Abstract
There have been encouraging recent successes in the development of safe and effective topical microbicides to prevent vaginal or rectal HIV-1 transmission, based on the use of anti-retroviral drugs. However, much work remains to be accomplished before a microbicide becomes a standard element of prevention science strategies. Animal models should continue to play an important role in pre-clinical testing, with emphasis on safety, pharmacokinetic and efficacy testing.
Introduction
Ideally, microbicide candidates should be thoroughly evaluated in vitro and in animal model testing systems before proceeding to human clinical trials. This goal has not always been accomplished, sometimes with serious consequences [1]. Animal models are generally valuable for drug development, but they are particularly important for testing prevention and therapeutic strategies against infection by HIV-1 and other sexually transmitted pathogens. The vaginal and rectal transmission of HIV-1 involves a complex, but still poorly understood, sequence of events involving viral attachment, cellular infection, and local amplification in a variety of target cells and tissues that are intimately associated with the blood and lymph systems. This level of biological complexity simply cannot be mimicked in cell or tissue culture models. Moreover, the specific receptor proteins that HIV-1 uses for attachment and entry are found only on the cells of a subset of primate species, so only these animals and/or specifically humanized mice can be used for testing the efficacy of vaginal and rectal microbicides. An additional concern is safety: Some compounds that are effective against HIV-1 infection in vitro can have cumulative toxicities, and may even have enhanced HIV-1 transmission rates when they were tested in human clinical trials [1,2]. Furthermore, the repeated exposure to a compound, alone or in combination with HIV-1, may induce inflammation in the vagina, leading to the recruitment of viral target cells into the mucosa. The latter phenomenon could increase the risk of infection, particularly in the period immediately after microbicide use is discontinued [1,3]. Effects like these may not be identifiable in single dose testing/challenge studies, so repeated exposure to the compounds should now be elements of a comprehensive pre-clinical safety and efficacy assessment for microbicide candidates. Animal models can also be used to acquire valuable information on the pharmacokinetics of vaginally- or rectally-delivered compounds, including the longevity of protective effects [4]. Information like this can guide the design of longer-lasting formulations that may become coitally-independent microbicides, i.e., ones that do not need to applied only a short time before intercourse [4,5]. Overall, animal models should be an essential element of the protocols used for selecting the safest and most effective microbicide candidates for large scale testing in women. Here, we review the currently most useful animal models that can be used for this purpose.
Small animal models; rodents and rabbits
Rodents are only distantly related to humans, and for various reasons their cells are non-permissive for the replication of HIV-1. Although rats and mice, and also rabbits, are certainly useful for preclinical safety screening of microbicide candidates, their reproductive tissues and cycles differ both anatomically and physiologically from those of humans or primates. Hence vaginal safety studies need to be evaluated with caution. In cycling women and nonhuman primates (NHPs), the vaginal epithelium is fully lined by layers of squamous epithelial cells that vary in thickness and cell-numbers during the menstrual cycle. In contrast, rodents have estrous, rather than menstrual, cycles meaning that they absorb, rather than shed, their endometria if pregnancy does not occur. Rodents also undergo anestrous periods in which they do not cycle; and during certain phases of the murine estrous cycle, columnar epithelial cells appear in the vaginal epithelium [6]. Similarly, portions of the rabbit vagina are lined by columnar epithelial cells [7], which may differ from human tissues in their inflammatory response to viruses or microbicide candidates. Nonetheless, if used correctly, rodents and rabbits are useful for a basic level of pre-clinical safety screening aimed at eliminating tissue-damaging or grossly inflammatory compounds from further consideration. In fact, since FDA approval requires testing in two different species, rabbit vaginal irritation tests are usually performed to assess safety of microbicides candidates prior to entering human trials [8], even though these animals are not infectable by HIV-1.
Early attempts to engineer rodents to become more susceptible to HIV-1 infection were not particularly successful [9]. Severe combined immune deficiency (SCID) mice, and mice transplanted with various human cells or tissues, have long been used in HIV-1 research, but the limited reconstitution and distribution of relevant viral target cells into mucosal tissues has limited their value to the microbicide field [9]. For example, the vaginal infection rates in SCID-Hu mice reconstituted with human peripheral blood lymphocytes were too variable to be reliable, due to the low levels of human cells populating the vaginal mucosa [10,11]. As peripheral blood lymphocytes differ from those of mucosal tissues in respect of phenotype, function and homing properties, it was not surprising that such models had serious limitations for studying mucosal biology and immunology.
To overcome some of the above problems, novel strains of mice have now been engineered by xenografting human fetal stem cells into specific immunodeficient strains of mice; these animals can be infected with HIV-1 after mucosal challenge [11–13]. Currently, the most promising murine models for microbicide research are those that utilize human CD34+ stem cells derived from fetal liver tissues implanted into mice deficient in the recombinase activating genes 1 and 2 (Rag-knockout mice), or into SCID mice. These mouse strains allow much better reconstitution and there is less rejection of the human cell grafts (reviewed in [14,15]). Both models are susceptible to vaginal HIV-1 transmission [16], and hence they are useful for efficacy screening of vaginal microbicide candidates [11,13,16]. However, Rag-deficient mice still contain functional murine NK cells, with uncertain consequences for HIV-1 transmission or immune responses, so additional mutations may be required to produce animals better suited for xenograft studies [15]. In contrast, non-obese diabetic (NOD) SCID mice lack murine T, B, and NK cells, allowing better reconstitution of primary and secondary lymphoid tissues, and the consequent generation of primary human adaptive immune responses to the infecting virus [15]. As these mice spontaneously develop tumors at a high rate, their use may be limited to short-term studies [15].
Although thorough comparative studies have yet to be performed, the most promising murine model for microbicide testing now appears to be the humanized bone marrow/liver/thymic (BLT) NOD/SCID mouse. Here, human fetal-liver derived CD34+ stem cells are transplanted into irradiated NOD/SCID mice that have been surgically implanted with autologous human fetal thymus and liver [17]. Unlike most of the other models, implanting both autologous thymic tissue and stem cells into immunodeficient, irradiated mice permits the stem cells to develop into fully functional, HLA-restricted T cell and dendritic cell subsets that behave more similarly to those of humans. These cells have been shown to reconstitute primary and secondary lymphoid tissues, including the lung, intestine and vagina [12,18,19]. Their reconstitution with fully functional human immune cells renders the BLT mice susceptible to HIV-1 infection, making them useful for studying virus transmission and, to a certain extent, also the pathogenesis, immunology and therapy of the subsequent infections [20]. BLT mice have been used recently for testing the efficacy of vaginal and rectal microbicide candidates, although for reasons that are not clear, the rectal challenge studies were performed by first abrading the rectum [19], whereas no mucosal trauma was involved in the vaginal transmission experiments [15]. The protocol difference is surprising, given that NHP studies have shown that the vulnerability of the rectum to viral challenge is several orders of magnitude greater than the vagina [21]. A similar conclusion was recently drawn from a meta-analysis of human transmission data [22]. Perhaps the murine rectum is unusually resistant to an atraumatic rectal inoculation with HIV-1.
