normal group and keratoconus-suspect group.
Table 2.
Videokeratography indices in the 3 groups at baseline.
| Group | ||||||
|---|---|---|---|---|---|---|
| Normal | KC Suspect | Early KC | ||||
| Variable* | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD |
| Central K | 2138 | 44.17 ± 1.58 | 276 | 45.13 ± 1.87 | 86 | 45.97 ± 2.90 |
| I-S | 2127 | 0.57 ± 0.52 | 277 | 1.20 ± 0.92 | 86 | 4.44 ± 2.76 |
| Log(KISA) | 2082 | 2.49 ± 1.25 | 258 | 2.94 ± 1.56 | 85 | 5.71 ± 1.65 |
I–S = inferior–superior; K = keratometry; KC = keratoconus; Log(KISA) = logarithm transformation of keratoconus percentage index; n = number of eyes
All P < .05 between normal and keratoconus-suspect groups and between normal and early keratoconus groups for each index
Figure 1.
Normality plot of central K, I–S, and logKISA values (blue = normal group; orange = keratoconus-suspect group; red = early keratoconus group) (I–S = inferior–superior; K = keratometry; Log(KISA) = logarithm transformation of keratoconus percentage index).
Progression to Keratoconus
Of the 2501 eyes enrolled at baseline, 1627 were followed for a median of 4.1 years. Table 3 shows the rates of progression to keratoconus in these eyes. As expected, the early keratoconus group had the highest rate of progression to clinically detectable keratoconus, with a small percentage of normal eyes progressing to keratoconus.
Table 3.
Progression rate of keratoconus by group.
| Progression, n (%) | ||||
|---|---|---|---|---|
| Group | Eyes (n) | KC Suspect | Early KC | KC |
| Normal | 1382 | 18 (1.3) | 9 (0.7) | 11 (0.8) |
| KC suspect | 174 | — | 22 (12.6) | 27 (15.5) |
| Early KC | 71 | — | — | 53 (74.6) |
KC = keratoconus
After adjusting for age, sex, race, family structure, family history of keratoconus, relatedness of 2 eyes, and repeated measures by the mixed model, the early keratoconus group had the highest estimated values for each index and the normal group had the lowest estimated values. Table 4 shows the parameters of the final models for each index. The estimated difference between the keratoconus-suspect group and the normal group was 0.12 for central K (P = .02), 0.28 for I–S (P < .0001), and 0.10 for log(KISA) (P = .10). Similarly, the difference between the normal group and the early keratoconus group was 1.42 for central K, 2.58 for I–S, and 2.59 for log(KISA) (all P < .0001). These results are consistent with baseline data; this indicates significant differences between the 3 groups, especially between the normal group and the early keratoconus group during the follow-up period. However, the progression of each index was not statistically different between groups according to the interaction terms. Evaluation of those younger than 30 years, who are believed to have high risk for keratoconus in clinical practice, showed more progression of log(KISA) values in early keratoconus eyes than in normal eyes (P = .003).
Table 4.
Final models for central K, I–S and KISA comparing the 3 groups by the mixed model at a 0.10 significance level.
| Index | Model |
|---|---|
| Central K | 44.17 + 0.005 × Time + 0.12 × KC Suspect + 1.42 × Early KC − 0.60 × Female + 0.63 × Family History − 0.26 × Black − 0.31 × Hispanic − 0.39 × Other |
| I-S | 0.28 + 0.006 × Time + 0.28 × KC Suspect + 2.58 × Early KC + 0.24 × Family History |
| Log(KISA) | 2.33 + 0.010 × Time + 0.10 × KC Suspect + 2.59 × Early KC + 0.22 × Female + 0.22 × Family History + 0.06 × Black − 0.15 × Hispanic − 0.04 × Other |
I–S = inferior–superior; K = keratometry; KC = keratoconus; Log(KISA) = logarithm transformation of keratoconus percentage index
Classification Rate of Quantitative Variables
Both cross-sectional and longitudinal data indicated significant differences in each quantitative index between the 3 groups. Thus, these variables can be useful in classifying keratoconus. In addition, age was significantly different between the groups. Table 5 shows the estimated classification rate for the quantitative variables using discriminant analysis. When the accuracy of classification by each index was tested alone, I–S alone classified more normals eyes and early keratoconus eyes than central K and KISA alone classified more early keratoconus eyes than central K. Using the backward method to select the best subset of predictive variables, all 3 indices and age were statistically significant in the model that remained in the subset. Using all 3 quantitative indices and age, 86.9% in the normal group, 75.3% in the early keratoconus group, and 44.6% in the keratoconus-suspect group could be classified, yielding a total classification rate of 68.9%. Thus, these indices may be useful to differentiate normal eyes from early keratoconus eyes; however, the ability to distinguish between normal eyes and keratoconus-suspect eyes is modest.
