The evaluation of 160 meta-analyses of animal studies on potential treatments for neurological disorders reveals that the number of statistically significant results was too large to be true, suggesting biases.
Abstract
Animal studies generate valuable hypotheses that lead to the conduct of preventive or therapeutic clinical trials. We assessed whether there is evidence for excess statistical significance in results of animal studies on neurological disorders, suggesting biases. We used data from meta-analyses of interventions deposited in Collaborative Approach to Meta-Analysis and Review of Animal Data in Experimental Studies (CAMARADES). The number of observed studies with statistically significant results (O) was compared with the expected number (E), based on the statistical power of each study under different assumptions for the plausible effect size. We assessed 4,445 datasets synthesized in 160 meta-analyses on Alzheimer disease (n = 2), experimental autoimmune encephalomyelitis (n = 34), focal ischemia (n = 16), intracerebral hemorrhage (n = 61), Parkinson disease (n = 45), and spinal cord injury (n = 2). 112 meta-analyses (70%) found nominally (p≤0.05) statistically significant summary fixed effects. Assuming the effect size in the most precise study to be a plausible effect, 919 out of 4,445 nominally significant results were expected versus 1,719 observed (p<10−9). Excess significance was present across all neurological disorders, in all subgroups defined by methodological characteristics, and also according to alternative plausible effects. Asymmetry tests also showed evidence of small-study effects in 74 (46%) meta-analyses. Significantly effective interventions with more than 500 animals, and no hints of bias were seen in eight (5%) meta-analyses. Overall, there are too many animal studies with statistically significant results in the literature of neurological disorders. This observation suggests strong biases, with selective analysis and outcome reporting biases being plausible explanations, and provides novel evidence on how these biases might influence the whole research domain of neurological animal literature.
Author Summary
Studies have shown that the results of animal biomedical experiments fail to translate into human clinical trials; this could be attributed either to real differences in the underlying biology between humans and animals, to shortcomings in the experimental design, or to bias in the reporting of results from the animal studies. We use a statistical technique to evaluate whether the number of published animal studies with “positive” (statistically significant) results is too large to be true. We assess 4,445 animal studies for 160 candidate treatments of neurological disorders, and observe that 1,719 of them have a “positive” result, whereas only 919 studies would a priori be expected to have such a result. According to our methodology, only eight of the 160 evaluated treatments should have been subsequently tested in humans. In summary, we judge that there are too many animal studies with “positive” results in the neurological disorder literature, and we discuss the reasons and potential remedies for this phenomenon.
Introduction
Animal research studies make a valuable contribution in the generation of hypotheses that might be tested in preventative or therapeutic clinical trials of new interventions. These data may establish that there is a reasonable prospect of efficacy in human disease, which justifies the risk to trial participants.
Several empirical evaluations of the preclinical animal literature have shown limited concordance between treatment effects in animal experiments and subsequent clinical trials in humans [1]–[4]. Systematic assessments of the quality of animal studies have attributed this translational failure, at least in part, to shortcomings in experimental design and in the reporting of results [5]. Lack of randomization, blinding, inadequate application of inclusion and exclusion criteria, inadequate statistical power, and inappropriate statistical analysis may compromise internal validity [6],[7].
These problems are compounded by different types of reporting biases [8]. First, bias against publication of “negative” results (publication bias) or publication after considerable delay (time lag bias) may exist [9]. Such findings may not be published at all, published with considerable delay, or published in low impact or low visibility national journals in comparison to studies with “positive” findings. Second, selective analysis and outcome reporting biases may emerge when there are many analyses that can be performed, but only the analysis with the “best” results is presented resulting in potentially misleading findings [10]. This can take many different representations such as analyzing many different outcomes but reporting only one or some of them, or using different statistical approaches to analyze the same outcome but reporting only one of them. Third, in theory “positive” results may be totally faked, but hopefully such fraud is not common. Overall, these biases ultimately lead to a body of evidence with an inflated proportion of published studies with statistically significant results.
Detecting these biases is not a straightforward process. There are several empirical statistical methods that try to detect publication bias in meta-analyses. The most popular of these are tests of asymmetry, which evaluate whether small or imprecise studies give different results from larger more precise ones [11]. However, these methods may not be very sensitive or specific in the detection of such biases, especially when few studies are included in a meta-analysis [11]–[13].
An alternative approach is the excess significance test. This examines whether too many individual studies in a meta-analysis report statistically significant results compared with what would be expected under reasonable assumptions about the plausible effect size [14]. The excess significance test has low power to detect bias in single meta-analyses with limited number of studies, but a major advantage is its applicability to many meta-analyses across a given field. This increases the power to detect biases that pertain to larger fields and disciplines rather than just single topics. Previous applications have found an excess of statistically significant findings in various human research domains [14]–[17], but it has not been applied to animal research studies.
Biases in animal experiments may result in biologically inert or even harmful substances being taken forward to clinical trials, thus exposing patients to unnecessary risk and wasting scarce research funds. It is important to understand the extent of potential biases in this field, as multiple interventions with seemingly promising results in animals accumulate in its literature. Therefore, in this paper, we probed whether there is evidence for excess statistical significance in animal studies of interventions for neurological diseases using a large database of 160 interventions and 4,445 study datasets.
Results
Description of Database
Our database included a total of 4,445 pairwise comparisons from 1,411 unique animal studies that were synthesized in 160 meta-analyses (Table S1). Two meta-analyses (n = 1,054 comparisons) pertained to Alzheimer disease (AD), 34 meta-analyses (n = 483) to experimental autoimmune encephalomyelitis (EAE), 16 meta-analyses (n = 1,403) to focal ischemia, 61 meta-analyses (n = 424) to intracerebral hemorrhage (ICH), 45 meta-analyses (n = 873) to Parkinson disease (PD), and two meta-analyses (n = 208) to spinal cord injury (SCI). The median number of comparisons in each meta-analysis was eight (interquartile range [IQR], 3–23). The median sample size in each animal study dataset was 16 (IQR, 11–20), while the median sample size in each meta-analysis was 135 (IQR, 48–376).
Summary Effect Sizes
Of the 160 meta-analyses, 112 (70%) had found a nominally (p≤0.05) statistically significant summary effect per fixed-effects synthesis, of which 108 meta-analyses favored the experimental intervention and only four meta-analyses favored the control intervention (94 and four for random effects synthesis). The proportion of the associations that had a nominally statistically significant effect using the fixed-effects summary ranged from 57% for ICH to 100% for AD, focal ischemia, and SCI. Table S1 provides information for all 160 meta-analyses. In 47 (29%) meta-analyses the respective most precise study had a nominally statistically significant result, as described in Table 1. The effect size of the most precise study in each meta-analysis was more conservative than the fixed-effects summary in 114 (71%) meta-analyses.
