Abstract
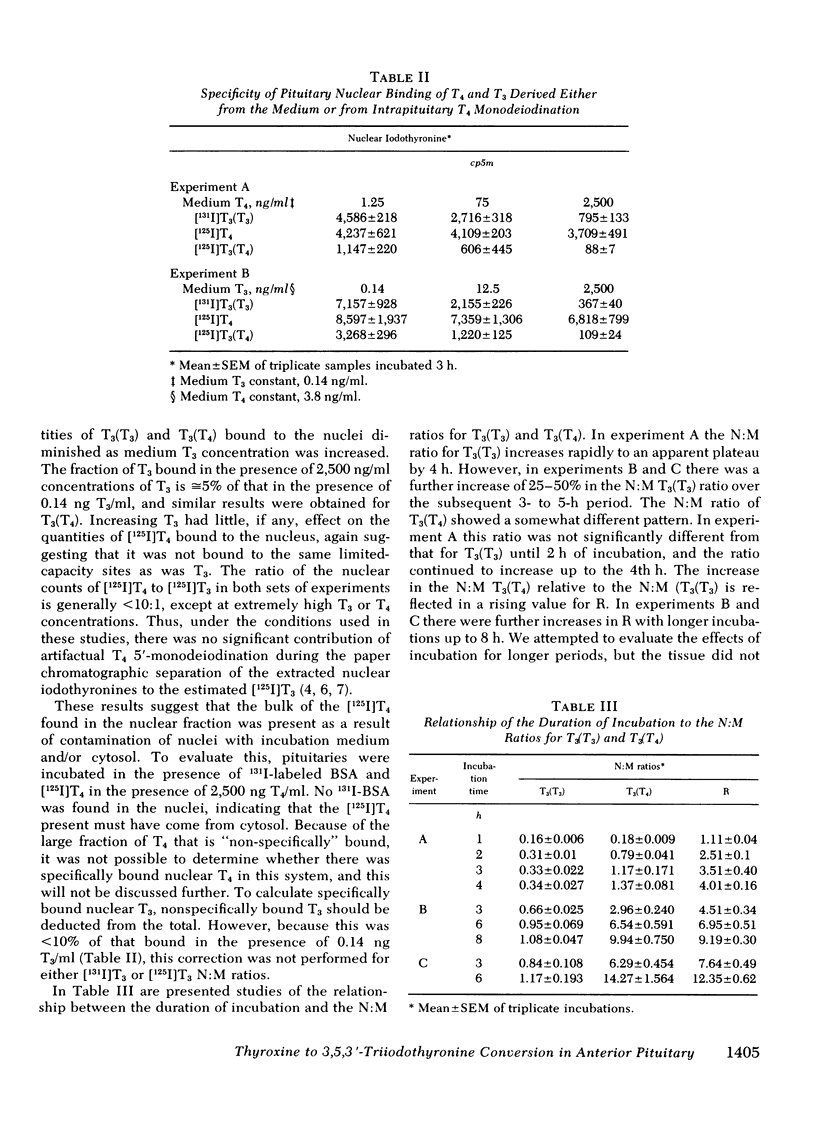
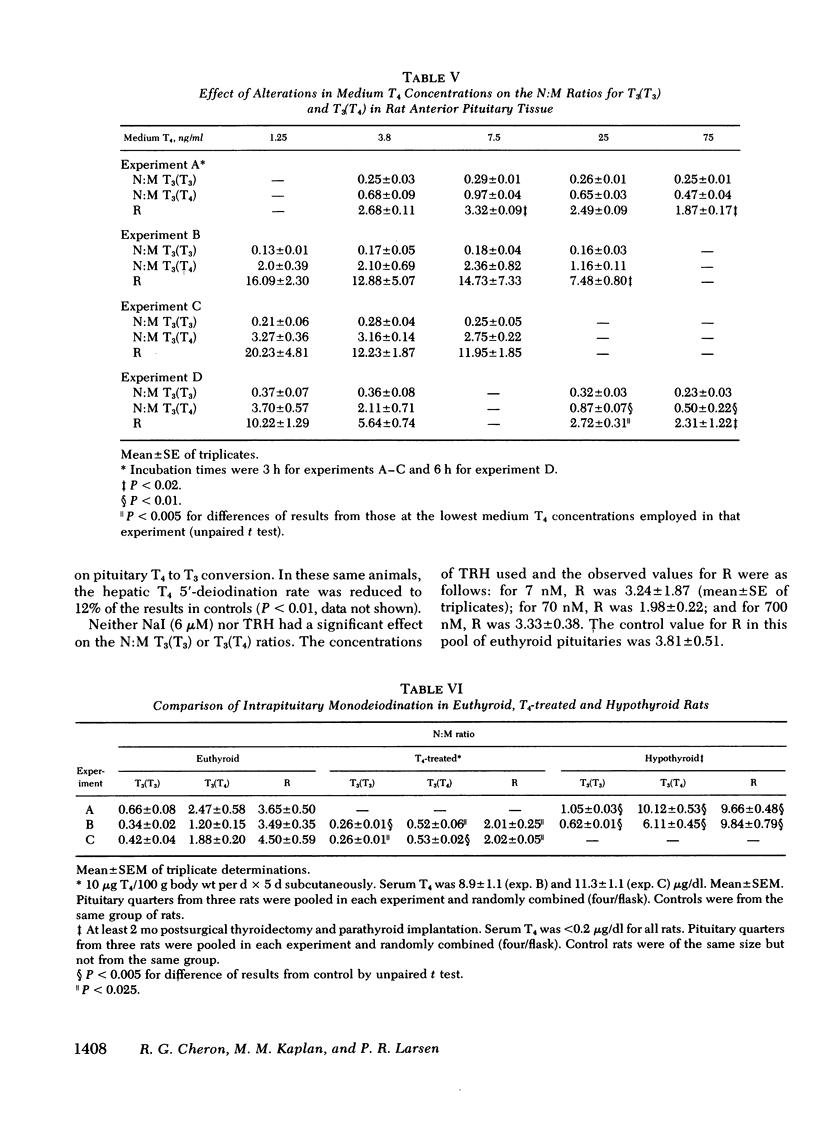
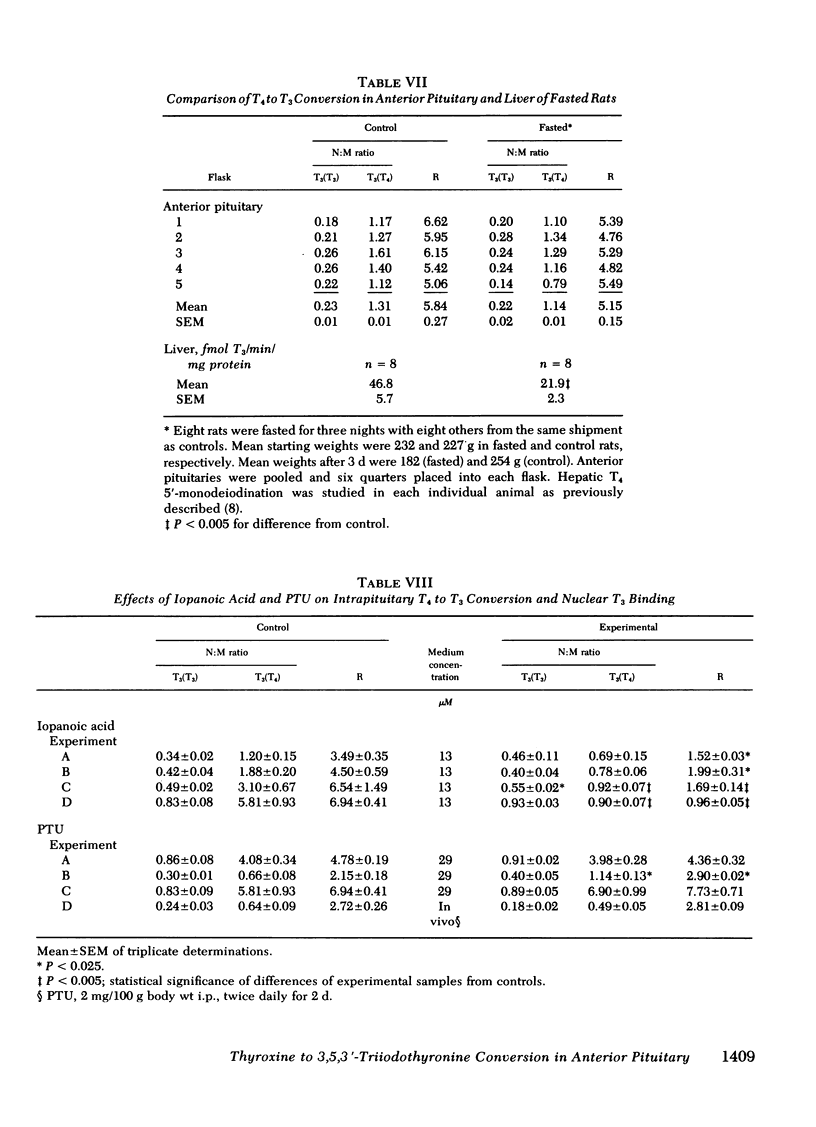
Our recent in vivo studies have suggested that intrapituitary l-thyroxine (T4) to 3,5,3′-triiodo-l-thyronine (T3) conversion with subsequent nuclear binding of T3 is an important pathway by which circulating T4 can inhibit thyrotropin release. The present studies were performed to evaluate various physiological and pharmacological influences on these two processes in rat anterior pituitary tissue. Intact pituitary fragments were incubated in buffer—1% bovine serum albumin containing 0.14 ng/ml [131I]T3 and 3.8 ng/ml [125I]T4. Nuclei were isolated after 3 h of incubation and the bound iodothyronines identified by paper chromatography. There was 0.3-1% [125I]T3 contaminating the medium [125I]T4, and this did not change during incubation. Nuclear [125I]T4 was not decreased by 650-fold excesses of medium T3 or T4, suggesting that it was nonspecifically bound. The ratio of nuclear to medium [131I]- and [125I]T3 were expressed as nuclear counts per minute per milligram wet weight of tissue:counts per minute per microliter medium. Intrapituitary T4 to T3 conversion was evidenced by the fact that the nuclear:medium (N:M) ratio for [131I]T3 was 0.45±0.21, whereas that for [125I]T3 was 2.23±1.28 (mean±SD, n = 51). A ratio (R), the N:M [125I]T3 divided by the N:M [131I]T3, was used as an index of intrapituitary T4 to T3 conversion. Increasing medium T3 concentrations up to 50 ng/ml caused a progressive decrease in the N:M ratio for both T3 isotopes, but no change in the value for R, indicating that both competed for the same limited-capacity nuclear receptors. Increasing concentrations of medium T4 caused no change in the N:M [131I]T3 but did cause a significant decrease in R in three of four experiments. These results suggest saturation of T4-5′-monodeiodination occurred at lower T4 concentrations than saturation of nuclear T3 binding sites. In hypothyroid rats, the N:M ratios for both [131I]T3 and [125I]T3 were increased (P < 0.005), but R was three-fold higher than in controls (P < 0.005). Animals given 10 μg T4/100 g body wt per d for 5 d had significantly