Abstract
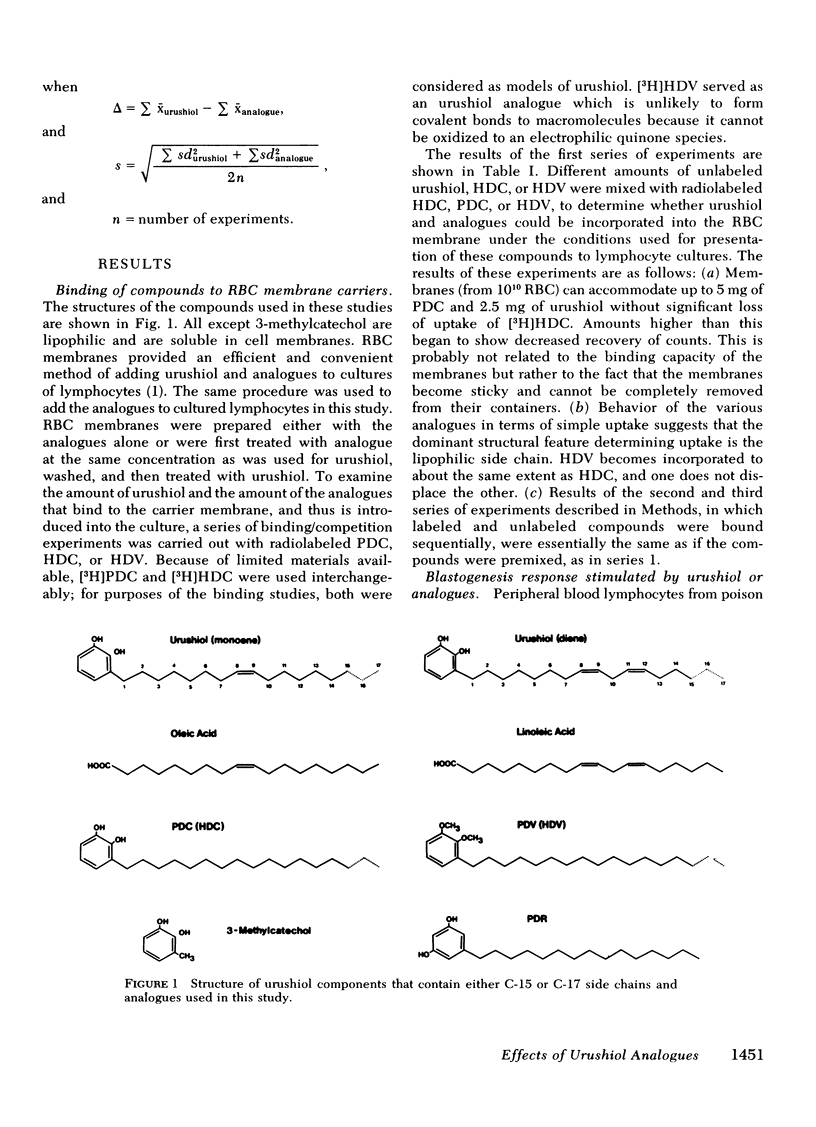
Studies were performed to ascertain the effect of urushiol analogues on the in vitro lymphocyte blastogenesis elicited by urushiol in peripheral blood lymphocytes taken from individuals sensitized to poison oak or ivy. Urushiol is a mixture of alkylcatechols composed of a catechol ring coupled to mono-, di-, or tri-unsaturated C-15 or C-17 carbon side chains. Each of these two moieties, catechol ring and side chain, was tested for its role in eliciting reactivity. Analogues tested represented the catechol ring (3-methylcatechol), the mono- or di-unsaturated side chain (oleic or linoleic acid), and the saturated side chain coupled to a catechol ring (pentadecylcatechol), a blocked catechol ring (heptadecylveratrole), or a resorcinol (pentadecylresorcinol). Urushiol with a blocked catechol ring (urushiol dimethyl ether) was also included.
