Abstract
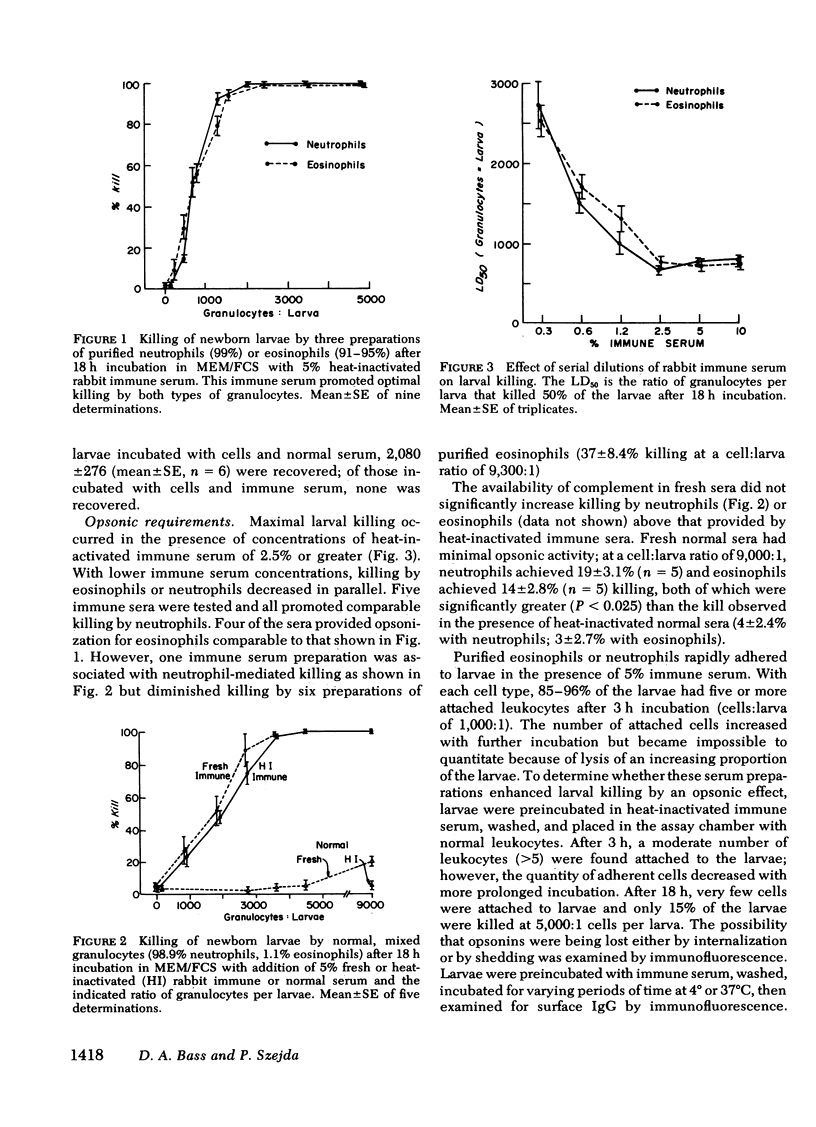
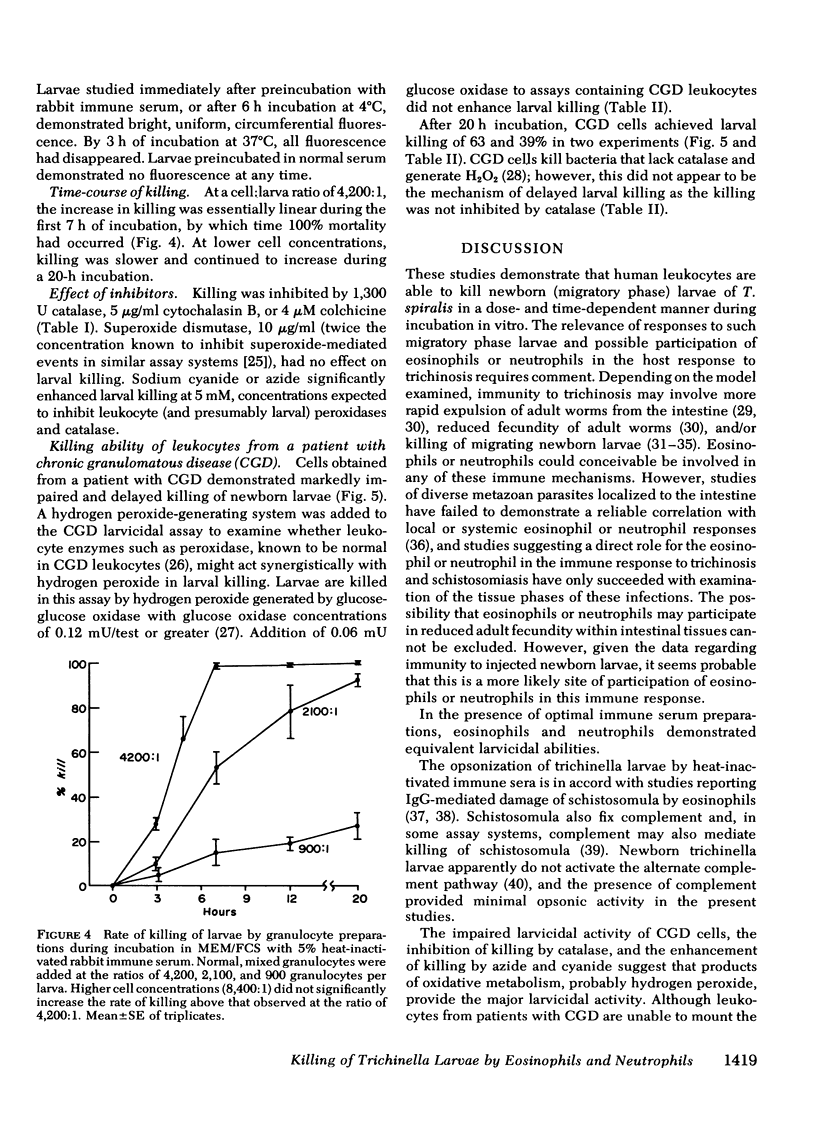
Eosinophil leukocytes have been reported to have a major role in host defense against invasive, migratory phases of helminth infestations, yet the relative larvicidal abilities of eosinophils and neutrophils have not been thoroughly examined. This study examined the killing of newborn (migratory phase) larvae of Trichinella spiralis during incubation by human granulocytes in vitro. The assay employed cultue of larvae with cells, sera, and reagents in microtiter wells with direct counting of surviving larvae after incubation. Killed larvae appeared to be lysed. Verification of the microplate assay was obtained by demonstrating complete loss of infectivity of larvae incubated with leukocytes and immune serum. In the presence of optimal immune serum concentrations, purified neutrophils or eosinophils achieved ≥95% killing of larvae at cell:larva ratios of 2,000:1 or greater. Fresh normal serum prompted slight (19%) killing by leukocytes at a cell:larva ratio of 9,000:1. Cells plus heat-inactivated normal serum and all sera preparations in the absence of leukocytes killed <8% of the larvae. The activity of immune serum was opsonic. Cells adhered to larvae that had been preincubated in immune serum, and immunofluorescent studies indicated that such preopsonized larvae were coated with immunoglobulin (Ig)G. However, preopsonized larvae lost opsonic activity and surface IgG during incubation for 3 h in medium lacking immune serum.
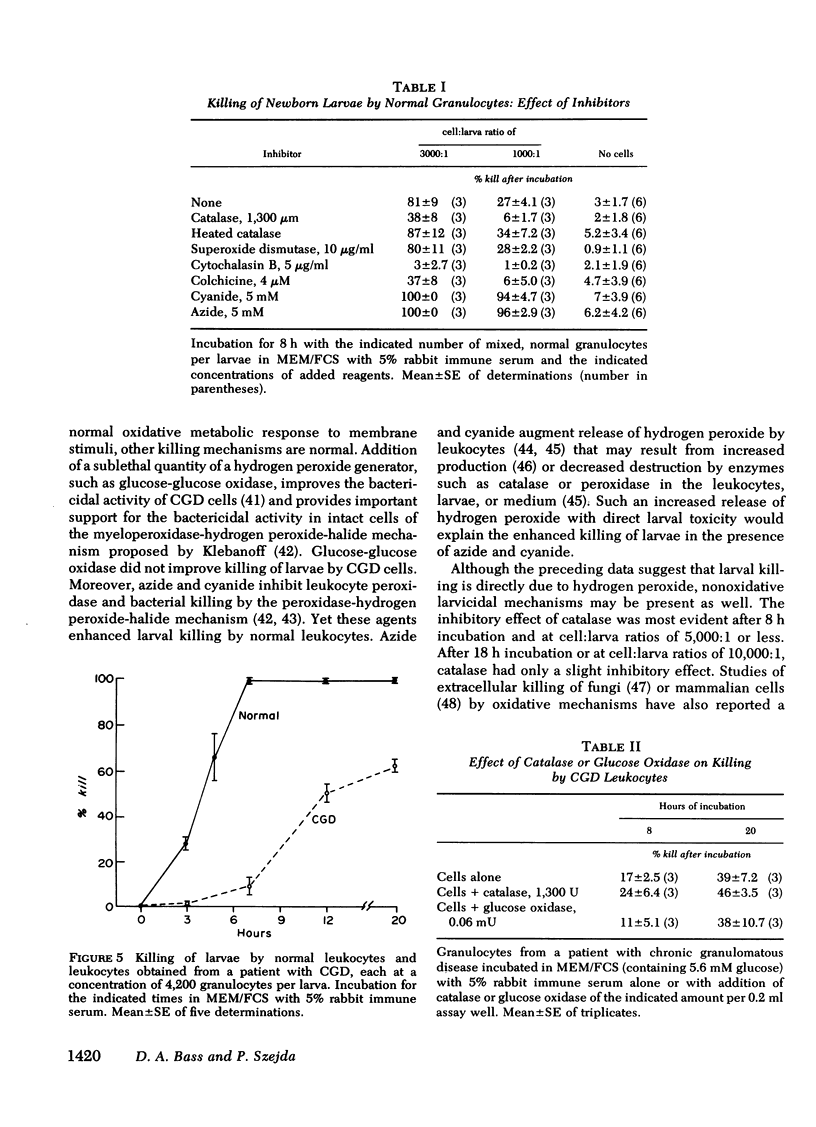
The rate of killing was dependent on the cell:larva ratio; at high leukocyte concentrations (4,200:1), 99% were killed within 7 h; at lower cell:larva ratios, killing increased steadily during a 20-h incubation period. Killing was inhibited by 20 μg catalase, 5 μg/ml cytochalasin B, or 5μM colchicine, but was unchanged by superoxide dismutase and was enhanced by azide or cyanide. Leukocytes from a patient with chronic granulomatous disease, lacking ability to mount a normal oxidative response, demonstrated a markedly suppressed larvicidal effect.
The data indicate that neutrophils are at least as effective as eosinophils in the killing of newborn larvae of T. spiralis. The killing appeared to be mediated by the oxidative metabolic burst with its generation of hydrogen peroxide.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYUM A. SEPARATION OF WHITE BLOOD CELLS. Nature. 1964 Nov 21;204:793–794. doi: 10.1038/204793a0. [DOI] [PubMed] [Google Scholar]