decreased N:M ratios for both [131I]T3 and [125I]T3, as well as a decreased value for R. In fasted rats, neither N:M ratio was depressed, although hepatic T4 to T3 conversion in the same animals was 50% of control (P < 0.005). Iopanoic acid (13 μM), but not 6-n-propylthiouracil (29 μM), decreased the N:M [125I]T3 with a significant decrease in the value for R (P < 0.025 or less). Neither sodium iodide (6 μM) nor thyrotropin-releasing hormone (7-700 nM) affected the T3 N:M ratios. These results indicate that intrapituitary T4 to T3 conversion is stimulated in hypothyroidism and depressed in T4-treated animals, whereas opposite changes occur in hepatic T4-5′-monodeiodination. Unlike liver, anterior pituitary T4-5′-monodeiodination is not affected by fasting or incubation with 6-n-propyl-2-thiouracil, but T4 to T3 conversion is inhibited in both by iopanoic acid. These results indicate that there are important differences between anterior pituitary and other tissues in the regulation of T4-5′-monodeiodination.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams G. M., Larsen P. R. Triiodothyronine and thyroxine in the serum and thyroid glands of iodine-deficient rats. J Clin Invest. 1973 Oct;52(10):2522–2531. doi: 10.1172/JCI107443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi F. Effect of dietary composition on fasting-induced changes in serum thyroid hormones and thyrotropin. Metabolism. 1978 Aug;27(8):935–942. doi: 10.1016/0026-0495(78)90137-3. [DOI] [PubMed] [Google Scholar]
- Balsam A., Ingbar S. H. The influence of fasting, diabetes, and several pharmacological agents on the pathways of thyroxine metabolism in rat liver. J Clin Invest. 1978 Aug;62(2):415–424. doi: 10.1172/JCI109143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. A., Kurcz M., Marshall S., Meites J. Effects of starvation in rats on serum levels of follicle stimulating hormone, luteinizing hormone, thyrotropin, growth hormone and prolactin; response to LH-releasing hormone and thyrotropin-releasing hormone. Endocrinology. 1977 Feb;100(2):580–587. doi: 10.1210/endo-100-2-580. [DOI] [PubMed] [Google Scholar]