Clearly these newer rodent models are far superior to earlier ones, and they will now play a valuable role in microbicide research. However, there are some disadvantages, including the high cost and complexity of the surgical techniques required to generate BLT mice on an individual basis. The CD34+ stem cells needed to make the mice are obtained from aborted human fetal liver tissue, which may prove problematic in respect of future supply. There are also differences in the anatomy and immunology of rodents and humans to consider. For example, the interactions of the engrafted human cells with HLA-mismatched murine tissues and cells need to be better understood. Differences in HIV-1 pathogenesis, in secondary and opportunistic infections, and in the virus-specific immune responses also need to be more fully explored, and compared with what happens in infected humans (although not all of these factors will influence transmission and microbicide protection studies). Nonetheless, the new generation of humanized mouse models does offer a promising alternative for the preclinical screening of compounds for HIV-1 prevention. Whether some clinicians and a small subset of funding agency employees will embrace the results of mouse model studies any more enthusiastically than they do nonhuman primate research remains, however, to be seen; arguably, there may be even more prejudice against research in mice than monkeys.
Nonhuman primate models
Nonhuman primates remain the premier animal model for studying the transmission, immunology, and pathogenesis of HIV-1 infection. They are, and should remain, essential for the pre-clinical safety and efficacy testing of candidate microbicides. The most widely used NHP models are macaques, which are old-world monkeys, including the rhesus (Macaca mulatta), pigtail (Macaca nemestrina), and cynomolgous (Macaca fascicularis) sub-species. As would be expected given inter-species genetic relationships, NHP have anatomical, immunological, and reproductive physiological properties broadly comparable to those of humans. HIV-1 can efficiently infect macaque cells and tissues using the same mechanisms and receptors it utilizes on human cells (CD4, CCR5, or CXCR4). However, it does not replicate efficiently, post entry, in macaque cells as they contain host restriction factors, most notably TRIM5α gene products, which likely evolved as a response to a HIV-like infection in the distant past. The TRIM5α proteins of rhesus macaques and other NHP species block viral replication by inhibiting the uncoating of the capsid protein, thereby preventing integration [23]. Since we understand neither the mechanisms of mucosal transmission nor the events required to establish a successful infection, detecting plasma viremia (i.e., sustained, productive virus replication) remains the only way to determine whether transmission occurred successfully, whatever the species involved. Accordingly, viruses other than HIV-1 must be used in NHP vaginal and rectal transmission models.
Virus strain selection
Depending on the microbicide candidate’s mechanism of action, several different challenge viruses can be used in NHP, but all of them are either simian immunodeficiency viruses (SIV) or SIV/HIV hybrids called SHIVs. We know that HIV-1 evolved from the SIV lineage [24]. Although numerous NHP species are naturally, and generally benignly, infected with various SIV strains, the introduction of SIV into a naïve host species results in disease and profound immunodeficiency. Over evolutionary time, the virus and host adapt to co-existence, the virus replicating and being transmitted but not causing (much) disease in its host. When humans became infected with SIV of chimpanzees (SIVcpz), the virus was pathogenic in the new host. Indeed, SIVcpz is also now known to be pathogenic in chimpanzees [25–27], perhaps because it has not been present in that species long enough for host adaptation to take place. Several chimp-human cross-species transmissions have now been documented, one of which lead to the emergence and spread of the HIV-1 Main Group virus that created the current AIDS epidemic. Similarly, the introduction of SIVsm from sooty mangabeys (its natural host, in which it is non-pathogenic) into rhesus macaques resulted in the emergence of SIVmac and an AIDS-like disease that closely recapitulates HIV-1 infection of humans [28]. The occurrence of opportunistic infections such as CMV, mycobacteria sp., candida, etc., and the infection and depletion of very specific mucosal and memory CD4+ T cell subsets, in both SIVmac-infected macaques and HIV-1-infected humans speak to the close similarities in the interplay between the virus and the host in these two species.
SIVmac is broadly similar to HIV-1 in its genetic sequence, target cell tropism, transmission mechanisms and pathogenic properties, and is suitable for testing various types of microbicide. However, there are differences between the envelope glycoproteins (Env) of SIVmac and HIV-1, such that viruses bearing HIV-1 Env are preferable for the testing of compounds that target the attachment and entry process (i.e., fusion or entry inhibitors). Given the dominant role of CCR5 in HIV-1 and SIVmac transmission, SHIVs that utilize this co-receptor (e.g., SHIV-162P3 [4,29,30], SHIV-BAL [31], SHIV-1157 [32], etc.) are the preferred option. Viruses such as SHIV-89.6P [33–35] SHIVsf33A [36,37] or SHIV-KU1 [4,38,39] that also or instead use CXCR4 may, however, be suitable for testing some entry inhibitors. Differences between the reverse transcriptase (RT) proteins of SIVmac and HIV-1 mean that some relevant antiviral compounds (notably the non-nucleoside RT inhibitors) must be tested against a SHIV encoding the HIV-1 RT enzyme (RT-SHIV) [40,41]. Overall, there are now several wild type or genetically engineered challenge viruses that are vaginally and/or rectally transmissible to macaques, and that can be used to assess microbicide efficacy. These studies include multiple challenge experiments aimed at assessing the duration of efficacy, or the safety of repetitive mucosal exposure to a test compound.
Vaginal and rectal transmission: Physical barriers
As has been the case in HIV-1 vaccine research, several different primate species are used for testing microbicide efficacy; the application of different doses of different challenge viruses given in different ways over different time periods adds further complexity. The lack of standardization and comparability of NHP models remains a major obstacle for HIV-1 prevention research; indeed, this problem has been ranked as one of the leading roadblocks hindering HIV-1 vaccine development [42]. There are additional concerns when it comes to testing microbicides to prevent heterosexual (vaginal) transmission in NHP models, arising both from the low transmission rate of HIV-1 and the subtle, yet potentially important, differences between the reproductive tissues and cycles of the various macaque sub-species and humans.