- Basten A., Beeson P. B. Mechanism of eosinophilia. II. Role of the lymphocyte. J Exp Med. 1970 Jun 1;131(6):1288–1305. doi: 10.1084/jem.131.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogitsh B. J. Schistosoma mansoni: cytochemistry of eosinophils in egg-caused early hepatic granulomas of mice. Exp Parasitol. 1971 Jun;29(3):493–500. doi: 10.1016/0014-4894(71)90058-0. [DOI] [PubMed] [Google Scholar]
- Boyer M. H., Spry C. J., Beeson P. B., Sheldon W. H. Mechanism of eosinophilia. IV. The pulmonary lesion resulting from intravenous injection of Trichinella spiralis. Yale J Biol Med. 1971 Jun;43(6):351–357. [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., David J. R., Franks D., Mahmoud A. A., David P. H., Sturrock R. F., Houba V. Antibody-dependent eosinophil-mediated damage to 51Cr-labeled schistosomula of Schistosoma mansoni: damage by purieid eosinophils. J Exp Med. 1977 Jan 1;145(1):136–150. doi: 10.1084/jem.145.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., Remold H. G., Houba V., David J. R., Franks D., David P. H., Sturrock R. F. Antibody-dependent eosinophil-mediated damage to 51Cr-labeled schistosomula of Schistosoma mansoni: mediation by IgG, and inhibition by antigen-antibody complexes. J Immunol. 1977 Jun;118(6):2230–2236. [PubMed] [Google Scholar]
- Butterworth A. E., Sturrock R. F., Houba V., Mahmoud A. A., Sher A., Rees P. H. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975 Aug 28;256(5520):727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Capron M., Bazin H. Specific IgE antibodies in immune adherence of normal macrophages to Schistosoma mansoni schistosomules. Nature. 1975 Feb 6;253(5491):474–475. doi: 10.1038/253474a0. [DOI] [PubMed] [Google Scholar]
- Capron M., Capron A., Torpier G., Bazin H., Bout D., Joseph M. Eosinophil-dependent cytotoxicity in rat schistosomiasis. Involvement of IgG2a antibody and role of mast cells. Eur J Immunol. 1978 Feb;8(2):127–133. doi: 10.1002/eji.1830080211. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J., Einstein A. B., Fefer A. Peroxidase-H2O2-halide system: Cytotoxic effect on mammalian tumor cells. Blood. 1975 Feb;45(2):161–170. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975 Jun 1;141(6):1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Role of the myeloperoxidase-H2O2-halide system in concanavalin A-induced tumor cell killing by human neutrophils. J Immunol. 1979 Jun;122(6):2605–2610. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Studies on the mechanism of antibody-dependent polymorphonuclear leukocyte-mediated cytotoxicity. J Immunol. 1977 Oct;119(4):1413–1418. [PubMed] [Google Scholar]