Table 1. Description of the 47 meta-analyses where the respective most precise study had a nominally statistically significant effect.
| Disease | Outcome | Intervention/Treatment | Cases/Controls | Standardized Mean Difference (95% CI) | ||
| Most Precise Studya | Summary Fixed Effect | Summary Random Effect | ||||
| EAE | Mean severity | Aminoguanidine | 70/82 | 1·10 (0·19–2·01) | 0·40 (0·04–0·77) | 0·45 (−0·22 to 1·12) |
| EAE | Mean severity | Complete freunds adjuvant | 44/42 | 2·79 (1·27–4·31) | 1·61 (0·79–2·43) | 1·85 (−0·09 to 3·78) |
| EAE | Mean severity | Estrogen hormone | 415/531 | 0·84 (0·32–1·36) | 1·10 (0·91–1·29) | 1·49 (1·08–1·90) |
| EAE | Mean severity | Fingolimod (FTY720) | 91/111 | 1·30 (0·07–2·53) | 2·39 (1·75–3·03) | 3·14 (1·29–4·98) |
| EAE | Mean severity | Immunoglobulin | 139/90 | 1·15 (0·67–1·62) | 0·89 (0·56–1·23) | 1·01 (0·22–1·80) |
| EAE | Mean severity | Insulin like growth factor | 38/38 | 4·04 (2·29–5·80) | 4·85 (3·85–5·85) | 5·00 (3·65–6·34) |
| EAE | Mean severity | MBP | 1,138/1,638 | 0·82 (0·32–1·32) | 0·75 (0·65–0·86) | 0·98 (0·77–1·19) |
| EAE | Mean severity | Minocycline | 22/25 | 2·16 (0·90–3·43) | 1·68 (0·91–2·45) | 1·72 (0·48–2·97) |
| EAE | Mean severity | Phenytoin | 11/11 | 2·59 (0·68–4·51) | 3·15 (1·70–4·59) | 3·15 (1·70–4·59) |
| EAE | Mean severity | Proteolipid protein | 19/28 | −1·27 (−2·07 to −0·48) | −0·97 (−1·62 to −0·32) | −0·92 (−1·78 to −0·05) |
| EAE | Mean severity | Prednisolone | 17/17 | 1·55 (0·12–2·98) | 1·54 (0·64–2·44) | 1·54 (0·64–2·44) |
| EAE | Mean severity | Rolipram | 117/109 | 0·56 (0·03–1·08) | 0·69 (0·41–0·97) | 0·63 (0·06–1·19) |
| Focal ischemia | Infarct volume | Hypothermia | 1,803/1,949 | 1·08 (0·42–1·74) | 1·39 (1·30–1·48) | 1·69 (1·53–1·86) |
| Focal ischemia | Infarct volume | Stem Cells | 1,611/1,657 | 0·91 (0·20–1·62) | 0·81 (0·71–0·91) | 1·21 (1·00–1·41) |
| Focal ischemia | Infarct volume | Growth factor | 981/1,026 | 0·78 (0·14–1·42) | 0·93 (0·82–1·04) | 1·09 (0·90–1·27) |
| Focal ischemia | Infarct volume | Nootropic | 120/128 | 1·44 (0·43–2·44) | 0·93 (0·60–1·26) | 0·97 (0·53–1·42) |
| Focal ischemia | Infarct volume | Minocycline | 335/337 | 1·20 (0·55–1·84) | 0·84 (0·65–1·03) | 0·83 (0·60–1·07) |
| Focal ischemia | Infarct volume | Melatonin | 276/303 | 1·33 (0·51–2·14) | 1·13 (0·90–1·35) | 1·13 (0·90–1·35) |
| Focal ischemia | Infarct volume | NOS donors | 280/356 | 1·58 (0·76–2·41) | 0·71 (0·50–0·92) | 0·76 (0·44–1·08) |
| ICH | Neurobehavioural score | Anti-inflammatory | 218/214 | 0·79 (0·10–1·48) | 1·19 (0·89–1·48) | 2·06 (1·34–2·77) |
| ICH | Neurobehavioural score | Calcium channel blockers | 108/108 | 1·06 (0·64–1·48) | 0·72 (0·42–1·02) | 0·47 (−0·04 to 0·98) |
| ICH | Neurobehavioural score | Growth factor | 70/70 | 4·03 (2·73–5·34) | 5·35 (4·55–6·15) | 6·37 (4·18–8·55) |
| ICH | Neurobehavioural score | Tuftsin fragment 1–3 | 29/26 | 1·33 (0·59–2·08) | 1·21 (0·62–1·79) | 1·21 (0·62–1·79) |
| ICH | Neurobehavioural score | PPAR-gamma agonist | 37/37 | 2·00 (0·94–3·06) | 3·36 (2·52–4·20) | 5·38 (1·82–8·95) |
| ICH | Neurobehavioural score | Psychomotor stimulant | 98/104 | 0·76 (0·27–1·25) | 0·17 (−0·15 to 0·48) | −0·36 (−1·19 to 0·48) |
| ICH | Neurobehavioural score | Stem cells | 408/371 | 2·81 (2·09–3·53) | 1·64 (1·45–1·84) | 1·77 (1·38–2·15) |
| ICH | Brain water content | Minocycline | 12/12 | 2·33 (0·73–3·94) | 2·62 (1·41–3·84) | 2·62 (1·41–3·84) |
| ICH | Brain water content | Growth factor | 18/18 | 5·76 (3·81–7·71) | 4·98 (3·51–6·45) | 4·95 (3·19–6·70) |
| ICH | Brain water content | Zinc protoporphyrin | 12/12 | 1·31 (0·01–2·60) | 1·40 (0·46–2·34) | 1·40 (0·46–2·34) |
| ICH | Brain water content | Atorvastatin | 18/18 | 3·01 (1·25–4·77) | 3·77 (2·28–5·26) | 4·12 (1·53–6·71) |
| ICH | Brain water content | Tuftsin fragment | 10/10 | 1·63 (0·09–3·17) | 1·83 (0·69–2·98) | 1·83 (0·69–2·98) |
| ICH | Brain water content | Stem cells | 32/32 | 1·60 (0·43–2·77) | 1·43 (0·64–2·22) | 1·43 (0·64–2·22) |
| ICH | Hematoma volume | Recombinant factor VIIa | 12/12 | −1·74 (−3·15 to −0·32) | −2·03 (−3·11 to −0·96) | −2·03 (−3·11 to −0·96) |
| ICH | Hematoma volume | Tuftsin fragment | 12/12 | 1·35 (0·15–2·56) | 1·57 (0·59–2·54) | 1·57 (0·59–2·54) |
| ICH | Hematoma volume | Rosiglitazone | 11/11 | 2·05 (0·07–4·02) | 3·01 (1·59–4·44) | 3·03 (1·06–5·01) |
| PD | Various | A86929 | 68/68 | 2·31 (0·20–4·42) | 1·55 (0·76–2·34) | 1·55 (0·76–2·34) |
| PD | Rotational behavior | Aplindore | 105/105 | 0·78 (0·04–1·53) | 1·73 (1·40–2·06) | 1·88 (1·25–2·51) |
| PD | Various | Ciladopa (AY27110) | 100/100 | 1·40 (0·55–2·25) | 0·002 (−0·38 to 0·38) | −0·37 (−1·36 to 0·62) |
| PD | Various | Bromocriptine | 436/490 | 0·97 (0·02–1·91) | 0·96 (0·76–1·17) | 0·95 (0·71–1·18) |
| PD | Rotational behavior | Dinapsoline | 131/131 | 1·47 (0·07–2·87) | 2·03 (1·59–2·47) | 2·30 (1·48–3·11) |
| PD | Various | Lisuride | 100/146 | 1·04 (0·18–1·90) | 1·39 (0·99–1·80) | 1·39 (0·99–1·80) |
| PD | Various | Pramipexole | 146/143 | 1·81 (0·45–3·16) | 1·42 (0·97–1·87) | 1·43 (0·83–2·03) |
| PD | Various | Quinelorane | 24/21 | 2·48 (0·88–4·07) | 1·06 (0·15–1·97) | 1·22 (−0·43 to 2·86) |
| PD | Various | Quinpirole | 320/312 | 1·47 (0·77–2·17) | 1·09 (0·87–1·31) | 1·08 (0·65–1·50) |
| PD | Various | Rotigotine | 75/83 | 1·81 (0·88–2·74) | 1·76 (1·21–2·32) | 1·76 (1·21–2·32) |
| PD | Various | SKF 80723 | 10/10 | 2·30 (0·51–4·09) | 3·04 (1·42–4·67) | 4·04 (−0·02 to 8·09) |
| PD | Various | Talipexole | 239/238 | 1·03 (0·33–1·73) | 0·79 (0·51–1·07) | 0·86 (0·45–1·27) |
Standardized mean difference and 95% CI of the most precise study (smallest standard error) in each meta-analysis.