The primate vagina is a dynamic and complex structure that has evolved to resist the inherent frictional forces, or outright trauma, associated with intercourse. Overall, the vaginal mucosal tissues have a multi-tiered system of physical, chemical and cellular barriers that respond to stimuli in ways that cannot be mimicked in cell culture systems. Unlike the rectum, the vagina has several natural defenses against the transmission of viruses including, but not limited to, HIV-1. As part of its normal function, the vagina is repeatedly exposed to foreign materials that are introduced during intercourse, including pathogens and, of course, semen, which is usually deposited by non-MHC matched males. The organ’s defenses include innate immune factors, virus-trapping mucus and even protective bacteria. Furthermore, the vaginal tract is lined by a mucosal epithelium consisting of 20 to 50 layers of squamous epithelial cells that varies during the menstrual cycle but is always thicker than the lining of the rectal tract. The vaginal epithelium thickens during the ovulatory phase in anticipation of the friction and trauma anticipated from intercourse. It also provides an important physical barrier to the passage of bacteria or viruses into the target cell-rich underlying tissues (lamina propria). However, the barrier effect of the epithelium is not absolute; the apparent absence of tight junctions between the superficial epithelial cells allows viruses and small particles to penetrate deeply into the epithelium, moving toward the target cell-rich environment of the basal lamina.
The physical barriers to HIV-1 infection are much more substantial in the vagina than the rectum, which no doubt contributes to the greatly increased risk of HIV-1 transmission posed by rectal intercourse; far fewer rectal than vaginal exposures are needed for HIV-1 transmission to occur [22]. However, transmission rates are estimates based on epidemiologic data, which vary widely depending on the questions asked and the populations sampled. In practice, it is impossible to estimate the actual vaginal or rectal HIV-1 transmission rates in humans with any accuracy. Thus, one can only rarely determine exactly when an individual became infected, what the level of infectious HIV-1 was in the semen at that particular moment, and what actually happened during the act of intercourse that might have caused local trauma or otherwise increased the chances of viruses encountering target cells. The experimental transmission of SIV/SHIV to macaques is valuable for addressing several of these issues. As with HIV-1-exposed women, the rate of SIV vaginal transmission to macaques is very low, at least when low challenge virus doses are used that mimic, in broad terms, those found in the semen of HIV-1 infected men. Thus, one problem that users of NHP models investigators have struggled to overcome is not vaginal transmission per se, but rather with the inefficiency of the process. In simple terms, if the challenge virus does not infect the control animals consistently and predictably, it is very difficult to design and execute a study intended to determine whether a microbicide (or a vaccine) reduces the rate of transmission significantly and reproducibly.
Hormone treatment and single versus multiple dosing issues in macaques
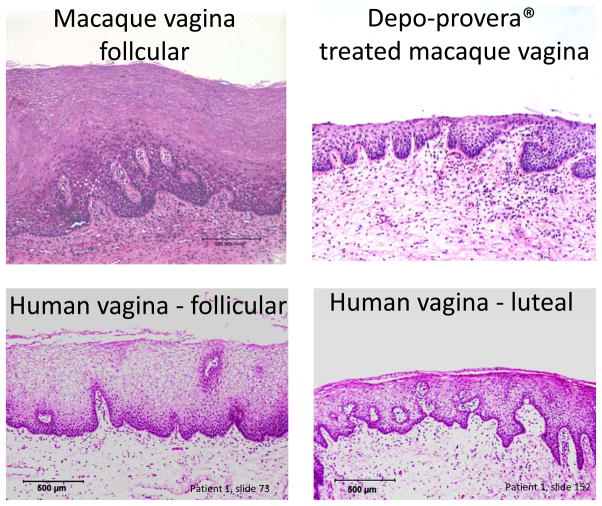
Although normal (non-hormone treated) rhesus macaques can be infected with SIV following atraumatic vaginal inoculation, 100–1000 times more virus is required to infect the animals intravaginally than intravenously [43–46]. Thus, large challenge virus doses are usually required to infect normal animals vaginally if only a single inoculation is given. Of particular relevance is that treating rhesus macaques with the progestin-based contraceptive (Depo-Provera) thins the vaginal epithelium and markedly increases their susceptibility to vaginal transmission of test viruses. The experimental application of Depo-Provera mimics the luteal phase of the natural menstrual cycle (Figure 1), a stage in which both rhesus [46], and more recently pigtail [47], macaques have been shown to be more vulnerable to vaginal SIV/SHIV transmission. There have long been suspicions that the same scenario applies to naturally cycling women, and that progesterone-based contraceptives may increase their risk of acquiring HIV-1 infection. Although both propositions remain controversial, a recent study indicated women taking Depoprovera are indeed at significantly increased risk of HIV transmission [48]. Further, there is mounting evidence that endogenous hormone levels may indeed influence HIV-1 transmission to women [49]. For example, there is significant epidemiological data showing that younger women are much more susceptible to HIV-1 infection [49], while women who are pregnant or postpartum are also more vulnerable [49,50]. Both these conditions (youth and pregnancy) are associated with progesterone-dominance and a thin vaginal mucosa that is mimicked in the macaque by the administration of exogenous progesterone. Furthermore, the vaginal epithelium of women is thinner and less keratinized during the luteal phase (menses) of the menstrual cycle, when progesterone levels are high, estrogen levels low [51]. Finally, mucus is now thought to be a barrier to HIV-1 vaginal transmission, but it is secreted in smaller volumes, and is of lower viscosity, during menses [52,53]. Again, however, formally proving that women are more susceptible to acquiring HIV-1, or other pathogens, during menses is, at best, very difficult. Moreover, sociological considerations complicate any biological interpretation of epidemiological data; for example, adolescent girls, women who have intercourse during menses, or pregnant women may simply use condoms relatively infrequently. Again, NHP models can play an important role in addressing various uncertainties about how HIV-1 is transmitted vaginally.
Figure 1.
Comparative histology of the vaginal mucosa of the rhesus macaque (top, A, B) and a naturally cycling women (bottom, C, D). A) Rhesus vagina in natural follicular phase; B) rhesus vagina 30 days after Depo-Provera administration; C) human vagina in follicular phase of the menstrual cycle; D) same vagina in the luteal phase. Since the epithelium of the rhesus vagina is normally thicker than that of women, Depo-Provera is administered to rhesus macaques to synchronize the menstrual cycle and produce a vaginal epithelium more closely resembling that of humans, especially during the luteal stage of the menstrual cycle.
The rhesus macaque progesterone-treatment model has been widely used over the past 15 years [54]. Among its applications has been to assess the protective efficacy of various vaginal microbicide candidates (ARVs and others), passively delivered neutralizing antibodies (given vaginally or systemically), and Env-based vaccines. Briefly, the animals are given a Depo-Provera injection 30 days before the animals are challenged vaginally with a pre-calibrated inoculum of the test virus, added atraumatically in a small volume (typically 300 TCID50 in 1 ml of saline). In addition to its simplicity, this model’s principal advantage is the consistency with which control animals are infected, which facilitates comparisons with the intervention group. Thus ~90% of the control animals are infected after a single vaginal challenge with what is considered to be an “intermediate” dose of virus. However, the Depo-Provera model is often criticized on three grounds.