Table 5.
Classification rate estimation for quantitative variables by discrimination analysis
| Classification Rate (%) | ||||
|---|---|---|---|---|
| Group | ||||
| Variable | Normal | KC Suspect |
Early KC |
Total |
| Central K | 71.0 | 36.2 | 34.9 | 47.4 |
| I–S | 89.4 | 38.3 | 68.6 | 65.4 |
| KISA | 68.4 | 30.3 | 78.8 | 59.1 |
| Central K, I–S, and KISA | 87.4 | 44.2 | 69.4 | 67.0 |
| Central K, I–S, KISA, and age | 86.9 | 44.6 | 75.3 | 68.9 |
I–S = inferior–superior; K = keratometry; KC = keratoconus; KISA = keratoconus percentage index
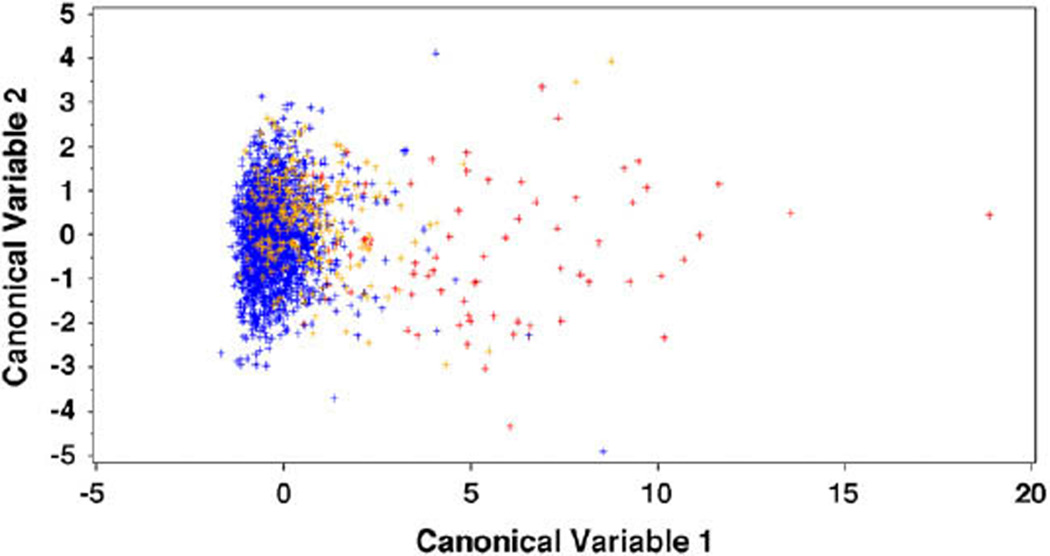
Both canonical correlations were significantly different from 0 (all P < .001). The first canonical variable was highly correlated with I–S and log(KISA), with a mean of −0.28, 0.61, and 4.88 in the normal group, keratoconus-suspect group, and early keratoconus group, respectively. The second canonical variable was more correlated with central K and age, with a mean of −0.04, 0.42, and −0.22, respectively. Figure 2 shows the scatterplot of the 2 canonical variables in all eyes; the scatterplot indicates that the first canonical variable was better at classifying the 3 groups. Therefore, I–S and KISA may be more useful in differentiating between the 3 groups.
Figure 2.
Scatterplot of canonical discriminant analysis (blue = normal group; orange = keratoconus-suspect group; red = early keratoconus group).
DISCUSSION
Using the cross-sectional data and longitudinal data together, we found that (1) there were significant differences in central K, I–S, and KISA values between eyes with early keratoconus, eyes with suspected keratoconus, and clinically normal eyes; (2) progression to keratoconus was significantly different between the 3 groups during the follow-up; and (3) using the videokeratography indices allowed classification of most normal eyes and early keratoconus eyes but the ability to differentiate between keratoconus-suspect eyes and normal eyes was modest. Moreover, I–S and KISA may be more useful in separating the groups.