PPAR, peroxisome proliferator-activated receptor; A86929, a dopamine receptor agonist; SKF 80723, a benzazepine D1 dopamine agonist.
Between-Study Heterogeneity
There was statistically significant heterogeneity at p≤0.10 for 83 (52%) meta-analyses (Table S1). There was moderate heterogeneity (I2 = 50%–75%) in 52 (33%) meta-analyses, and high heterogeneity (I2>75%) in 22 (14%). The lowest proportion of significant heterogeneity was observed in meta-analyses of ICH (36%) and PD (42%), while all other areas had significant heterogeneities above 70%. Uncertainty around the heterogeneity estimates was often large, as reflected by wide 95% CI of I2.
Small-Study Effects
There was evidence of small-study effects in 74 (46%) meta-analyses (Table S1). These pertained to AD (n = 2 meta-analyses), EAE (n = 14), focal ischemia (n = 9), ICH (n = 27), PD (n = 21), and SCI (n = 1).
Excess of Significance
When the plausible effect was assumed to be that of the most precise study in each meta-analysis, there was evidence (p≤0.10) of excess significance in 49 (31%) meta-analyses (AD n = 2, EAE n = 13, focal ischemia n = 11, ICH n = 10, PD n = 12, SCI n = 1) (Table 2), despite the generally low power of the excess significance test. Under the assumptions of the summary fixed effect being the plausible effect, there was evidence of excess significance in 23 meta-analyses.
Table 2. Observed and expected number of “positive” studies in the 49 meta-analyses with a significant excess of “positive” studies under the assumption that the plausible effect size equals the effect of the most precise study in each meta-analysis.
| Disease | Outcome | Intervention/Treatment | Studies | Comparisons | Observed Positive | Most Precise Study Effect | Summary Fixed Effect | ||
| Expected Positivea | p-Value* | Expected Positiveb | p-Value* | ||||||
| AD | Morris water maze | Various | 82 | 250 | 76 | 45·8 | 3·8 • 10−6 | 51·3 | 2·2 • 10−4 |
| AD | Plaque area | Various | 318 | 804 | 342 | 40·6 | <1 • 10−9 | 226 | <1 • 10−9 |
| EAE | Mean severity | Bone marrow | 2 | 24 | 15 | 1·2 | <1 • 10−9 | 14·5 | 0·99 |
| EAE | Mean severity | Cobra venom factor | 2 | 8 | 4 | 1·17 | 0·02 | 3·95 | 0·99 |
| EAE | Mean severity | Cyclosporin | 8 | 14 | 6 | 3·05 | 0·10 | 4·43 | 0·39 |
| EAE | Mean severity | Estrogen hormone | 7 | 42 | 22 | 15·8 | 0·06 | 22·3 | NP |
| EAE | Mean severity | Glatiramer acetate | 9 | 20 | 13 | 2·4 | 4 • 10−8 | 11·5 | 0·65 |
| EAE | Mean severity | Interleukin 10 | 7 | 34 | 9 | 4·6 | 0·04 | 2·15 | 2 • 10−4 |
| EAE | Mean severity | Interleukin 4 | 3 | 10 | 2 | 0·52 | 0·09 | 1·51 | 0·65 |
| EAE | Mean severity | Lovastatin | 2 | 7 | 4 | 0·93 | 7·7 • 10−3 | 4·59 | NP |
| EAE | Mean severity | Methylprednisolone | 4 | 10 | 6 | 3·15 | 0·08 | 0·69 | 1·8 • 10−5 |
| EAE | Mean severity | Mitoxantrone | 4 | 30 | 20 | 5·29 | <1 • 10−9 | 25·4 | NP |
| EAE | Mean severity | Rolipram | 3 | 9 | 7 | 2·16 | 1 • 10−3 | 2·99 | 8·2 • 10−3 |
| EAE | Mean severity | Spinal cord protein | 2 | 5 | 2 | 0·32 | 0·04 | 0·34 | 0·04 |
| EAE | Mean severity | Vitamin D | 6 | 13 | 9 | 1·88 | 1·1 • 10−5 | 9·19 | NP |
| Focal ischemia | Infarct volume | Hypothermia | 107 | 221 | 135 | 101 | 7·2 • 10−6 | 145 | NP |
| Focal ischemia | Infarct volume | Stem cells | 110 | 211 | 84 | 67·6 | 0·02 | 63·7 | 3·3 • 10−3 |
| Focal ischemia | Infarct volume | Thrombolytic | 83 | 202 | 60 | 10·2 | <1 • 10−9 | 41·1 | 1·6 • 10−3 |
| Focal ischemia | Infarct volume | Anti-oxidants | 18 | 34 | 14 | 6·31 | 2·8 • 10−3 | 13·2 | 0·86 |
| Focal ischemia | Infarct volume | Growth factor | 78 | 128 | 53 | 32·7 | 9·7 • 10−5 | 42·7 | 0·06 |
| Focal ischemia | Infarct volume | Anti-inflammatory | 23 | 44 | 31 | 2·36 | <1 • 10−9 | 20 | 1·2 • 10−3 |
| Focal ischemia | Infarct volume | Estrogens | 33 | 100 | 46 | 10·4 | <1 • 10−9 | 25·6 | 9·7 • 10−6 |
| Focal ischemia | Infarct volume | Urokinase | 12 | 26 | 18 | 2·26 | <1 • 10−9 | 10·1 | 2 • 10−3 |
| Focal ischemia | Infarct volume | NXY-059 | 10 | 29 | 14 | 1·54 | <1 • 10−9 | 11·1 | 0·26 |
| Focal ischemia | Infarct volume | NOS inhibitors | 65 | 148 | 63 | 34·6 | 2·6 • 10−7 | 33 | 5 • 10−8 |
| Focal ischemia | Infarct volume | Tacrolimus (FK506) | 29 | 95 | 55 | 15·8 | <1 • 10−9 | 50·3 | 0·36 |
| ICH | Neurobehavioural score | Minocycline | 3 | 9 | 3 | 0·45 | 8·5 • 10−3 | 0·46 | 9 • 10−3 |
| ICH | Neurobehavioural score | Anti-inflammatory | 7 | 26 | 15 | 7·32 | 1·7 • 10−3 | 13·6 | 0·70 |
| ICH | Neurobehavioural score | Anti-oxidants | 9 | 32 | 12 | 1·6 | 2 • 10−8 | 2·03 | 3 • 10−7 |
| ICH | Neurobehavioural score | Atorvastatin | 2 | 6 | 3 | 0·31 | 2·5 • 10−3 | 2·97 | 0·99 |
| ICH | Neurobehavioural score | Iron chelator | 6 | 14 | 7 | 1·9 | 1·2 • 10−3 | 6·35 | 0·79 |
| ICH | Brain water content | Anti-inflammatory | 7 | 13 | 4 | 1·04 | 0·02 | 4·05 | NP |