- Carlson H. E., Drenick E. J., Chopra I. J., Hershman J. M. Alterations in basal and TRH-stimulated serum levels of thyrotropin, prolactin, and thyroid hormones in starved obese men. J Clin Endocrinol Metab. 1977 Oct;45(4):707–713. doi: 10.1210/jcem-45-4-707. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. A study of extrathyroidal conversion of thyroxine (T4) to 3,3',5-triiodothyronine (T3) in vitro. Endocrinology. 1977 Aug;101(2):453–463. doi: 10.1210/endo-101-2-453. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Carlson H. E., Solomon D. H. Comparison of inhibitory effects of 3,5,3'-triiodothyronine (T3), thyroxine (T4), 3,3,',5'-triiodothyronine (rT3), and 3,3'-diiodothyronine (T2) on thyrotropin-releasing hormone-induced release of thyrotropin in the rat in vitro. Endocrinology. 1978 Aug;103(2):393–402. doi: 10.1210/endo-103-2-393. [DOI] [PubMed] [Google Scholar]
- Conversion of thyroxine into tri-iodothyronine by rat liver homogenate. Biochem J. 1975 Sep;150(3):489–493. doi: 10.1042/bj1500489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxson M. S., Hall T. D., Kletzky O. A., Jaramillo J. E., Nicoloff J. T. Decreased serum thyrotropin induced by fasting. J Clin Endocrinol Metab. 1977 Sep;45(3):560–568. doi: 10.1210/jcem-45-3-560. [DOI] [PubMed] [Google Scholar]
- Frumess R. D., Larsen P. R. Correlation of serum triiodothyronine (T3) and thyroxine (T4) with biologic effects of thyroid hormone replacement in propylthiouracil-treated rats. Metabolism. 1975 Apr;24(4):547–554. doi: 10.1016/0026-0495(75)90079-7. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C. Regulation of thyrotropin production by mouse pituitary thyrotropic tumor cells in vitro by physiological levels of thyroid hormones. Endocrinology. 1978 Apr;102(4):1122–1128. doi: 10.1210/endo-102-4-1122. [DOI] [PubMed] [Google Scholar]
- Gordon A., Spira O. Triiodothyronine binding in rat anterior pituitary, posterior pituitary, median eminence and brain. Endocrinology. 1975 Jun;96(6):1357–1365. doi: 10.1210/endo-96-6-1357. [DOI] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Vagenakis A. G., Braverman L. E. Effect of starvation, nutriment replacement, and hypothyroidism on in vitro hepatic T4 to T3 conversion in the rat. Metabolism. 1978 Nov;27(11):1680–1690. doi: 10.1016/0026-0495(78)90290-1. [DOI] [PubMed] [Google Scholar]