- Clark R. A., Olsson I., Klebanoff S. J. Cytotoxicity for tumor cells of cationic proteins from human neutrophil granules. J Cell Biol. 1976 Sep;70(3):719–723. doi: 10.1083/jcb.70.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley D. G., Savage A. M., Lewis F. A. Host responses induced and elicited by cercariae, schistosomula, and cercarial antigenic preparations. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):88–95. doi: 10.4269/ajtmh.1977.26.88. [DOI] [PubMed] [Google Scholar]
- Daimond R. D., Krzesicki R. Mechanisms of attachment of neutrophils to Candida albicans pseudohyphae in the absence of serum, and of subsequent damage to pseudohyphae by microbicidal processes of neutrophils in vitro. J Clin Invest. 1978 Feb;61(2):360–369. doi: 10.1172/JCI108946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., McPhail L. C., Shirley P. S. Effect of cyanide on NADPH oxidation by granules from human polymorphonuclear leukocytes. Blood. 1977 Mar;49(3):445–454. [PubMed] [Google Scholar]
- Dean D. A., Wistar R., Murrell K. D. Combined in vitro effects of rat antibody and neutrophilic leukocytes on schistosomula of Schistosoma mansoni. Am J Trop Med Hyg. 1974 May;23(3):420–428. doi: 10.4269/ajtmh.1974.23.420. [DOI] [PubMed] [Google Scholar]
- Dennis D. T., Despommier D. D., Davis N. Infectivity of the newborn larva of Trichinella spiralis in the rat. J Parasitol. 1970 Oct;56(5):974–977. [PubMed] [Google Scholar]
- Despommier D. D., Campbell W. C., Blair L. S. The in vivo and in vitro analysis of immunity to Trichinella spiralis in mice and rats. Parasitology. 1977 Feb;74(1):109–119. doi: 10.1017/s0031182000047570. [DOI] [PubMed] [Google Scholar]
- Despommier D. Immunity to Trichinella spiralis. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):68–75. doi: 10.4269/ajtmh.1977.26.68. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Peroxidase-mediated mammalian cell cytotoxicity. J Exp Med. 1973 Jul 1;138(1):318–323. doi: 10.1084/jem.138.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove D. I., Civil R. H. Trichinella spiralis: effects on the host-parasite relationship in mice of BCG (attenuated Mycobacterium bovis). Exp Parasitol. 1978 Apr;44(2):181–189. doi: 10.1016/0014-4894(78)90096-6. [DOI] [PubMed] [Google Scholar]