| ICH | Brain water content | Anti-oxidants | 5 | 29 | 9 | 3·12 | 2·6 • 10−3 | 2·15 | 1·7 • 10−4 |
| ICH | Brain water content | Deferoxamine | 2 | 12 | 6 | 1·05 | 2·6 • 10−4 | 5·58 | 0·99 |
| ICH | Brain water content | Estradiol | 2 | 4 | 2 | 0·28 | 0·03 | 1·06 | 0·29 |
| ICH | Hematoma volume | Anti-inflammatory | 5 | 8 | 2 | 0·4 | 0·06 | 0·84 | 0·2 |
| PD | Various | PHNO | 15 | 41 | 6 | 2·05 | 0·02 | 14·8 | NP |
| PD | Various | 7-OH-DPAT | 4 | 7 | 4 | 0·8 | 4·4 • 10−3 | 4·12 | NP |
| PD | Rotational behavior | Aplindore | 4 | 7 | 7 | 3·49 | 7·7 • 10−3 | 6·99 | 0·99 |
| PD | Various | Apomorphine | 54 | 156 | 63 | 32·2 | 2 • 10−8 | 30·3 | <1 • 10−9 |
| PD | Various | Cabergoline | 6 | 14 | 4 | 0·91 | 0·01 | 1·9 | 0·11 |
| PD | Various | Pergolide | 8 | 14 | 4 | 1·3 | 0·03 | 3·25 | 0·75 |
| PD | Various | Piribedil | 12 | 42 | 5 | 2·17 | 0·06 | 10·4 | NP |
| PD | Various | Ropinirole | 13 | 33 | 9 | 4·22 | 0·03 | 19·1 | NP |
| PD | Various | S32504 | 15 | 36 | 14 | 2·5 | 5 • 10−8 | 12·7 | 0·73 |
| PD | Various | SKF 83959 | 3 | 4 | 2 | 0·39 | 0·05 | 0·96 | 0·25 |
| PD | Various | SKF 38393 | 14 | 38 | 12 | 6·33 | 0·03 | 5·62 | 9·2 • 10−3 |
| PD | Various | SKF 82958 | 10 | 16 | 7 | 2·12 | 2·7 • 10−3 | 5·73 | 0·6 |
| SCI | Neurobehavior-motor | Stem cells | 89 | 186 | 85 | 16·5 | <1 • 10−9 | 85·8 | NP |
Expected number of statistically significant studies using the effect of the most precise study of each meta-analysis as the plausible effect size.
Expected number of statistically significant studies using the summary fixed effects estimate of each meta-analysis as the plausible effect size.
p-value of the excess statistical significance test· All statistical tests were two-sided.
7-OH-DPAT, a dopamine D3 receptor agonist; NP, not pertinent, because the estimated E is larger than the O, and there is no evidence of excess statistical significance based on the assumption made for the plausible effect size; NXY-059, disufenton sodium; PHNO, a dopamine D2 receptor agonist; S32504, a dopamine D3/D2 receptor agonist; SKF 82958, a benzazepine D1/D5 dopamine agonist; SKF 38393, a benzazepine D1/D5 dopamine agonist; SKF 83959, a benzazepine D1/D2 dopamine agonist.
When the excess of significance was examined in aggregate across all 4,445 studies (Table 3), excess significance was present when assuming as plausible effect the effect of the most precise study (p<1.10−9). The observed number of “positive” studies was O = 1,719, while the expected was E = 919. Excess significance was also documented in studies of each of the six disease categories. An excess of “positive” studies was observed also when assuming the summary fixed effect as the plausible effect size (p<1.10−9).
Table 3. Observed and expected number of “positive” studies by type of neurological disease.
| Disease | Comparisons | Observed Positive | Most Precise Study Effect | Summary Fixed Effect | ||
| Expected Positivea | p-Value* | Expected Positiveb | p-Value* | |||
| All | 4,445 | 1,719 | 919 | <1 • 10−9 | 1,450 | <1 • 10−9 |
| AD | 1,054 | 418 | 86·4 | <1 • 10−9 | 277 | <1 • 10−9 |
| EAE | 483 | 212 | 131 | <1 • 10−9 | 187 | 0·02 |
| Focal ischemia | 1,403 | 626 | 370 | <1 • 10−9 | 517 | <1 • 10−9 |
| ICH | 424 | 145 | 108 | 5·6 • 10−5 | 130 | 0·11 |
| PD | 873 | 228 | 202 | 0·04 | 254 | NP |
| SCI | 208 | 90 | 21·6 | <1 • 10−9 | 90·3 | NP |
Expected number of statistically significant studies using the effect of the most precise study of each meta-analysis as the plausible effect size.
Expected number of statistically significant studies using the summary fixed effects estimate of each meta-analysis as the plausible effect size.
p-Value of the excess statistical significance test. All statistical tests were two-sided.
NP, not pertinent, because the estimated E is larger than the O, and there is no evidence of excess statistical significance based on the assumption made for the plausible effect size.
Similar results were observed in analyses according to methodological or reporting characteristics of included studies (Table 4). Under the assumption of the effect of the most precise study being the plausible effect, there was evidence of excess significance in all subgroups. However, the strongest excesses of significance (as characterized by the ratio of O over E) were recorded specifically in meta-analyses where small-study effects had also been documented (O/E = 2.94), in those meta-analyses with the least precise studies (O/E = 2.94 in the bottom quartile of weight), and in those meta-analyses where the corresponding studies included a statement about the presence of conflict of interest (O/E = 3.27). Under the assumption of the summary fixed effects being the plausible effect size, excess significance was still formally documented in the large majority of subgroups, but none had such extreme O/E ratios (Table 4).