First, the “intermediate” challenge dose is considered still to be too high to be physiologically relevant, leading to arguments that underestimating the potential of a moderately protective microbicide could cause the rejection of a concept that may actually have succeeded in human females. However, a substantial number of different compounds have now been shown to be highly protective in the Depo-Provera model, provoking the counter-question of what there is to be gained (all other things being equal) by advancing less potent microbicide compounds that have failed a stringent test.
A second concern is that exogenous progesterone treatment thins the vaginal epithelium “too much” in macaques, thereby creating an artificial state not representative of what happens in women. However, this notion may be a misconception based upon early studies in which high progesterone doses (200 mg progesterone rods implanted monthly) were used [54]. Nowadays, the most widely used Depo-Provera dose is only 30 mg/monkey, which in a 10 kg macaque would translate to 3 mg/kg. The lower dose results in a vaginal epithelium more closely resembling that of women in the luteal phase (Figure 1). That dose is also similar to the one given as contraception to human females, 150 mg, which equates to 3 mg/kg for a 50 kg (110 lb) woman. Perhaps only with very small (4–5 kg) macaques would “excessive” thinning of the vaginal epithelium occur under these conditions. Progesterone doses should now routinely be calibrated according to the animal’s weight, to further increase the consistency of this model. Nonetheless, even if the vaginal epithelium is thinner in the Depo-Provera treated macaques than arises during menses in naturally cycling women, the outcome is an even more stringent NHP model of microbicide efficacy. Thus, if a compound is safe and effective under these conditions, it is an encouraging indicator of its likely safety and effectiveness in women.
A final concern about the use of Depo-Provera is that it may suppress local immune responses, which could compromise the success of some vaccination regimens in particular. This issue is more controversial, with conflicting data that are hard to interpret unequivocally [55–57]. Whatever the merits of the argument, it is less relevant to the testing of microbicides that are intended to stop the initial transmission of the test virus before immune responses can develop and intervene.
A counter-argument in favor of the Depo-Provera model is the finding that the number of transmitted/founder (T/F) viruses infecting control animals is close to one, very similar to what happens in sexually infected women. Thus however the viral RNA, and/or infectious virus, content of the experimental inoculum compares to what is present in semen, only 1 or 2 viruses successfully infect both monkeys and women, fewer than occurs in rectal transmission and far fewer than initiate intravenous infection [58–60]. Finally, the recent human study showing that Depo-provera is also associated with increased transmission rates in women [48]suggest that this model should be revisited.
Multiple “low dose” challenge models in macaques
In response to criticisms of the Depo-Provera model, a “multiple low dose” model of SHIV transmission was developed in pigtail macaques [61]. By administering a relatively small challenge dose (10 TCID50) weekly for several weeks, it was found that all the control animals could be infected vaginally without exogenous hormone treatment [61]. It has been argued that, when analyzing the experimental outcome statistically, the number of exposures required to infect all the controls replaces the number of animals infected by a single dose, thereby reducing the number of animals needed to yield a robust endpoint. Such an analysis is more straightforward when each exposure has an equal likelihood of resulting in transmission. In practice, however, the susceptibility of individual animals, or all of them, might vary from challenge to challenge over a multi-week period. As noted above, there are likely to be differences in the susceptibility of macaques, and possibly women, during the different stages of the menstrual cycle. The worst-case scenario is that the majority of the animals may actually become infected during the menstrual stage of their cycles, the occurrence of which will vary across a test group, and in respect of when the experiment was initiated. If this were, in fact, a concern, a possible solution would be to ensure that the test groups were carefully matched in respect of the average stage of their menstrual cycles at the start of the experiment.
The multiple “low dose” model has been less successfully applied to rhesus macaques. Some groups (mostly in unpublished observations, including our own) have failed to infect sufficient numbers of rhesus macaques using the same stock and dose of challenge virus that was employed successfully in the pigtail studies (Figure 2). Two recent published studies involved SHIV-162P3 vaginal challenges of rhesus macaques without the use of Depo-Provera, and we have also carried out similar experiments on an exploratory basis. In all three studies, a “high dose” inoculum was actually used (300 TCID50), at least initially, with the challenges given weekly. In our own study and the one reported by Cheng-Mayer et al., the control animals became infected only gradually and inconsistently, to the extent that the latter group increased the challenge virus inoculum twice on an ad hoc basis, eventually to as high as 3000 TCID50 [62]. In the end, most of the control animals did become infected, but the use of an ultra-high challenge dose is inconsistent with the goal of finding a protocol that better mimics what happens in women. Indeed, to infect the remaining control animals, challenge virus doses far in excess of those ever used in the Depo-Provera model were required, essentially negating one of the major arguments against the latter – i.e., the “high” challenge dose. Moreover, the ultra-high inoculum may have compromised any chance of a successful outcome to the study (the intent was to see whether combining a microbicide with a vaccine was more protective than when each intervention was used alone; the desired outcome was not observed). In the second study, Lagenaur et al. used a constant 300 TCID50 inoculum of the same virus (SHIV-162P3) and eventually infected 11/12 animals over 6 weekly challenges, yet the remaining animal resisted an additional 6 weekly challenges [63]. Furthermore, titration studies performed with the same stock and dose (300 TCID50) of the same virus, by Ranajit Pal at Applied Biosystems Laboratories (unpublished observations), yielded infection rates comparable to that seen in the study by Lagenaur et al., but quite different from what we (Veazey, unpublished) and Cheng-Mayer et al. found (Fig. 2). Of note, and possible significance, is that both the Pal and Lagenaur et al. protocols involved treating the macaques with antibiotics prior to vaginal challenge, as a device to increase transmission rates [63]. In another study, Bomsel et al. reported that vaginal challenge with very much lower inocula of SHIV-162P3 (20–30 TCID50) caused efficient infections [64]. It is hard to reconcile those infection rates with the ones seen in the other experiments, although a possibly relevant variable is that Bomsel et al. used macaques of Chinese origin, whereas Indian macaques were used in all the other studies (Fig. 2).
Figure 2.