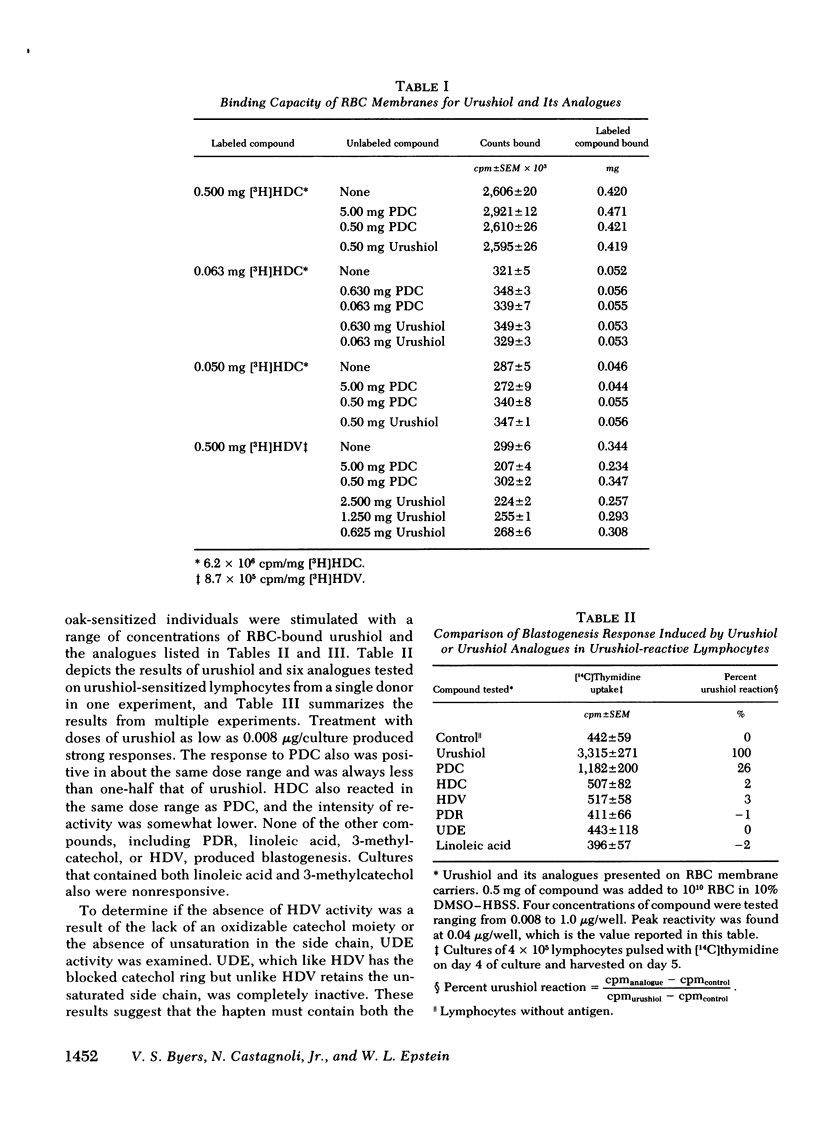
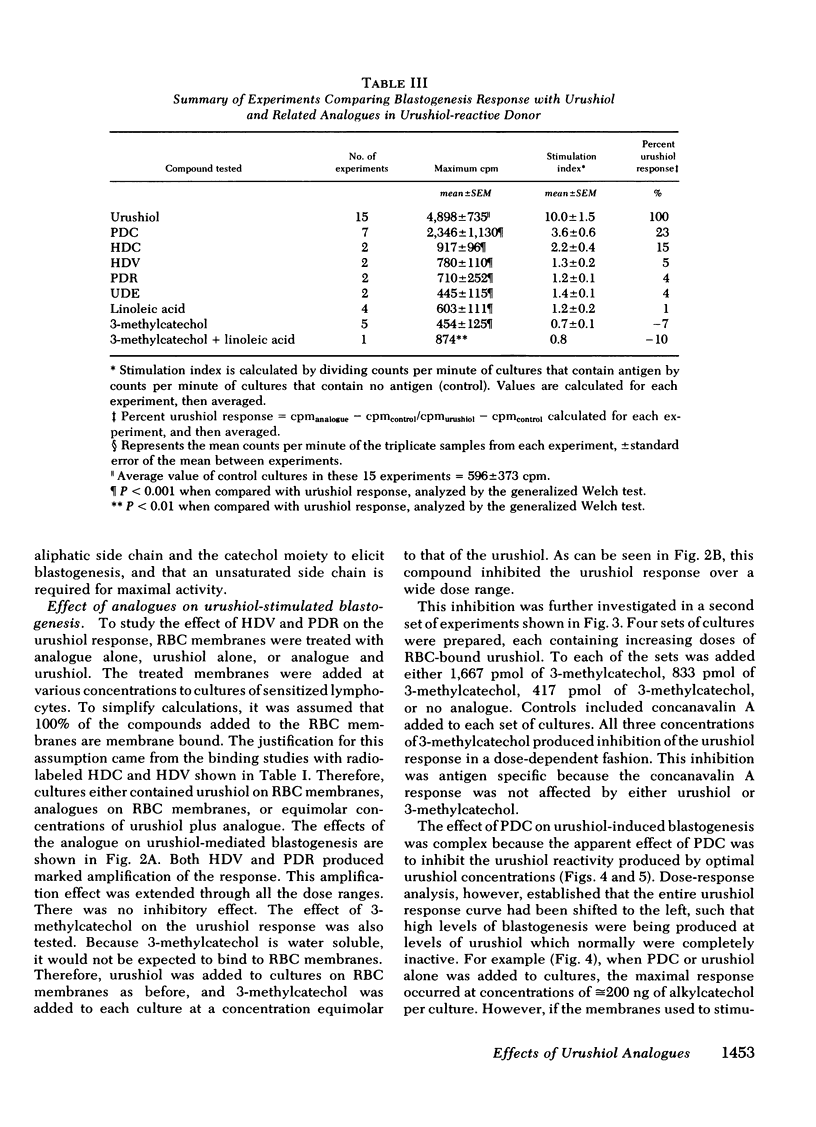
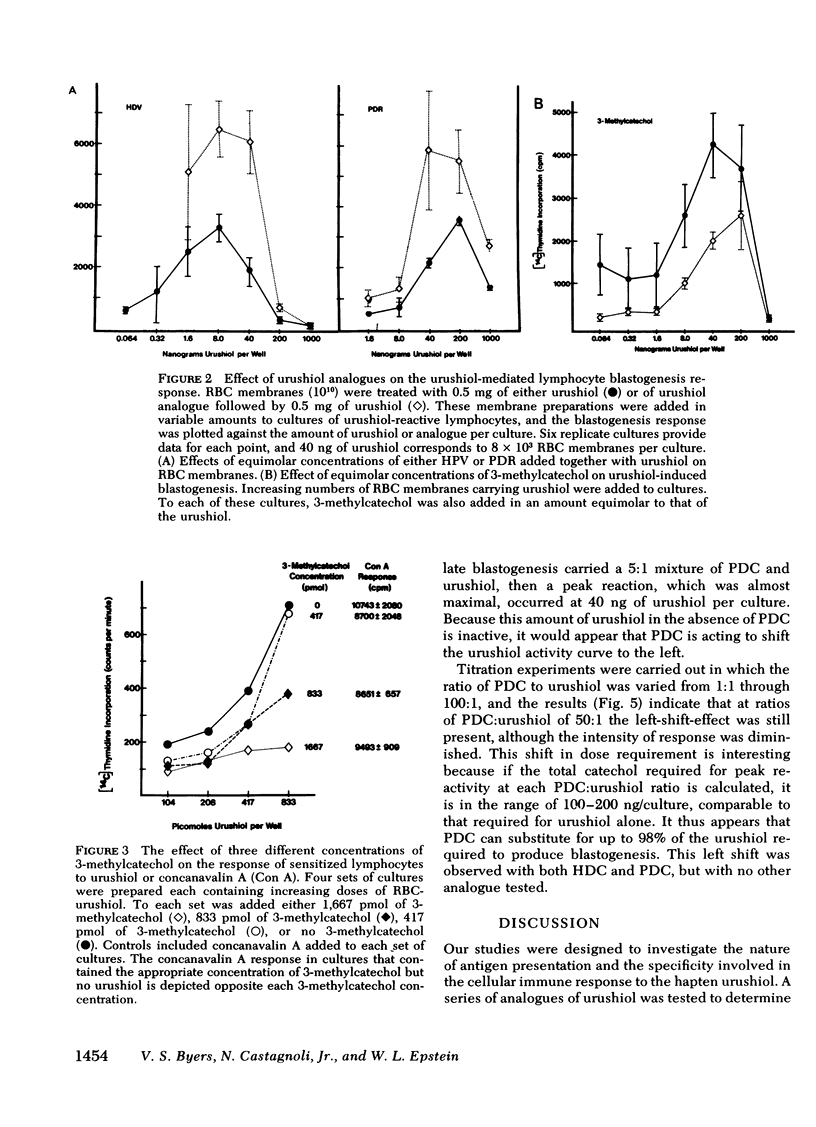
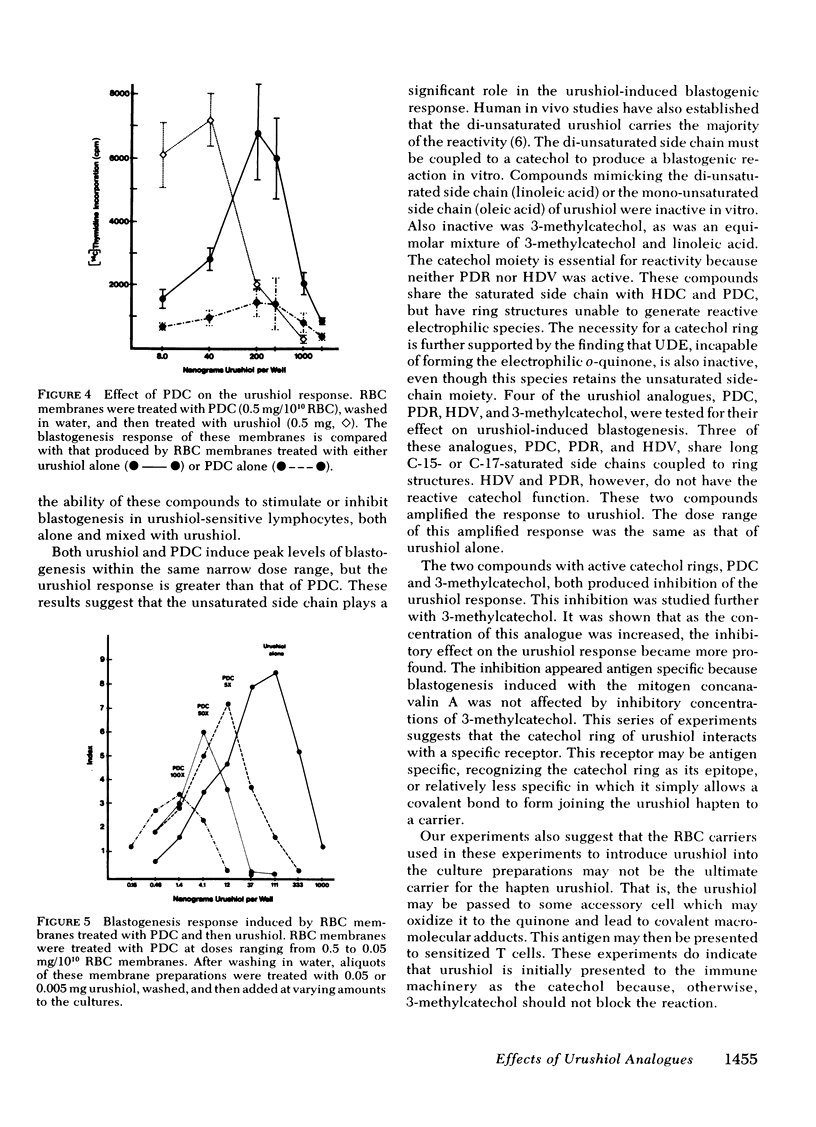
Of these, only pentadecylcatechol evoked reactivity in sensitized lymphocytes, and this reactivity was only a fraction of that evoked by urushiol. This suggested that the system has some requirement for the side chain, and that the catechol ring is critical for reactivity. This was further investigated by testing the ability of some of these analogues to inhibit urushiol-specific blastogenesis. No inhibition was noted with compounds bearing the saturated side chain with modified ring structures (pentadecylresorcinol and heptadecylveratrole). However, both 3-methylcatechol and pentadecylcatechol (at equimolar concentrations) blocked reactivity. The results of our experiments suggested that