- Grove D. I., Hamburger J., Warren K. S. Kinetics of immunological responses, resistance to reinfection, and pathological reactions to infection with Trichinella spiralis. J Infect Dis. 1977 Oct;136(4):562–570. doi: 10.1093/infdis/136.4.562. [DOI] [PubMed] [Google Scholar]
- Grove D. I., Mahmoud A. A., Warren K. S. Eosinophils and resistance to Trichinella spiralis. J Exp Med. 1977 Mar 1;145(3):755–759. doi: 10.1084/jem.145.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover W. H., Winkler H. H., Normansell D. E. Phagocytic properties of isolated human eosinophils. J Immunol. 1978 Aug;121(2):718–725. [PubMed] [Google Scholar]
- Hsü S. Y., Hsü H. F., Isacson P., Cheng H. F. In vitro schistosomulicidal effect of immune serum and eosinophils, neutrophils and lymphocytes. J Reticuloendothel Soc. 1977 Mar;21(3):153–162. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Baehner R. L. Improvement of leukocyte bactericidal activity in chronic granulomatous disease. Blood. 1970 Mar;35(3):350–355. [PubMed] [Google Scholar]
- Jones C. E., Rubin R. Nematospiroides dubius: mechanisms of host immunity. I. Parasite counts, histopathology, and serum transfer involving orally or subcutaneously sensitized mice. Exp Parasitol. 1974 Jun;35(3):434–452. doi: 10.1016/0014-4894(74)90050-2. [DOI] [PubMed] [Google Scholar]
- Kaplan E. L., Laxdal T., Quie P. G. Studies of polymorphonuclear leukocytes from patients with chronic granulomatous disease of childhood: bactericidal capacity for streptococci. Pediatrics. 1968 Mar;41(3):591–599. [PubMed] [Google Scholar]
- Kassis A. I., Aikawa M., Mahmoud A. F. Mouse antibody-dependent eosinophil and macrophage adherence and damage to schistosomula of Schistosoma mansoni. J Immunol. 1979 Feb;122(2):398–405. [PubMed] [Google Scholar]
- Kazura J. W., Grove D. I. Stage-specific antibody-dependent eosinophil-mediated destruction of Trichinella spiralis. Nature. 1978 Aug 10;274(5671):588–589. doi: 10.1038/274588a0. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970 Sep 11;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1974 Jun 25;249(12):3724–3728. [PubMed] [Google Scholar]
- Lichtenberg F., Sher A., Gibbons N., Doughty B. L. Eosinophil-enriched inflammatory response to schistosomula in the skin of mice immune to Schistosoma mansoni. Am J Pathol. 1976 Sep;84(3):479–500. [PMC free article] [PubMed] [Google Scholar]