The use of quantitative videokeratography-derived indices may be a more reproducible way of quantifying keratoconus and its early phenotypes. Some studies12–14 suggest that topographic features might be useful in detecting keratoconus before the development of the slitlamp findings. Several quantitative indices (eg, KPI, KCI%, central K, I–S, and KISA) have been used.12,13 Rabinowitz et al.7 developed 3 indices (central K, I–S, and R versus L) and distinguished eyes with keratoconus from normal eyes in a small preliminary study (28 family members of 5 patients with keratoconus). Their results support that the 3 indices may be descriptors of the earliest stages of keratoconus. However, the sensitivity and accuracy of quantitative indices for studying subclinical changes and early keratoconus must be confirmed by longitudinal studies and serial topographic analysis.
Our preliminary data indicate that the I–S index may be useful in distinguishing cases of suspected keratoconus and early keratoconus from normal eyes.15 To our knowledge, our analysis is the first to identify early keratoconus and suspected keratoconus using a large-scale data set and longitudinal data. Using both cross-sectional data and longitudinal study design, our study provides useful information about the value of 3 quantitative indices: central K, I–S, and KISA. All 3 indices were significantly different between the 3 groups and progressed to keratoconus at different rates. A previous study by our group16 showed that I–S and KISA progressed quicker in the clinically normal fellow eyes of unilateral keratoconus patients and in eyes with the AB/SRAX videokeratography pattern. This is consistent with the results we obtained here, which showed that I–S and KISA can more correctly classify early keratoconus and suspected keratoconus. Although central K was also significantly different between groups in this study, it seems that this index does not have much predictive value. In addition, KISA was calculated proportionally by central K and I–S but did not show an advantage over than I–S in predicting progression. It is hard to distinguish the impact of KISA from that of I–S or other combinations of indices, which may limit our explanation of the use of these indices. We have not exhaustively examined all quantitative indices for early keratoconus detection; however, our studies suggest that all the indices may be valuable adjuncts for identifying the early stages of keratoconus by videokeratography before the case progresses to clinical keratoconus.
Our results and conclusions are based on the data from many eyes and may not be straightforward in an individual case. However, useful information can be gained by estimating the probability distribution of an eye having keratoconus given a value of an index, such as I–S. For example, with an I–S value of 1.0 or greater the probabilities are approximately 20%, 58% and 90% of being normal, keratoconus suspect and early keratoconus cases, respectively. Thus, the predictive value of these indices is guided and may be beneficial to clinicians; however, their use is not recommended to confirm a diagnosis of keratoconus without the appropriate accompanying clinical signs of the disease.
Although we were able to identify early keratoconus using videokeratography indices, the classification rate was modest for suspected keratoconus because of the high variations of the indices. Thus, videokeratography alone might not be sufficient to classify keratoconus subtypes to ultimately define subclinical keratoconus. Preliminary data froma small subset of these keratoconus subtypes suggests that a combination of videokeratography variables and wavefront Zernike polynomials better separate these subgroups than videokeratography alone.15 This is supported by a recent study by Bühren et al.,5 who found that higher-order aberrations can be useful in separating these groups. Combining these 2 technologies to develop more robust criteria for detecting patients with subclinical keratoconus is the subject of current research by our group.
The other limitation of this study was that the sample size was very uneven between the 3 groups, which limited the power of the statistical tests. To estimate the impact of this, we calculated the power by comparing the central K values in the early keratoconus group and the keratoconus-suspect group based on the mean and standard deviation estimated in the sample. The power was 97% for an even sample size and 87% for an uneven sample size. Although it decreased slightly, the power remained high enough (>80%) for testing the difference. Because the normal group had a larger sample size, we expected that the comparisons between that group and the other groups would have higher power.
Although this study clearly has limitations and it is not intended to encourage clinicians to rely solely on indices for diagnostic purposes, we hope that by adopting the classification scheme we outlined (a combination of videokeratography maps and clinical signs) clinicians will be able to more easily recognize eyes with early keratoconus and suspected keratoconus that are at higher risk for refractive surgery. This system may also be helpful in laying the foundation for more clearly defining high-risk suspected keratoconus patterns in the future.
Acknowledgments
Supported by National Eye Institute grant NEI 09052 and the Eye Defects Research Foundation, Inc.
Biography

First author:
Xiaohui Li, MD, MS
Cornea-Genetic Eye Institute and the Medical Genetics Institute, Cedars-Sinai Medical Center, Los Angeles, California, USA
Footnotes
No author has a financial or proprietary interest in any material or method mentioned.