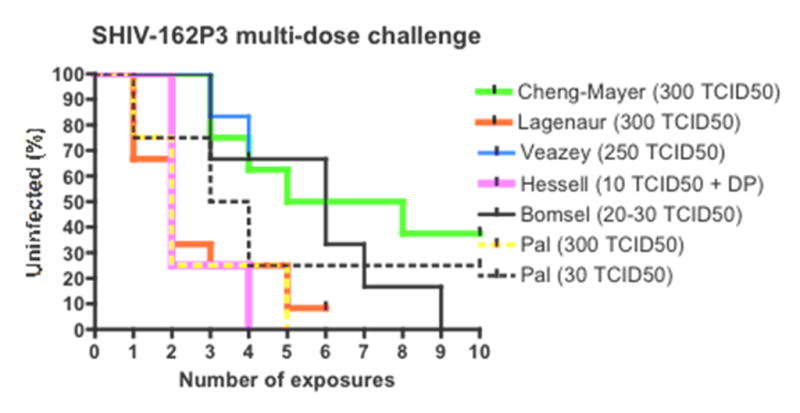
The Kaplan-Meier plots show the remaining fraction of uninfected control animals (y axis) after the number of vaginal exposures to SHIV-162P3 (x axis). The infectious doses (TCID50) used per inoculation in each study are given in the legends to the right of the diagram. DP= Depo-Provera used. Unpublished data (Veazey et al. and Pal et al.) are plotted together with results from the literature (Cheng-Mayer [62]; Lagenaur [63]; Hessell [73]; Bomsel [64]). In the Cheng-Mayer et al. study, doses higher than 300 TCID50 were given after ten exposures, to infect the remaining control animals. They are not included in the diagram.
Finally, another potential confounding variable could be the time of year when the animals are used. Rhesus macaques are seasonable breeders [65], and their susceptibility to vaginal transmission may vary at different times of the year. Whether the various factors noted above do actually affect virus transmission is not known, as no comparative studies have yet been performed. The markedly different outcomes of similar, but clearly not identical, experiments emphasizes the need for now developing a standardized vaginal multiple challenge protocol for both microbicide and vaccine research (Fig. 2).
Nonetheless, there are genuine logistical concerns to following protocols that last several months, and that require many challenges of the control animals. For example, unless the control animals all become infected in the first few challenges, it would be impossible to use such an approach to evaluate a vaginal ring that secretes an ARV for a period of approximately one month, but no longer. Similarly, testing the effect of a vaccine whose immunogenicity waned over a period of a few months would also be problematic. The practical implications of using such a large amount of a carefully calibrated challenge virus stock needs also to be borne in mind, particularly given the value of comparative data obtained from multiple studies using the same stock. Overall, to date, the multiple “low dose” model has only been successfully used in pigtail macaques, which appear to be more susceptible to vaginal SIV transmission.
Alternative macaque models
As an alternative to rhesus macaques, the use of two other sub-species should be considered – pig-tailed macaques and cynomolgus macaques. Pigtail macaques have been widely used for vaginal microbicide safety and efficacy testing [66–68]. Some investigators prefer pigtails for vaginal transmission studies because like humans, pigtails give birth all year round, whereas rhesus breed seasonally [65]. However, pigtails are generally considered to be much more susceptible than rhesus to SIV infection, with higher viral loads and a more rapid progression to AIDS [69]. These early observations led to a decline in demand for pigtails in the US National Primate Research Centers. A perception arose SIV infections in this model were more extreme, from a pathogenicity perspective, than HIV-1 infections of humans, limiting the information that could be gained. More recently, it was shown that pigtails are also more susceptible to disease progression after SHIV-162P3 infection [70], a virus that is only weakly pathogenic in rhesus macaques [71]. It now appears that pigtails are also the more susceptible to vaginal transmission, as they can be infected by relatively low SIV doses without hormone manipulation [61]. One final point is that pigtails are currently in very short supply in the USA; their limited availability alone may render these animals unsuitable for large scale studies of microbicides, for example for screening new compounds. However, discussions of how to increase the availability of these animals in Primate Centers are now underway.
Cynomolgus macaques have been fairly widely used for AIDS virus research, and may be more extensively used for microbicide studies in the future. These animals are smaller and less expensive than rhesus or pigtails, and are currently widely available. Like pigtails, cynomolgus macaques have regular menstrual cycles and breed all-year round. Whether cynomolgus macaques more resemble pigtails or rhesus in their susceptibility to SIV transmission remains to be determined in urgently needed comparative studies. Of note is that the smaller size of the cynomolgus vaginal vault makes colposcopy and vaginal biopsies more difficult than with larger macaques, which may limit their utility for vaginal safety assessments [72]. Nonetheless, the basic anatomy, vaginal and rectal flora, and vaginal pH of cynomolgus macaques and humans are all similar, implying that this model may be suitable for microbicide efficacy testing [72]. Indeed, some such studies have already been performed successfully, using rectal, and more recently vaginal, challenges [30,37].
Summary
We believe that rhesus macaques are presently still the model of choice for microbicide safety and efficacy testing. This is due in part to their availability, but also to the vast experience and knowledge accumulated during over 20 years use of these animals for prevention science, pathogenesis and immunology research. It remains debatable as to whether the Depo-Provera or “multiple dose” models should be the standardized method of choice for preclinical microbicide studies. If it is possible to overcome the limitations of the Depo-Provera model, these steps should certainly be taken. One approach may be to lower the challenge virus inoculum, to address the disparity between the quantity of virus given to macaques and the amount that women are exposed to. In a passive NAb transfer study, Hessell et al. used a modified protocol in which rhesus macaques received regular injections of Depo-Provera (every 3 weeks) but were then exposed weekly to a far smaller SHIV-162P3 inoculum (5–10 TCID50) than is normally used (Fig. 2) [73]. The protocol was successful in that the protective concentrations of NAb were also significantly lower than had been previously found in experiments employing a higher inoculum of the same or similar challenge virus. Another approach is to use lower or weight-adjusted dosages of Depo-Provera to create a more consistent model that better approximates the hormone levels and vaginal histology of “average women” during menses.
Our own preference remains to use the Depo-Provera model. Not least of the arguments, as noted above, is that if a microbicide can safely protect animals with a thin vaginal mucosa, and against a higher viral challenge than is ever found in semen, it is much more likely to be safe and effective in women than a compound that is only effective under less stringent conditions. There are too many candidates in the pipeline for all of them to be tested in human clinical trials, but the subset that has demonstrated considerable efficacy in the most stringent of the available models should be taken particularly seriously. Further, clinical trials in women recently indicated Depo-provera also increases HIV transmission rates [48], giving further credibility to this model
In summary, there remains a substantial need to validate and standardize an animal model for topical microbicide testing. Critics may argue that the macaque models have not correctly predicted the success or failures of vaccines or microbicides in humans, but this is simply not a true assertion. The recent success of Tenofovir and Truvada in protecting humans was predated by similar outcomes in the rhesus macaque model in multiple studies [74–77]. And the clinical failure of the N-9 microbicide candidate would have been predicted had an NHP model been used correctly for repeated exposure experiments. Currently there are several species, viruses, and model systems to choose from, and in some cases, selecting an inappropriate viral challenge has led to disappointing results when a comparable vaccine was later tested for efficacy in humans. But some errors are avoidable, or should be, once the underlying science is apparent. For example, vaccine challenges in macaques using a CXCR4-using virus that was selected because of its efficient replication in PBMC cultures (that express high levels of CXCR4) and that is neutralization-sensitive, does not mimic HIV-1 infection of humans by neutralization-resistant R5 viruses. The development and improvement of NHP models goes hand in hand with increased understanding of the biology and immunology of HIV-1 transmission and pathogenesis. Conversely, discoveries in the NHP models themselves drive a further appreciation of what happens in HIV-1-infected humans. The different elements of AIDS research must continue to work together in this regard.