Table 4. Observed and expected number of “positive” studies for all neurological diseases in subgroups.
| Subgroup | Studies | Observed Positive (O) | Most Precise Study Effect | Summary Fixed Effect | ||||
| Expected Positive (E)a | O/E Ratio | p-Value* | Expected Positive (E)b | O/E Ratio | p-Value* | |||
| Heterogeneity | ||||||||
| I2≤50% | 1,098 | 264 | 217 | 1·22 | 5.6 • 10−4 | 317 | 0·83 | NP |
| I2>50% | 3,347 | 1,455 | 702 | 2·07 | <1 • 10−9 | 1,140 | 1·28 | <1 • 10−9 |
| Small-study effects | ||||||||
| Yes | 2,773 | 1,139 | 387 | 2·94 | <1 • 10−9 | 953 | 1·20 | <1 • 10−9 |
| No | 1,672 | 580 | 532 | 1·09 | 0·01 | 501 | 1·16 | 3·5 • 10−5 |
| Meta-analysis (fixed-effect) | ||||||||
| Significant | 4,055 | 1,644 | 871 | 1·89 | <1 • 10−9 | 1,420 | 1·16 | <1 • 10−9 |
| Non-significant | 390 | 75 | 47·5 | 1·58 | 6·9 • 10−5 | 30·2 | 2·48 | <1 • 10−9 |
| Weight (1/SE2) of the most precise studyc | ||||||||
| Q1 | 1,275 | 553 | 188 | 2·94 | <1 • 10−9 | 422 | 1·31 | <1 • 10−9 |
| Q2 | 1,051 | 402 | 204 | 1·97 | <1 • 10−9 | 293 | 1·37 | <1 • 10−9 |
| Q3 | 1,032 | 424 | 229 | 1·85 | <1 • 10−9 | 350 | 1·21 | 1·8 • 10−6 |
| Q4 | 1,087 | 340 | 298 | 1·14 | 4·7 • 10−3 | 390 | 0·87 | NP |
| Random allocation | ||||||||
| Yes | 994 | 415 | 200 | 2·08 | <1 • 10−9 | 346 | 1·20 | 6·7 • 10−6 |
| No | 3,185 | 1,207 | 641 | 1·88 | <1 • 10−9 | 1,020 | 1·18 | <1 • 10−9 |
| Blinded induction | ||||||||
| Yes | 208 | 74 | 42·6 | 1·74 | 4·4 • 10−7 | 79·4 | 0·93 | NP |
| No | 2,615 | 1,108 | 465 | 2·38 | <1 • 10−9 | 845 | 1·31 | <1 • 10−9 |
| Blinded outcome | ||||||||
| Yes | 1,236 | 482 | 230 | 2·10 | <1 • 10−9 | 418 | 1·15 | 1·3 • 10−4 |
| No | 2,943 | 1,140 | 610 | 1·87 | <1 • 10−9 | 947 | 1·20 | <1 • 10−9 |
| Sample size calculation | ||||||||
| Yes | 39 | 8 | 3·87 | 2·07 | 0·05 | 14·9 | 0·54 | NP |
| No | 4,140 | 1,614 | 837 | 1·93 | <1 • 10−9 | 1,350 | 1·20 | <1 • 10−9 |
| Animal welfare compliance | ||||||||
| Yes | 2,436 | 943 | 467 | 2·02 | <1 • 10−9 | 805 | 1·17 | <1 • 10−9 |
| No | 1,743 | 679 | 373 | 1·82 | <1 • 10−9 | 559 | 1·21 | <1 • 10−9 |
| Conflict of interest | ||||||||
| Yes | 319 | 160 | 49·2 | 3·25 | <1 • 10−9 | 109 | 1·47 | 1 • 10−8 |
| No | 3,860 | 1,462 | 791 | 1·85 | <1 • 10−9 | 1,260 | 1·16 | <1 • 10−9 |
Expected number of statistically significant studies using the effect of the most precise study of each meta-analysis as the plausible effect size.
Expected number of statistically significant studies using the summary fixed effects estimate of each meta-analysis as the plausible effect size.
p-Value of the excess statistical significance test· All statistical tests were two-sided.
Quartiles of the weight of the most precise study in each meta-analysis.
NP, not pertinent, because the estimated E is larger than the O, and there is no evidence of excess statistical significance based on the assumption made for the plausible effect size.
Interventions with Strong Evidence of Association
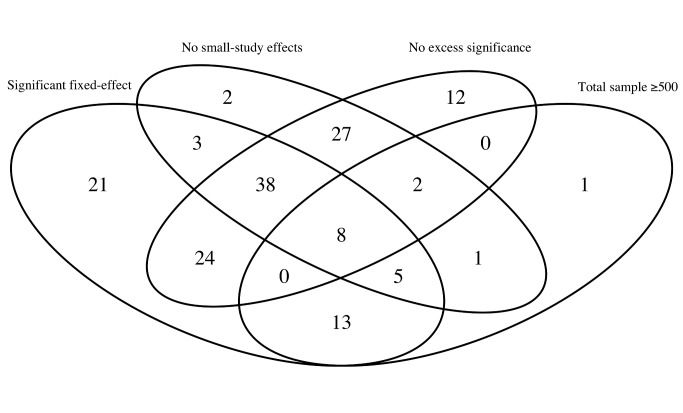
Only 46 meta-analyses (29%) found interventions with a nominally significant effect per fixed-effects synthesis and no evidence of small-study effects or excess significance (when this calculation was based on the plausible effect being that of the most precise study) (Figure 1). Of those, only eight had a total sample size of over 500 animals: one pertained to EAE (myelin basic protein [MBP]), four pertained to focal ischemia (minocycline, melatonin, nicotinamide, nitric oxide species [NOS] donors), one pertained to ICH (stem cells), and two to PD (bromocriptine, quinpirole).
Figure 1. Venn diagrams of the meta-analyses of animal studies of neurological disorders.
We plotted the number of studies with a total sample size of at least 500 animals; those which showed a nominally (p≤0.05) statistically significant effect per fixed-effects synthesis; those that had no evidence of small-study effects; and those that had no evidence of excess significance. The numbers represent the studies that have two or more of the above characteristics according to the respective overlapping areas.
Discussion
We evaluated 160 meta-analyses of animal studies describing six neurological conditions, most of which had found a nominally (p≤0.05) statistically significant fixed-effects summary favoring the experimental intervention. The number of nominally statistically significant results in the component studies of these meta-analyses was too large to be true, and this evidence of excess significance was present in studies across all six neurological diseases. Overall, only eight of the 160 meta-analyses had nominally significant results, no suggestion of bias related to small-study effects or excess of significant findings, and evidence procured from over 500 animals.
Animal studies represent a considerable proportion of the biomedical literature with approximately five million papers indexed in PubMed [8]. These studies are conducted to do a first-pass evaluation of the effectiveness and safety of therapeutic interventions. However, there is great discrepancy between the intervention effects found in preclinical animal studies and those found in clinical trials of humans with most of these interventions rarely achieving successful translation [2],[3],[18]. Possible explanations for this failure include differences in the underlying biology and pathophysiology between humans and animals, but also the presence of biases in study design or reporting of the animal literature.
Our empirical evaluation of animal studies on neurological disorders found a significant excess of nominally statistically significant studies, which suggests the presence of strong study design or reporting biases. Prior evaluations of animal studies had also noted that alarmingly the vast majority of the published studies had statistically significant associations, and had suggested high prevalence of publication bias [9],[19], resulting in spurious claims of effectiveness. We observed excessive nominally significant results in all subgroup categories defined by random allocation of treatment, blinded induction of treatment, blinded assessment of the outcome, sample size calculation, or compliance to animal welfare. This suggests that the excess of significance in animal studies of neurological disorders may reflect reporting biases that operate regardless of study design features. It is nevertheless possible that reporting biases are worst in fields with poor study quality, although this was not clear in our evaluation. Deficiencies in random allocation and blinded induction of the treatment or blinded assessment of the outcome have been associated with inflated efficacy estimates in other evaluations of animal research [20],[21].