- Hillier A. P. Deiodination of thyroid hormones by the perfused rat liver. J Physiol. 1972 Apr;222(2):475–485. doi: 10.1113/jphysiol.1972.sp009809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M. Subcellular alterations causing reduced hepatic thyroxine-5'-monodeiodinase activity in fasted fats. Endocrinology. 1979 Jan;104(1):58–64. doi: 10.1210/endo-104-1-58. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in liver and kidney homogenates from hyperthyroid and hypothyroid rats. Endocrinology. 1978 Jul;103(1):156–161. doi: 10.1210/endo-103-1-156. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in rat liver homogenates. J Clin Invest. 1978 Feb;61(2):459–471. doi: 10.1172/JCI108957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVEY H. A., ROBERTS S. Influence of thyroid function on the metabolism of the anterior pituitary gland. Am J Physiol. 1957 Apr;189(1):86–90. doi: 10.1152/ajplegacy.1957.189.1.86. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Dick T. E., Markovitz B. P., Kaplan M. M., Gard T. G. Inhibition of intrapituitary thyroxine to 3.5.3'-triiodothyronine conversion prevents the acute suppression of thyrotropin release by thyroxine in hypothyroid rats. J Clin Invest. 1979 Jul;64(1):117–128. doi: 10.1172/JCI109430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R. Direct immunoassay of triiodothyronine in human serum. J Clin Invest. 1972 Aug;51(8):1939–1949. doi: 10.1172/JCI107000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R., Dockalova J., Sipula D., Wu F. M. Immunoassay of thyroxine in unextracted human serum. J Clin Endocrinol Metab. 1973 Aug;37(2):177–182. doi: 10.1210/jcem-37-2-177. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Frumess R. D. Comparison of the biological effects of thyroxine and triiodothyronine in the rat. Endocrinology. 1977 Apr;100(4):980–988. doi: 10.1210/endo-100-4-980. [DOI] [PubMed] [Google Scholar]
- Larsen P. R. Technical aspects of the estimation of triiodothyronine in human serum: evidence of conversion of thyroxine to triiodothyronine during assay. Metabolism. 1971 Jun;20(6):609–624. doi: 10.1016/0026-0495(71)90009-6. [DOI] [PubMed] [Google Scholar]