Acknowledgments
This work was supported in part by NIH Grants U19AI76982 and U19AI076981. Animal infectivity data labeled Pal in Figure 2 was kindly provided by Dr. Ranajit Pal, Advanced Biosciences Laboratory (ABL) under NIH contract No. HHSN272200800020C with NIAID.
References cited
- 1.Hillier SL, Moench T, Shattock R, Black R, Reichelderfer P, Veronese F. In vitro and in vivo: the story of nonoxynol 9. J Acquir Immune Defic Syndr. 2005;39:1. doi: 10.1097/01.qai.0000159671.25950.74. [DOI] [PubMed] [Google Scholar]
- 2.Van Damme L, Govinden R, Mirembe FM, Guedou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359:463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 3.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 4.Veazey RS, Ketas TJ, Dufour J, Moroney-Rasmussen T, Green LC, Klasse PJ, Moore JP. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis. 2010;202:739–744. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klasse PJ, Shattock RJ, Moore JP. Which topical microbicides for blocking HIV-1 transmission will work in the real world? PLoS Med. 2006;3:e351. doi: 10.1371/journal.pmed.0030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb JCt, Newbold RR, Stumpf WE, McLachlan JA. Transitional changes in the surface epithelium of the cycling mouse vagina, cervix and uterus: scanning electron microscopic studies. Biol Reprod. 1978;19:701–711. doi: 10.1095/biolreprod19.4.701. [DOI] [PubMed] [Google Scholar]
- 7.Barberini F, Correr S, De Santis F, Motta PM. The epithelium of the rabbit vagina: a microtopographical study by light, transmission and scanning electron microscopy. Arch Histol Cytol. 1991;54:365–378. doi: 10.1679/aohc.54.365. [DOI] [PubMed] [Google Scholar]
- 8.van de Wijgert JH, Shattock RJ. Vaginal microbicides: moving ahead after an unexpected setback. AIDS. 2007;21:2369–2376. doi: 10.1097/QAD.0b013e3282ef83fd. [DOI] [PubMed] [Google Scholar]
- 9.Shacklett BL. Can the new humanized mouse model give HIV research a boost. PLoS Med. 2008;5:e13. doi: 10.1371/journal.pmed.0050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Cruz OJ, Uckun FM. Limitations of the human-PBL-SCID mouse model for vaginal transmission of HIV-1. Am J Reprod Immunol. 2007;57:353–360. doi: 10.1111/j.1600-0897.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 11.Neff CP, Kurisu T, Ndolo T, Fox K, Akkina R. A topical microbicide gel formulation of CCR5 antagonist maraviroc prevents HIV-1 vaginal transmission in humanized RAG-hu mice. PLoS One. 2011;6:e20209. doi: 10.1371/journal.pone.0020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olesen R, Wahl A, Denton PW, Garcia JV. Immune reconstitution of the female reproductive tract of humanized BLT mice and their susceptibility to human immunodeficiency virus infection. J Reprod Immunol. 2011;88:195–203. doi: 10.1016/j.jri.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denton PW, Garcia JV. Novel humanized murine models for HIV research. Curr HIV/AIDS Rep. 2009;6:13. doi: 10.1007/s11904-009-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 15.Berges BK, Rowan MR. The utility of the new generation of humanized mice to study HIV-1 infection: transmission, prevention, pathogenesis, and treatment. Retrovirology. 2011;8:65. doi: 10.1186/1742-4690-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akkina R, Berges BK, Palmer BE, Remling L, Neff CP, Kuruvilla J, Connick E, Folkvord J, Gagliardi K, Kassu A, et al. Humanized Rag1−/− gammac−/− mice support multilineage hematopoiesis and are susceptible to HIV-1 infection via systemic and vaginal routes. PLoS One. 2011;6:e20169. doi: 10.1371/journal.pone.0020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 18.Denton PW, Estes JD, Sun Z, Othieno FA, Wei BL, Wege AK, Powell DA, Payne D, Haase AT, Garcia JV. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5:e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Z, Denton PW, Estes JD, Othieno FA, Wei BL, Wege AK, Melkus MW, Padgett-Thomas A, Zupancic M, Haase AT, et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med. 2007;204:705–714. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denton PW, Othieno F, Martinez-Torres F, Zou W, Krisko JF, Fleming E, Zein S, Powell DA, Wahl A, Kwak YT, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol. 2011;85:7582–7593. doi: 10.1128/JVI.00537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trichel AM, Roberts ED, Wilson LA, Martin LN, Ruprecht RM, Murphey-Corb M. SIV/DeltaB670 transmission across oral, colonic, and vaginal mucosae in the macaque. J Med Primatol. 1997;26:3. doi: 10.1111/j.1600-0684.1997.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 22.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39:1048–1063. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 24.Gao F, Bailes E, Robertson DL, Chen Y, Rodenburg CM, Michael SF, Cummins LB, Arthur LO, Peeters M, Shaw GM, et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 25.O’Neil SP, Novembre FJ, Hill AB, Suwyn C, Hart CE, Evans-Strickfaden T, Anderson DC, deRosayro J, Herndon JG, Saucier M, et al. Progressive infection in a subset of HIV-1-positive chimpanzees. J Infect Dis. 2000;182:1051–1062. doi: 10.1086/315823. [DOI] [PubMed] [Google Scholar]