REFERENCES
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Klyce SD. Computer-assisted corneal topography; high-resolution graphic presentation and analysis of keratoscopy. [Accessed May 7, 2009];Inv Ophthalmol Vis Sci. 1984 25:1426–1435. Available at: http://www.iovs.org/cgi/reprint/25/12/1426. [PubMed] [Google Scholar]
- 3.Bafna S, Kohnen T, Koch DD. Axial, instantaneous, and refractive formulas in computerized videokeratography of normal corneas. J Cataract Refract Surg. 1998;24:1184–1190. doi: 10.1016/s0886-3350(98)80009-6. [DOI] [PubMed] [Google Scholar]
- 4.Koch DD, Wakil JS, Samuelson SW, Haft EA. Comparison of the accuracy and reproducibility of the keratometer and the EyeSys Corneal Analysis System Model. J Cataract Refract Surg. 1992;18:342–347. doi: 10.1016/s0886-3350(13)80068-5. [DOI] [PubMed] [Google Scholar]
- 5.Bühren J, Kühne C, Kohnen T. Defining subclinical keratoconus using corneal first-surface higher-order aberrations. Am J Ophthalmol. 2007;143:381–389. doi: 10.1016/j.ajo.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 6.Maguire LJ, Lowry JC. Identifying progression of subclinical keratoconus by serial topography analysis. Am J Ophthalmol. 1991;112:41–45. doi: 10.1016/s0002-9394(14)76210-5. [DOI] [PubMed] [Google Scholar]
- 7.Rabinowitz YS, Garbus J, McDonnell PJ. Computer-assisted corneal topography in family members of patients with keratoconus. Arch Ophthalmol. 1990;108:365–371. doi: 10.1001/archopht.1990.01070050063032. [DOI] [PubMed] [Google Scholar]
- 8.Randleman JB. Post-laser in-situ keratomileusis ectasia: current understanding and future directions. Curr Opin Ophthalmol. 2006;17:406–412. doi: 10.1097/01.icu.0000233963.26628.f0. [DOI] [PubMed] [Google Scholar]
- 9.Binder PS, Lindstrom RL, Stulting RD, Donnenfeld E, Wu H, McDonnell P, Rabinowitz Y. Keratoconus and corneal ectasia after LASIK [letter] J Cataract Refract Surg. 2005;31:2035–2037. doi: 10.1016/j.jcrs.2005.12.002. reply by Donnenfeld E, Wu H, McDonnell P, Rabinowitz Y, 2037–2038. [DOI] [PubMed] [Google Scholar]
- 10.Rabinowitz YS, Yang H, Brickman Y, Akkina J, Riley C, Rotter JI, Elashoff J. Videokeratography database of normal human corneas. Br J Ophthalmol. 1996;80:610–616. doi: 10.1136/bjo.80.7.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasheed K, Rabinowitz YS, Remba D, Remba MJ. Interobserver and intraobserver reliability of a classification scheme for corneal topographic patterns. [Accessed May 7, 2009];Br JOphthalmol. 1998 82:1401–1406. doi: 10.1136/bjo.82.12.1401. Available at: http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1722448&blobtype=pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabinowitz YS, Rasheed K. KISA% index: a quantitative videokeratography algorithm embodying minimal topographic criteria for diagnosing keratoconus. J Cataract Refract Surg. 1999;25:1327–1335. doi: 10.1016/s0886-3350(99)00195-9. erratum 2000; 26:480. [DOI] [PubMed] [Google Scholar]
- 13.Maeda N, Klyce SD, Smolek MK, Thompson HW. Automated keratoconus screening with corneal topography analysis. [Accessed May 7, 2009];Invest Ophthalmol Vis Sci. 1994 35:2749–2757. Available at: http://www.iovs.org/cgi/reprint/35/6/2749.pdf. [PubMed] [Google Scholar]
- 14.Maguire LJ, Bourne WM. Corneal topography of early keratoconus. Am J Ophthalmol. 1989;108:107–112. doi: 10.1016/0002-9394(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 15.Jafri B, Li X, Yang H, Rabinowitz YS. Higher order wavefront aberrations and topography in early and suspected keratoconus. J Refract Surg. 2007;23:774–781. doi: 10.3928/1081-597X-20071001-06. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Rabinowitz YS, Rasheed K, Yang H. Longitudinal study of the normal eyes in unilateral keratoconus patients. Ophthalmology. 2004;111:440–446. doi: 10.1016/j.ophtha.2003.06.020. [DOI] [PubMed] [Google Scholar]