- OPPENHEIMER J. H., SQUEF R., SURKS M. I., HAUER H. BINDING OF THYROXINE BY SERUM PROTEINS EVALUATED BY EQUILIBRUM DIALYSIS AND ELECTROPHORETIC TECHNIQUES. ALTERATIONS IN NONTHYROIDAL ILLNESS. J Clin Invest. 1963 Nov;42:1769–1782. doi: 10.1172/JCI104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWENS C. W., BELCHER R. V. A COLORIMETRIC MICRO-METHOD FOR THE DETERMINATION OF GLUTATHIONE. Biochem J. 1965 Mar;94:705–711. doi: 10.1042/bj0940705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Koerner D., Surks M. I. Limited binding capacity sites for L-triiodothyronine in rat liver nuclei. Nuclear-cytoplasmic interrelation, binding constants, and cross-reactivity with L-thyroxine. J Clin Invest. 1974 Mar;53(3):768–777. doi: 10.1172/JCI107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- Portnay G. I., O'Brian J. T., Bush J., Vagenakis A. G., Azizi F., Arky R. A., Ingbar S. H., Braverman L. E. The effect of starvation on the concentration and binding of thyroxine and triiodothyronine in serum and on the response to TRH. J Clin Endocrinol Metab. 1974 Jul;39(1):191–194. doi: 10.1210/jcem-39-1-191. [DOI] [PubMed] [Google Scholar]
- Reichlin S., Volpert E. M., Werner S. C. Hypothalamic influence of thyroxine monodeiodination by rat anterior pituitary gland. Endocrinology. 1966 Feb;78(2):302–306. doi: 10.1210/endo-78-2-302. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Shapiro L. E. Dose-dependent depletion of nuclear receptors by L-triiodothyronine: evidence for a role in induction of growth hormone synthesis in cultured GH1 cells. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3877–3881. doi: 10.1073/pnas.73.11.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action in cell culture: domonstration of nuclear receptors in intact cells and isolated nuclei. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3488–3492. doi: 10.1073/pnas.70.12.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadlow A. R., Surks M. I., Schwartz H. L., Oppenheimer J. H. Specific triiodothyronine binding sites in the anterior pituitary of the rat. Science. 1972 Jun 16;176(4040):1252–1254. doi: 10.1126/science.176.4040.1252. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Dick T. E., Larsen P. R. The contribution of local tissue thyroxine monodeiodination to the nuclear 3,5,3'-triiodothyronine in pituitary, liver, and kidney of euthyroid rats. Endocrinology. 1978 Oct;103(4):1196–1207. doi: 10.1210/endo-103-4-1196. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Kaplan M. M., Cheron R. G., Dick T. E., Larsen P. R. Thyroxine to 3,5,3'-triiodothyronine conversion by rat anterior pituitary and liver. Metabolism. 1978 Nov;27(11):1601–1607. doi: 10.1016/0026-0495(78)90282-2. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest. 1978 May;61(5):1247–1259. doi: 10.1172/JCI109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Pituitary nuclear 3,5,3'-triiodothyronine and thyrotropin secretion: an explanation for the effect of thyroxine. Science. 1977 Nov 11;198(4317):617–620. doi: 10.1126/science.199941. [DOI] [PubMed] [Google Scholar]
- Surks M. I., DeFesi C. R. Determination of the cell number of each cell type in the anterior pituitary of euthyroid and hypothyroid rats. Endocrinology. 1977 Sep;101(3):946–958. doi: 10.1210/endo-101-3-946. [DOI] [PubMed] [Google Scholar]
- Vinik A. I., Kalk W. J., McLaren H., Hendricks S., Pimstone B. L. Fasting blunts the TSH response to synthetic thyrotropin-releasing hormone (TRH). J Clin Endocrinol Metab. 1975 Mar;40(3):509–511. doi: 10.1210/jcem-40-3-509. [DOI] [PubMed] [Google Scholar]
- Visser T. J. A tentative review of recent in vitro observations of the enzymatic deiodination of iodothyronines and its possible physiological implications. Mol Cell Endocrinol. 1978 May;10(3):241–247. doi: 10.1016/0303-7207(78)90039-4. [DOI] [PubMed] [Google Scholar]
- Weeke J., Orskov H. Synthesis of 125I monolabelled 3, 5, 3'-triiodothyronine and thyroxine of maximum specific activity for radioimmunoassay. Scand J Clin Lab Invest. 1973 Dec;32(4):357–360. doi: 10.3109/00365517309084359. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. J., Izumi M., Larsen P. R. Isolation of labeled triiodothyronine from serum using affinity chromatography: application to the extimation of the peripheral T4 to T3 conversion in rats. Metabolism. 1978 Mar;27(3):303–313. doi: 10.1016/0026-0495(78)90110-5. [DOI] [PubMed] [Google Scholar]