- 26.Novembre FJ, Saucier M, Anderson DC, Klumpp SA, O’Neil SP, Brown CR, 2nd, Hart CE, Guenthner PC, Swenson RB, McClure HM. Development of AIDS in a chimpanzee infected with human immunodeficiency virus type 1. J Virol. 1997;71:4086–4091. doi: 10.1128/jvi.71.5.4086-4091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudicell RS, Holland Jones J, Wroblewski EE, Learn GH, Li Y, Robertson JD, Greengrass E, Grossmann F, Kamenya S, Pintea L, et al. Impact of simian immunodeficiency virus infection on chimpanzee population dynamics. PLoS Pathog. 2010;6:e1001116. doi: 10.1371/journal.ppat.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apetrei C, Kaur A, Lerche NW, Metzger M, Pandrea I, Hardcastle J, Falkenstein S, Bohm R, Koehler J, Traina-Dorge V, et al. Molecular epidemiology of simian immunodeficiency virus SIVsm in U.S. primate centers unravels the origin of SIVmac and SIVstm. J Virol. 2005;79:8991–9005. doi: 10.1128/JVI.79.14.8991-9005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harouse JM, Gettie A, Eshetu T, Tan RC, Bohm R, Blanchard J, Baskin G, Cheng-Mayer C. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3) J Virol. 2001;75:1990–1995. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M, Marx PA, Dufour J, Colonno RJ, Shattock RJ, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 31.Pal R, Taylor B, Foulke JS, Woodward R, Merges M, Praschunus R, Gibson A, Reitz M. Characterization of a simian human immunodeficiency virus encoding the envelope gene from the CCR5-tropic HIV-1 Ba-L. J Acquir Immune Defic Syndr. 2003;33:300–307. doi: 10.1097/00126334-200307010-00003. [DOI] [PubMed] [Google Scholar]
- 32.Song RJ, Chenine AL, Rasmussen RA, Ruprecht CR, Mirshahidi S, Grisson RD, Xu W, Whitney JB, Goins LM, Ong H, et al. Molecularly cloned SHIV-1157ipd3N4: a highly replication- competent, mucosally transmissible R5 simian-human immunodeficiency virus encoding HIV clade C Env. J Virol. 2006;80:8729–8738. doi: 10.1128/JVI.00558-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai CC, Emau P, Jiang Y, Tian B, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89. 6P in macaques. AIDS Res Hum Retroviruses. 2003;19:535–541. doi: 10.1089/088922203322230897. [DOI] [PubMed] [Google Scholar]
- 34.Jiang YH, Emau P, Cairns JS, Flanary L, Morton WR, McCarthy TD, Tsai CC. SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89. 6P in macaques. AIDS Res Hum Retroviruses. 2005;21:207–213. doi: 10.1089/aid.2005.21.207. [DOI] [PubMed] [Google Scholar]
- 35.Reimann KA, Li JT, Veazey R, Halloran M, Park IW, Karlsson GB, Sodroski J, Letvin NL. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckner CM, Gettie A, Tan RC, Eshetu T, Ratterree M, Blanchard J, Cheng-Mayer C, Harouse JM. Infection of macaques with a molecular clone, SHIVSF33A2, provides evidence for tissue specific variants. J Med Primatol. 2002;31:164–170. [PubMed] [Google Scholar]
- 37.Boadi T, Schneider E, Chung S, Tsai L, Gettie A, Ratterree M, Blanchard J, Neurath AR, Cheng-Mayer C. Cellulose acetate 1,2-benzenedicarboxylate protects against challenge with pathogenic X4 and R5 simian/human immunodeficiency virus. AIDS. 2005;19:1587–1594. doi: 10.1097/01.aids.0000186020.24426.62. [DOI] [PubMed] [Google Scholar]
- 38.Veazey RS, Ketas TA, Klasse PJ, Davison DK, Singletary M, Green LC, Greenberg ML, Moore JP. Tropism-independent protection of macaques against vaginal transmission of three SHIVs by the HIV-1 fusion inhibitor T-1249. Proc Natl Acad Sci U S A. 2008;105:10531–10536. doi: 10.1073/pnas.0802666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buch S, Pinson D, Hou Y, Adany I, Li Z, Mukherjee S, Jia F, Mackay G, Silverstein P, Kumar A, et al. Neuropathogenesis of chimeric simian human immunodeficiency virus infection in rhesus macaques. J Med Primatol. 2000;29:96–106. doi: 10.1034/j.1600-0684.2000.290302.x. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Y, Tian B, Agy MB, Saifuddin M, Tsai CC. Macaca fascicularis are highly susceptible to an RT-SHIV following intravaginal inoculation: a new model for microbicide evaluation. J Med Primatol. 2009;38 (Suppl 1):39. doi: 10.1111/j.1600-0684.2009.00374.x. [DOI] [PubMed] [Google Scholar]
- 41.Stolte-Leeb N, Loddo R, Antimisiaris S, Schultheiss T, Sauermann U, Franz M, Mourtas S, Parsy C, Storer R, La Colla P, et al. Topical Nonnucleoside Reverse Transcriptase Inhibitor MC 1220 Partially Prevents Vaginal RT-SHIV Infection of Macaques. AIDS Res Hum Retroviruses. 2011;27:933–943. doi: 10.1089/AID.2010.0339. [DOI] [PubMed] [Google Scholar]
- 42.Thomas C. Roadblocks in HIV research: five questions. Nature Medicine. 2009;15:855– 859. doi: 10.1038/nm0809-855. [DOI] [PubMed] [Google Scholar]
- 43.Miller CJ, Alexander NJ, Sutjipto S, Lackner AA, Gettie A, Hendrickx AG, Lowenstine LJ, Jennings M, Marx PA. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J Virol. 1989;63:4277–4284. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller CJ, Alexander NJ, Vogel P, Anderson J, Marx PA. Mechanism of genital transmission of SIV. a hypothesis based on transmission studies and the location of SIV in the genital tract of chronically infected female rhesus macaques. Journal of Medical Primatology. 1992;21:64. [PubMed] [Google Scholar]
- 45.Marx PA, Compans RW, Gettie A, Staas JK, Gilley RM, Mulligan MJ, Yamschikov GV, Chen D, Eldridge JH. Protection against vaginal SIV transmission with microencapsulated vaccine. Science. 1993;260:1323–1327. doi: 10.1126/science.8493576. [DOI] [PubMed] [Google Scholar]
- 46.Sodora DL, Gettie A, Miller CJ, Marx PA. Vaginal transmission of SIV. assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Research & Human Retroviruses. 1998;14 (Suppl 1):S119–123. [PubMed] [Google Scholar]
- 47.Vishwanathan SA, Guenthner PC, Lin CY, Dobard C, Sharma S, Adams DR, Otten RA, Heneine W, Hendry RM, McNicholl JM, et al. High Susceptibility to Repeated, Low- Dose, Vaginal SHIV Exposure Late in the Luteal Phase of the Menstrual Cycle of Pigtail Macaques. J Acquir Immune Defic Syndr. 2011;57:261–264. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 48.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, de Bruyn G, Nakku-Joloba E, Ngure K, Kiarie J, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2011 doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science. 2005;308:1582–1583. doi: 10.1126/science.1112489. [DOI] [PubMed] [Google Scholar]