We also documented very prominent excess of significant results (observed “positive” results being three times the number of expected) for interventions that had also evidence of small-study effects and in meta-analyses with the least precise studies. Both of these observations are commensurate with reporting bias being the explanation for the excess significance, with bias being more prominent in smaller studies and becoming more discernible when sufficiently precise studies are also available.
Conventional publication bias (non-publication of neutral/negative results), may exist in the literature of animal studies on neurological disorders. Our evaluation showed that 46% of the meta-analyses had evidence of small-study effects, which may signal publication bias. However, this association is not specific, and the Egger test used to evaluate small-study effects is underpowered especially when it evaluates few and small studies in a meta-analysis [13]. It is also likely that selective outcome or analysis reporting biases exist. The animal studies on neurological disorders used many different outcomes and methods to measure each outcome as can be seen across the Table S1, and they may have used different statistical analysis techniques and applied several different rules for inclusion and exclusion of data. Thus, it is possible that individual studies may have measured different outcomes, tested a variety of inclusion and exclusion criteria and performed several statistical analyses, but reported only a few findings guided in part by the significance of the results. Detection of such biases is difficult and no formal well-developed statistical test exists. Evidence is usually indirect and requires access to the study protocol or even communication with the investigators.
In contrast to the above, we found eight interventions with strong and statistically significant benefits in animal models and without evidence of small-study effects or excess significance. However, the data for these interventions may still have compromised internal validity; having identified one of these, melatonin, as a candidate treatment for stroke, we tested efficacy in an animal study designed specifically to avoid some of the prevalent sources of bias. Under these circumstances melatonin had no significant effect on outcome [22].
It is interesting to discuss whether human experimental evidence for these interventions is more promising than the generally disappointing results seen for most interventions that have previously given some signal of effectiveness in animals. A meta-analysis of 33 animal studies showed that administration of MBP reduced the severity of EAE, which is an animal model for multiple sclerosis. However, a phase III randomized clinical trial (RCT) in humans showed no significant differences between MBP and placebo [23]. Minocycline, a tetracycline antibiotic with potential neuroprotective effects, showed improvements in stroke scales in two human RCTs, but these were small phase II trials [24],[25] and have not been confirmed in larger studies. Several animal studies of melatonin, an endogenously produced antioxidant, have reported a beneficial effect on infarct volume [26], but RCTs with clinical endpoints in humans don't exist. A small RCT did not show significant differences in oxidative or inflammatory stress parameters between melatonin and placebo [27]. Administration of nicotinamide, the amide of vitamin B3, to animals with focal ischemia reduced the infarct volume [19], but RCTs have not evaluated clinical outcomes in relation to nicotinamide [28]. Several animal studies of NOS donors, like the anti-anginal drug nicorandil, have shown reductions in infarct volume, and RCTs have also shown that nicorandil improves cardiac function in patients with acute myocardial infarction [29],[30]. Granulocyte-colony stimulating factor (G-CSF), a growth factor that stimulates the bone marrow to produce granulocytes and stem cells, has been reported in animal studies to improve the neurobehavioral score in animals with ICH. Some similar evidence exists from RCTs of stroke patients, but it consists of small phase II trials [31],[32], and an unpublished phase III trial was neutral (Ringelstein P et al., International Stroke Conference, Feb 2012). Bromocriptine and quinpirole are dopamine agonists that have been successfully used in animal studies of PD [33]. Bromocriptine is approved to treat PD in humans [34], but no human trial exists for quinpirole. In spite of this patchy record, interventions with strong evidence of efficacy and no hints of bias in animals may be prioritized for further testing in humans.
Some limitations should be acknowledged in our work. First, asymmetry and excess significance tests offer hints of bias, not definitive proof thereof. Most individual animal studies were small with a median total sample size of 16 animals, and a median number of eight comparisons in each meta-analysis. Therefore, the interpretation of the excess significance test for the results of a single meta-analysis should be very cautious. A negative test for excess significance does not exclude the potential for bias [14]. The most useful application of the excess significance test is to give an overall impression about the average level of bias affecting the whole field of animal studies on neurological disorders.
Second, the exact estimation of excess statistical significance is influenced by the choice of plausible effect size. We performed analyses using different plausible effect sizes, including the effect of the most precise study in each meta-analysis, and the summary fixed effect; these yielded similar findings. Effect inflation may affect even the results of the most precise studies, since often these were not necessarily very large or may have had inherent biases themselves, or both. Thus, our estimates of the extent of excess statistical significance are possibly conservative, and the problem may be more severe.
Third, we evaluated a large number of meta-analyses on six neurological conditions, but our findings might not necessarily be representative of the whole animal literature. However, biases and methodological deficits have been described for many animal studies regardless of disease domain [1],[2],[21].
In conclusion, the literature of animal studies on neurological disorders is probably subject to considerable bias. This does not mean that none of the observed associations in the literature are true. For example, we showed evidence of eight strong and statistically significant associations without evidence of small-study effects or excess significance. However, only two (NOS donors and focal ischemia, and bromocriptine and PD) of even these eight associations seem to have convincing RCT data in humans. We support measures to minimize bias in animal studies and to maximize successful translation into human applications of promising interventions [35]. Study design, conduct, and reporting of animal studies can be improved by following published guidelines for reporting animal research [35],[36]. Publication and selective reporting biases may be diminished by preregistering experimental animal studies. Access to the study protocol and also to raw data and analyses would allow verification of their results, and make their integration with other parallel or future efforts easier. Systematic reviews and meta-analyses and large consortia conducting multi-centre animal studies should become routine to ensure the best use of existing animal data, and to aid in the selection of the most promising treatment strategies to enter human clinical trials.
Materials and Methods
Study Identification
We used data from published and unpublished meta-analyses of interventions tested in animal studies of six neurological diseases (AD, EAE, focal ischemia, ICH, PD, and SCI) deposited in the database of Collaborative Approach to Meta-Analysis and Review of Animal Data in Experimental Studies (CAMARADES), which is an international collaboration established in 2004 with an aim to support meta-analyses of animal data [20]. The database is representative of the literature, and includes details of each individual experiment. Out of the total of 14 published meta-analyses of animal stroke studies, 11 are part of the CAMARADES database. Animal studies report a variety of outcome measures often measured from the same cohort of animals. To ensure independence, we only used one outcome analysis per animal cohort. Where multiple outcomes were reported from a single cohort, we chose the one that was most frequently found in each meta-analysis. We abstracted the following information from each study: publication year, intervention, outcome, animal cohort, effect size and standard error, number of affected animals in the treatment and control group, and ten binary methodological criteria (peer-reviewed publication, statement of control of temperature, random allocation to treatment or control, blinded induction of treatment, blinded assessment of outcome, use of anesthetic without significant intrinsic neuroprotective activity, appropriate animal model, sample size calculation, compliance with animal welfare regulations, and statement of potential conflict of interest), which were based on relevant guidelines for reporting animal research [35]–[38].