although both the side chain and the catechol ring are required for reactivity, the latter is most critical. Unsaturation in the side chain is important for maximal reactivity because the saturated catechols were only partially as active as the urushiol oil. There may be a greater dose requirement for the catechol ring than for the side chain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byers V. S., Epstein W. L., Castagnoli N., Baer H. In vitro studies of poison oak immunity. I. In vitro reaction of human lymphocytes to urushiol. J Clin Invest. 1979 Nov;64(5):1437–1448. doi: 10.1172/JCI109602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Haer H., Kirkpatrick C. H., Dawson C. R., Khurana R. G. Comparison of the contact allergenicity of the four pentadecylcatechols derived from poison ivy urushiol in human subjects. J Allergy Clin Immunol. 1972 Jan;49(1):27–35. doi: 10.1016/0091-6749(72)90120-0. [DOI] [PubMed] [Google Scholar]
- Ozato K., Ziegler H. K., Henney C. S. Liposomes as model membrane systems for immune attack. I. Transfer of antigenic determinants to lymphocyte membranes after interactions with hapten-bearing liposomes. J Immunol. 1978 Oct;121(4):1376–1382. [PubMed] [Google Scholar]
- Thomas D. W., Shevach E. M. Nature of the antigenic complex recognized by T lymphocytes. VI. The effect of anti-TNP antibody on T cell responses to TNP-conjugated macrophages. J Immunol. 1978 Sep;121(3):1145–1151. [PubMed] [Google Scholar]
- Thomas D. W., Shevach E. M. Nature of the antigenic complex recognized by T lymphocytes. VII. Evidence for an association between TNP-conjugated macrophage membrane components and Ia antigens. J Immunol. 1978 Sep;121(3):1152–1156. [PubMed] [Google Scholar]