- 50.Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, Nalugoda F, Kiddugavu M, Sewankambo N, Quinn TC, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 51.Mauck CK, Callahan MM, Baker J, Arbogast K, Veazey R, Stock R, Pan Z, Morrison CS, Chen-Mok M, Archer DF, et al. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;60:15. doi: 10.1016/s0010-7824(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 52.Mahalingam A, Jay JI, Langheinrich K, Shukair S, McRaven MD, Rohan LC, Herold BC, Hope TJ, Kiser PF. Inhibition of the transport of HIV in vitro using a pH-responsive synthetic mucin-like polymer system. Biomaterials. 2011;32:8343–8355. doi: 10.1016/j.biomaterials.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol. 2009;83:11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 55.Genesca M, McChesney MB, Miller CJ. Depo-provera treatment does not abrogate protection from intravenous SIV challenge in female macaques immunized with an attenuated AIDS virus. PLoS One. 2010;5:e9814. doi: 10.1371/journal.pone.0009814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trunova N, Tsai L, Tung S, Schneider E, Harouse J, Gettie A, Simon V, Blanchard J, Cheng-Mayer C. Progestin-based contraceptive suppresses cellular immune responses in SHIV-infected rhesus macaques. Virology. 2006;352:169–177. doi: 10.1016/j.virol.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Sanders-Beer B, Babas T, Mansfield K, Golightly D, Kramer J, Bowlsbey A, Sites D, Nieves-Duran L, Lin S, Rippeon S, et al. Depo-Provera does not alter disease progression in SIVmac-infected female Chinese rhesus macaques. AIDS Res Hum Retroviruses. 2010;26:433–443. doi: 10.1089/aid.2009.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A. 2011;108:11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H, Bar KJ, Wang S, Decker JM, Chen Y, Sun C, Salazar-Gonzalez JF, Salazar MG, Learn GH, Morgan CJ, et al. High Multiplicity Infection by HIV-1 in Men Who Have Sex with Men. PLoS Pathog. 2010;6:e1000890. doi: 10.1371/journal.ppat.1000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Otten RA, Adams DR, Kim CN, Jackson E, Pullium JK, Lee K, Grohskopf LA, Monsour M, Butera S, Folks TM. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. J Infect Dis. 2005;191:164–173. doi: 10.1086/426452. [DOI] [PubMed] [Google Scholar]
- 62.Cheng-Mayer C, Huang Y, Gettie A, Tsai L, Ren W, Shakirzyanova M, Sina ST, Trunova N, Blanchard J, Jenkins LM, et al. Delay of simian human immunodeficiency virus infection and control of viral replication in vaccinated macaques challenged in the presence of a topical microbicide. AIDS. 2011;25:1833–1841. doi: 10.1097/QAD.0b013e32834a1d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lagenaur LA, Sanders-Beer BE, Brichacek B, Pal R, Liu X, Liu Y, Yu R, Venzon D, Lee PP, Hamer DH. Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal Immunol. 2011 doi: 10.1038/mi.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, Mouz N, Amacker M, Chalifour A, Diomede L, et al. Immunization with HIV-1 gp41 Subunit Virosomes Induces Mucosal Antibodies Protecting Nonhuman Primates against Vaginal SHIV Challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 65.Patton DL, Sweeney YT, Agnew KJ, Balkus JE, Rabe LK, Hillier SL. Development of a nonhuman primate model for Trichomonas vaginalis infection. Sex Transm Dis. 2006;33:743–746. doi: 10.1097/01.olq.0000218871.89901.61. [DOI] [PubMed] [Google Scholar]
- 66.Ambrose Z, Boltz V, Palmer S, Coffin JM, Hughes SH, Kewalramani VN. In vitro characterization of a simian immunodeficiency virus-human immunodeficiency virus (HIV) chimera expressing HIV type 1 reverse transcriptase to study antiviral resistance in pigtail macaques. J Virol. 2004;78:13553–13561. doi: 10.1128/JVI.78.24.13553-13561.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patton DL, Sweeney YT, Balkus JE, Hillier SL. Vaginal and rectal topical microbicide development: safety and efficacy of 1. 0% Savvy (C31G) in the pigtailed macaque. Sex Transm Dis. 2006;33:691–695. doi: 10.1097/01.olq.0000216022.18321.d3. [DOI] [PubMed] [Google Scholar]
- 68.Patton DL, Cosgrove Sweeney YT, McCarthy TD, Hillier SL. Preclinical safety and efficacy assessments of dendrimer-based (SPL7013) microbicide gel formulations in a nonhuman primate model. Antimicrob Agents Chemother. 2006;50:1696–1700. doi: 10.1128/AAC.50.5.1696-1700.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenberg YJ, White BD, Papermaster SF, Zack P, Jarling PB, Eddy GA, Burke DS, Lewis MG. Variation in T-lymphocyte activation and susceptibility to SIVPBj-14-induced acute death in macaques. J Med Primatol. 1991;20:206–210. [PubMed] [Google Scholar]
- 70.Batten CJ, De Rose R, Wilson KM, Agy MB, Chea S, Stratov I, Montefiori DC, Kent SJ. Comparative evaluation of simian, simian-human, and human immunodeficiency virus infections in the pigtail macaque (Macaca nemestrina) model. AIDS Res Hum Retroviruses. 2006;22:580–588. doi: 10.1089/aid.2006.22.580. [DOI] [PubMed] [Google Scholar]
- 71.Pahar B, Wang X, Dufour J, Lackner AA, Veazey RS. Virus-specific T cell responses in macaques acutely infected with SHIV(sf162p3) Virology. 2007;363:36. doi: 10.1016/j.virol.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patton DL, Sweeney YC, Tsai CC, Hillier SL. Macaca fascicularis vs. Macaca nemestrina as a model for topical microbicide safety studies. J Med Primatol. 2004;33:105–108. doi: 10.1111/j.1600-0684.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 73.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parikh UM, Dobard C, Sharma S, Cong ME, Jia H, Martin A, Pau CP, Hanson DL, Guenthner P, Smith J, et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol. 2009;83:10358–10365. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Subbarao S, Otten RA, Ramos A, Kim C, Jackson E, Monsour M, Adams DR, Bashirian S, Johnson J, Soriano V, et al. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis. 2006;194:904–911. doi: 10.1086/507306. [DOI] [PubMed] [Google Scholar]
- 76.Cranage M, Sharpe S, Herrera C, Cope A, Dennis M, Berry N, Ham C, Heeney J, Rezk N, Kashuba A, et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 2008;5:e157. doi: 10.1371/journal.pmed.0050157. discussion e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsai C-C, Follis KE, Sabo A, Beck TW, Grant RF, Bischofberger N, Benveniste RE, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]