Estimation of Summary Effect and Heterogeneity
For each meta-analysis, we estimated the summary effect size and its confidence intervals using both fixed and random effect models [39]. All outcomes included in the database were measured on a continuous scale, and hence we used the standardized mean difference as the effect size. We also tested for between-study heterogeneity estimating the p-value of the χ2-based Cochran Q test, and the I2 metric of inconsistency. Q is obtained by the weighted sum of the squared differences of the observed effect in each study minus the fixed summary effect [40]. I2 ranges from 0% to 100% and describes the percentage of variation across studies that is attributed to heterogeneity rather than chance [41]. The corresponding 95% CIs were also calculated [42].
Asymmetry Tests for Small-Study Effects
We evaluated whether there is evidence for small-study effects, i.e., whether smaller studies give substantially different estimates of effect size compared to larger studies. This may offer a hint for publication or other selective reporting biases, but they may also reflect genuine heterogeneity, chance or other reasons for differences between small and large studies [11]. We applied the regression asymmetry test proposed by Egger et al [43]. A p≤0.10 with more conservative effect in larger studies was considered evidence for small-study effects.
Evaluation of Excess Significance
We applied the excess significance test, which is an exploratory test that evaluates whether there is a relative excess of formally significant findings in the published literature due to any reason. This test evaluates whether the observed number of studies (O) with nominally statistically significant results (“positive” studies, p≤0.05) within a meta-analysis differs from their expected number (E). “Positive” findings were counted in both directions, i.e., when the experimental intervention is more beneficial compared to the control, as well as when the experimental intervention is harmful. If there is no excess significance bias, then O = E. The greater the difference between O and E, the greater is the extent of excess significance bias.
We used a binomial test, as previously presented in detail [14]. This test evaluates whether the number of “positive” studies, among those in a meta-analysis, is too large based on the power that these studies have to detect plausible effects at α = 0.05. The O versus E comparison is performed separately for each meta-analysis, and it is also extended to many meta-analyses after summing O and E across meta-analyses.
E is calculated in each meta-analysis by the sum of the statistical power estimates for each component study. The estimated power of each component study depends on the plausible effect size for the tested animal study association. The true effect size for any meta-analysis is not known. We performed the main analysis using the effect size of the most precise study (with the smallest standard error) in a meta-analysis as the plausible effect. The estimate from this most precise study, other things being equal, should be closer to the true estimate than the results of less precise studies, especially if biases affect predominantly the literature of smaller studies (small-study effects) [44]–[46]. Additionally, we conducted sensitivity analysis using as the plausible effect size the fixed effects summary from each meta-analysis. In the presence of bias these summary fixed effects may be larger than the true effects, and this situation may arise even for the effect estimate of the most precise study, albeit to a smaller extent. Therefore, all these assumptions tend to be conservative in testing for excess significance. We did not use the random effects summary, because it tends to be overtly inflated in the presence of small-study effects, as the small studies receive increased relative weight in random effects calculations [47].
The power of each study was calculated using the Wilcoxon rank sum test under the family of Lehmann alternative hypotheses in a freely available software [48], which is an exact calculation suitable for the very small sample sizes that are typical of many animal studies [49]. Excess significance for single meta-analyses was claimed at p≤0.10 (one-sided p≤0.05 with O>E as previously proposed), since the test is expected to have low power to detect a specific excess of significant findings especially when there are a few “positive” studies [14].
We assessed excess significance in aggregate across all six neurological diseases, and separately in each one of them, as selective reporting bias may affect different research domains to a different extent. These domains may also have different typical magnitudes of effect sizes for the tested interventions, and different analytical biases even if research is sometimes conducted by the same teams across various domains.
The excess significance test was also performed in subgroups: for meta-analyses with I2≤50% and >50%, as values exceeding 50% are typically considered evidence of large heterogeneity beyond chance [50]; by evidence or not of small-study effects as per Egger's test; by whether the summary fixed effect of the respective meta-analysis was nominally statistically significant or not; by quartiles of the weight of the most precise study for each meta-analysis; by use of random allocation of the treatment or not; by use of blinded induction of the treatment or not; by use of blinded assessment of the outcome or not; by whether the sample size calculation was described or not; by whether the study reported to comply to the animal welfare regulations or not; and by whether potential conflicts of interest were reported or not.
Supporting Information
Analytical description of the 160 meta-analyses with observed and expected numbers of “positive” study datasets.
(XLSX)
Acknowledgments
We would like to acknowledge the support of the Edinburgh MRC Trials Methodology Hub; and Hanna Vesterinen, Kieren Egan, Joseph Frantzias, and Ana Antonic for data collection.
Abbreviations
- AD
Alzheimer disease
- CAMARADES
Collaborative Approach to Meta-Analysis and Review of Animal Data in Experimental Studies
- E
number of expected studies with statistically significant results
- EAE
experimental autoimmune encephalomyelitis
- ICH
intracerebral hemorrhage
- IQR
interquartile range
- MBP
myelin basic protein
- NOS
nitric oxide species
- O
number of observed studies with statistically significant results
- PD
Parkinson disease
- RCT
randomized clinical trial
- SCI
spinal cord injury
Funding Statement
No specific and/or direct funding was received for this study. No funding bodies played any role in the design, writing or decision to publish this manuscript. The authors were personally salaried by their institutions during the period of writing though no specific salary was set aside or given for the writing of this paper. There are no current external funding sources for this study.