- Mackenzie C. D., Preston P. M., Ogilvie B. M. Immunological properties of the surface of parasitic nematodes. Nature. 1978 Dec 21;276(5690):826–828. doi: 10.1038/276826a0. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S., Peters P. A. A role for the eosinophil in acquired resistance to Schistosoma mansoni infection as determined by antieosinophil serum. J Exp Med. 1975 Oct 1;142(4):805–813. doi: 10.1084/jem.142.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Brukner L. H., Silverstein S. C., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. I. Pharmacologic triggering of effector cells and the release of hydrogen peroxide. J Exp Med. 1979 Jan 1;149(1):84–99. doi: 10.1084/jem.149.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegram P. S., Jr, DeChatelet L. R., McCall C. E. Comparison of myeloperoxidase activity in leukocytes from normal subjects and patients with chronic granulomatous disease. J Infect Dis. 1978 Nov;138(5):699–702. doi: 10.1093/infdis/138.5.699. [DOI] [PubMed] [Google Scholar]
- Perrudet-Badoux A., Binaghi R. A. Immunity against newborn Trichinella spiralis larvae in previously infected mice. J Parasitol. 1978 Feb;64(1):187–189. [PubMed] [Google Scholar]
- Ramalho-Pinto F. J., McLaren D. J., Smithers S. R. Complement-mediated killing of schistosomula of Schistosoma mansoni by rat eosinophils in vitro. J Exp Med. 1978 Jan 1;147(1):147–156. doi: 10.1084/jem.147.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J. A. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J Clin Invest. 1977 Dec;60(6):1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg E. J., Steerenberg P. A. Immunogenicity of the parenteral stages of Trichinella spiralis. J Parasitol. 1976 Feb;62(1):164–166. [PubMed] [Google Scholar]
- Specht D., Widmer E. A. Response of mouse liver to infection with tetrathyridia of Mesocestoides (Cestoda). J Parasitol. 1972 Jun;58(3):431–437. [PubMed] [Google Scholar]
- Vadas M. A., David J. R., Butterworth A., Pisani N. T., Siongok T. A. A new method for the purification of human eosinophils and neutrophils, and a comparison of the ability of these cells to damage schistosomula of Schistosoma mansoni. J Immunol. 1979 Apr;122(4):1228–1236. [PubMed] [Google Scholar]
- Zatti M., Rossi F., Patriarca P. The H2O2-production by polymorphonuclear leukocytes during phagocytosis. Experientia. 1968 Jul 15;24(7):669–670. doi: 10.1007/BF02138302. [DOI] [PubMed] [Google Scholar]
- von Lichtenberg F., Sher A., McIntyre S. A lung model of schistosome immunity in mice. Am J Pathol. 1977 Apr;87(1):105–123. [PMC free article] [PubMed] [Google Scholar]