References
- 1. van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, et al. (2010) Can animal models of disease reliably inform human studies? PLoS Med 7: e1000245 doi:10.1371/journal.pmed.1000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perel P, Roberts I, Sena E, Wheble P, Briscoe C, et al. (2007) Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ 334: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pound P, Ebrahim S, Sandercock P, Bracken MB, Roberts I (2004) Where is the evidence that animal research benefits humans? BMJ 328: 514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, et al. (2006) 1,026 experimental treatments in acute stroke. Ann Neurol 59: 467–477. [DOI] [PubMed] [Google Scholar]
- 5. Sena E, van der Worp HB, Howells D, Macleod M (2007) How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci 30: 433–439. [DOI] [PubMed] [Google Scholar]
- 6. Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA (2005) Systematic review and metaanalysis of the efficacy of FK506 in experimental stroke. J Cereb Blood Flow Metab 25: 713–721. [DOI] [PubMed] [Google Scholar]
- 7. Macleod MR, van der Worp HB, Sena ES, Howells DW, Dirnagl U, et al. (2008) Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke 39: 2824–2829. [DOI] [PubMed] [Google Scholar]
- 8. Ioannidis JP (2012) Extrapolating from animals to humans. Sci Transl Med 4: 151ps115. [DOI] [PubMed] [Google Scholar]
- 9. Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR (2010) Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol 8: e1000344 doi:10.1371/journal.pbio.1000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ioannidis JP (2008) Why most discovered true associations are inflated. Epidemiology 19: 640–648. [DOI] [PubMed] [Google Scholar]
- 11. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, et al. (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343: d4002. [DOI] [PubMed] [Google Scholar]
- 12. Ioannidis JP, Trikalinos TA (2007) The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ 176: 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I (2006) The case of the misleading funnel plot. BMJ 333: 597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ioannidis JP, Trikalinos TA (2007) An exploratory test for an excess of significant findings. Clin Trials 4: 245–253. [DOI] [PubMed] [Google Scholar]
- 15. Tsilidis KK, Papatheodorou SI, Evangelou E, Ioannidis JP (2012) Evaluation of excess statistical significance in meta-analyses of 98 biomarker associations with cancer risk. J Natl Cancer Inst 104: 1867–1878. [DOI] [PubMed] [Google Scholar]
- 16. Ioannidis JP (2011) Excess significance bias in the literature on brain volume abnormalities. Arch Gen Psychiatry 68: 773–780. [DOI] [PubMed] [Google Scholar]
- 17. Kavvoura FK, McQueen MB, Khoury MJ, Tanzi RE, Bertram L, et al. (2008) Evaluation of the potential excess of statistically significant findings in published genetic association studies: application to Alzheimer's disease. Am J Epidemiol 168: 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hackam DG, Redelmeier DA (2006) Translation of research evidence from animals to humans. JAMA 296: 1731–1732. [DOI] [PubMed] [Google Scholar]
- 19. Macleod MR, O'Collins T, Howells DW, Donnan GA (2004) Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 35: 1203–1208. [DOI] [PubMed] [Google Scholar]
- 20. Crossley NA, Sena E, Goehler J, Horn J, van der Worp B, et al. (2008) Empirical evidence of bias in the design of experimental stroke studies: a metaepidemiologic approach. Stroke 39: 929–934. [DOI] [PubMed] [Google Scholar]
- 21. Bebarta V, Luyten D, Heard K (2003) Emergency medicine animal research: does use of randomization and blinding affect the results? Acad Emerg Med 10: 684–687. [DOI] [PubMed] [Google Scholar]
- 22. O'Collins VE, Macleod MR, Cox SF, Van Raay L, Aleksoska E, et al. (2011) Preclinical drug evaluation for combination therapy in acute stroke using systematic review, meta-analysis, and subsequent experimental testing. J Cereb Blood Flow Metab 31: 962–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Freedman MS, Bar-Or A, Oger J, Traboulsee A, Patry D, et al. (2011) A phase III study evaluating the efficacy and safety of MBP8298 in secondary progressive MS. Neurology 77: 1551–1560. [DOI] [PubMed] [Google Scholar]
- 24. Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, et al. (2007) Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology 69: 1404–1410. [DOI] [PubMed] [Google Scholar]
- 25. Padma Srivastava MV, Bhasin A, Bhatia R, Garg A, Gaikwad S, et al. (2012) Efficacy of minocycline in acute ischemic stroke: a single-blinded, placebo-controlled trial. Neurol India 60: 23–28. [DOI] [PubMed] [Google Scholar]
- 26. Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA (2005) Systematic review and meta-analysis of the efficacy of melatonin in experimental stroke. J Pineal Res 38: 35–41. [DOI] [PubMed] [Google Scholar]
- 27. Kucukakin B, Wilhelmsen M, Lykkesfeldt J, Reiter RJ, Rosenberg J, et al. (2010) No effect of melatonin to modify surgical-stress response after major vascular surgery: a randomised placebo-controlled trial. Eur J Vasc Endovasc Surg 40: 461–467. [DOI] [PubMed] [Google Scholar]
- 28. Whitney EJ, Krasuski RA, Personius BE, Michalek JE, Maranian AM, et al. (2005) A randomized trial of a strategy for increasing high-density lipoprotein cholesterol levels: effects on progression of coronary heart disease and clinical events. Ann Intern Med 142: 95–104. [DOI] [PubMed] [Google Scholar]
- 29. Ono H, Osanai T, Ishizaka H, Hanada H, Kamada T, et al. (2004) Nicorandil improves cardiac function and clinical outcome in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: role of inhibitory effect on reactive oxygen species formation. Am Heart J 148: E15. [DOI] [PubMed] [Google Scholar]
- 30. Sugimoto K, Ito H, Iwakura K, Ikushima M, Kato A, et al. (2003) Intravenous nicorandil in conjunction with coronary reperfusion therapy is associated with better clinical and functional outcomes in patients with acute myocardial infarction. Circ J 67: 295–300. [DOI] [PubMed] [Google Scholar]
- 31. England TJ, Abaei M, Auer DP, Lowe J, Jones DR, et al. (2012) Granulocyte-colony stimulating factor for mobilizing bone marrow stem cells in subacute stroke: the stem cell trial of recovery enhancement after stroke 2 randomized controlled trial. Stroke 43: 405–411. [DOI] [PubMed] [Google Scholar]
- 32. Shyu WC, Lin SZ, Lee CC, Liu DD, Li H (2006) Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ 174: 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rooke ED, Vesterinen HM, Sena ES, Egan KJ, Macleod MR (2011) Dopamine agonists in animal models of Parkinson's disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 17: 313–320. [DOI] [PubMed] [Google Scholar]
- 34. Libman I, Gawel MJ, Riopelle RJ, Bouchard S (1987) A comparison of bromocriptine (Parlodel) and levodopa-carbidopa (Sinemet) for treatment of “de novo” Parkinson's disease patients. Can J Neurol Sci 14: 576–580. [PubMed] [Google Scholar]
- 35. Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, et al. (2012) A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412 doi:10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stroke Therapy Academic Industry Roundtable (STAIR) (1999) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 30: 2752–2758. [DOI] [PubMed] [Google Scholar]
- 38. Macleod MR, Fisher M, O'Collins V, Sena ES, Dirnagl U, et al. (2009) Good laboratory practice: preventing introduction of bias at the bench. Stroke 40: e50–52. [DOI] [PubMed] [Google Scholar]
- 39. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 40. Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10: 101–129. [Google Scholar]
- 41. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 42. Ioannidis JP, Patsopoulos NA, Evangelou E (2007) Uncertainty in heterogeneity estimates in meta-analyses. BMJ 335: 914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sena ES, Briscoe CL, Howells DW, Donnan GA, Sandercock PA, et al. (2010) Factors affecting the apparent efficacy and safety of tissue plasminogen activator in thrombotic occlusion models of stroke: systematic review and meta-analysis. J Cereb Blood Flow Metab 30: 1905–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR (2010) Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol 8: e1000344 doi:10.1371/journal.pbio.1000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vesterinen HM, Sena ES, ffrench-Constant C, Williams A, Chandran S, et al. (2010) Improving the translational hit of experimental treatments in multiple sclerosis. Mult Scler 16: 1044–1055. [DOI] [PubMed] [Google Scholar]
- 47.Higgins J, Green S, editors (2008) Cochrane Handbook for Systematic Reviews of Interventions. Chichester, England: The Cochrane Collaboration and John Wiley & Sons Ltd.
- 48. Erdfelder E, Faul F, Buchner A (1996) GPOWER: A general power analysis program. Behav Res Methods Instrum Comput 28: 1–11. [Google Scholar]
- 49. Heller G (2006) Power calculations for preclinical studies using a K-sample rank test and the Lehmann alternative hypothesis. Stat Med 25: 2543–2553. [DOI] [PubMed] [Google Scholar]
- 50. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analytical description of the 160 meta-analyses with observed and expected numbers of “positive” study datasets.
(XLSX)