Abstract
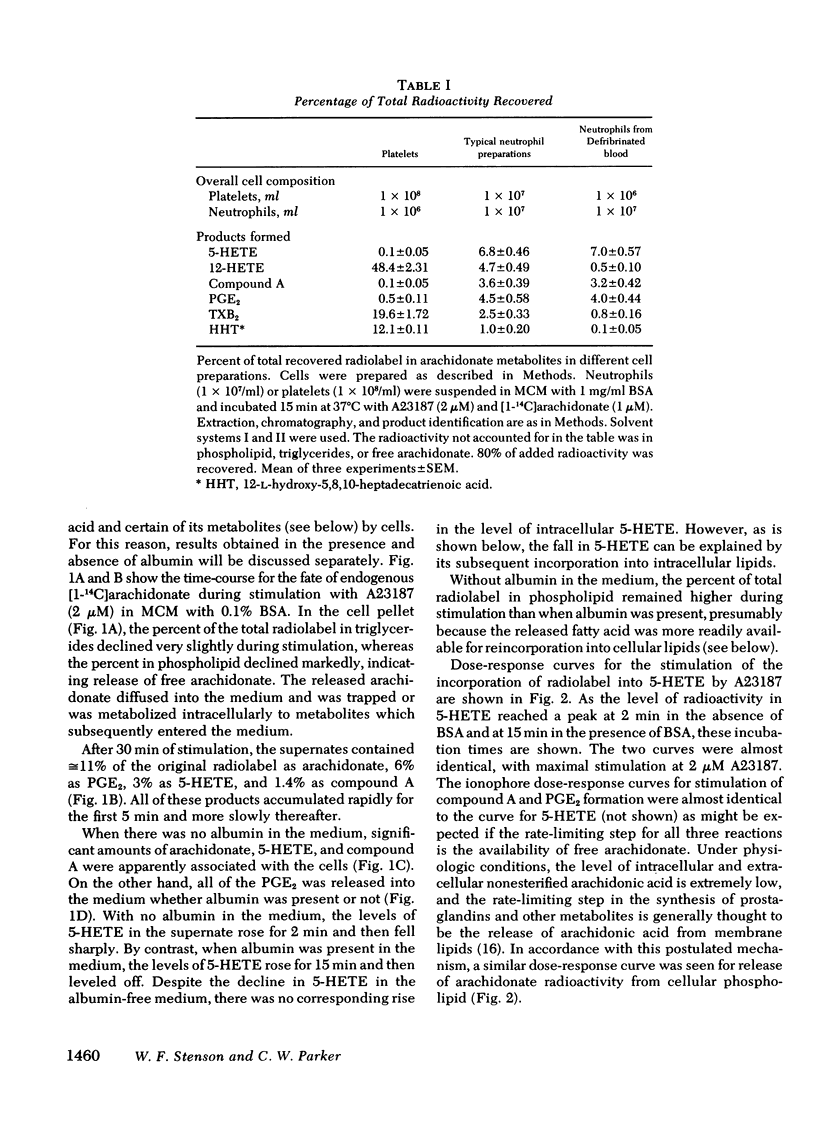
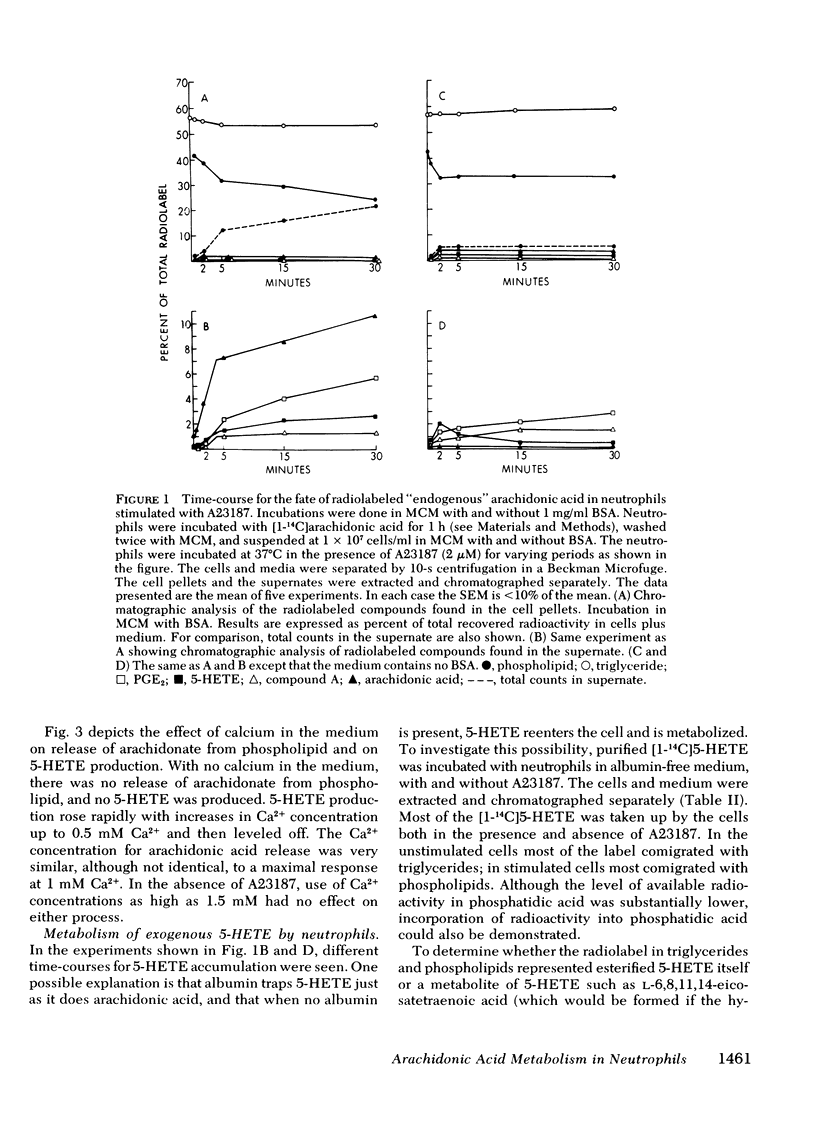
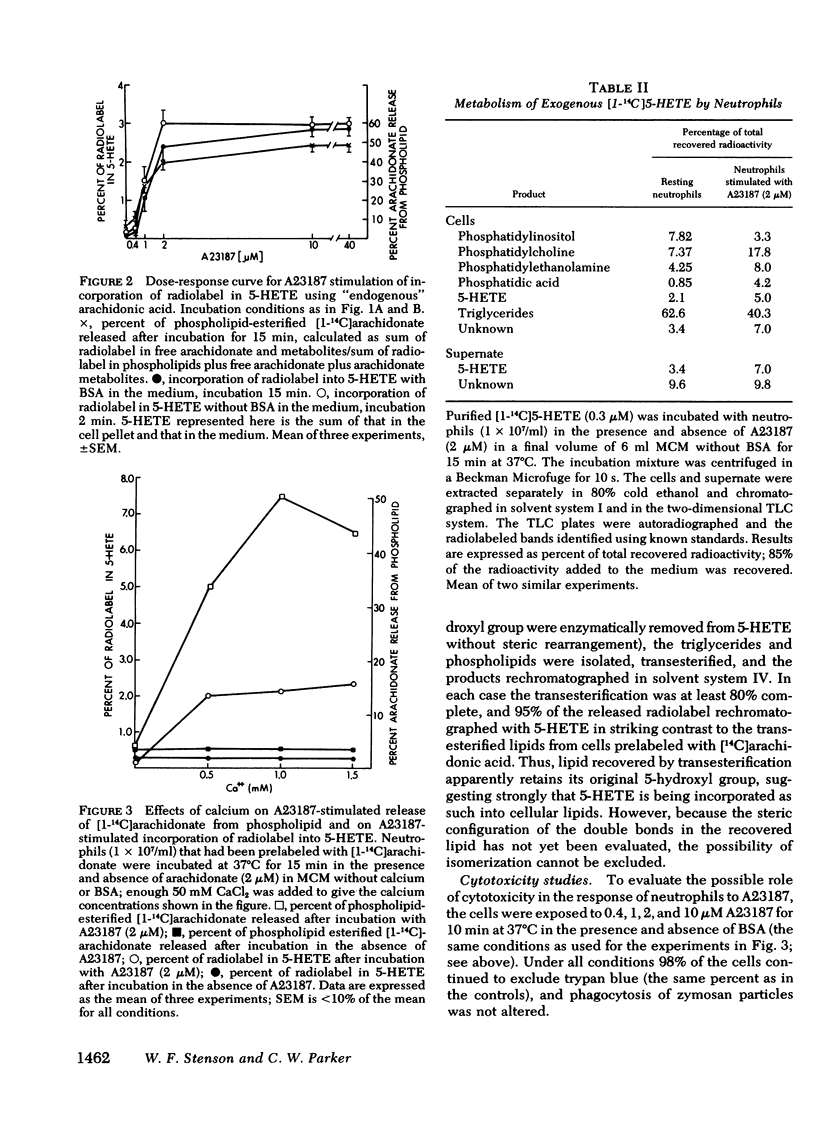
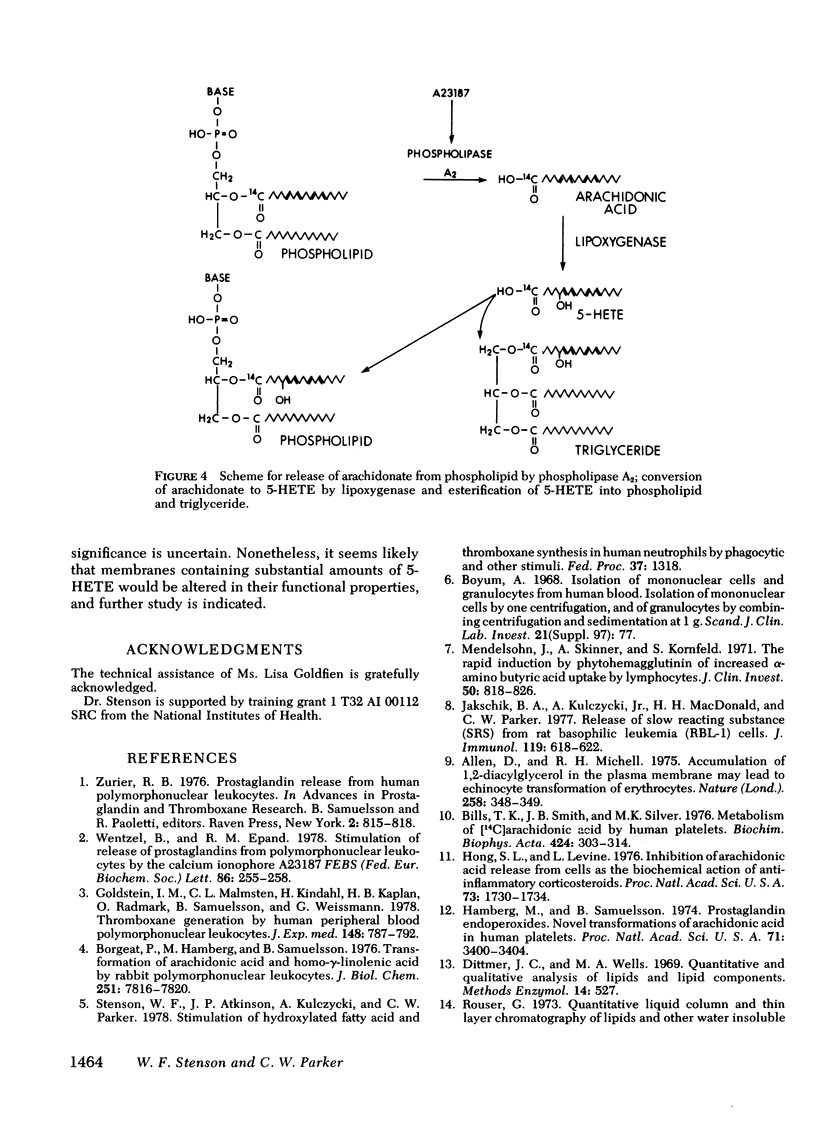
[14C]Arachidonic acid incubated with human neutrophils was esterified into phospholipids and triglycerides. Stimulation of these labeled neutrophils with ionophore A23187 (2 microM) results in release of [14C]arachidonate from phospholipid and its metabolism to prostaglandin E2 and 5-hydroxy-6,8,11,14-eicosatetraenoic acid (5-HETE), a lipoxygenase product. The released arachidonate is also metabolized to a polar lipid of unknown composition here disignated compound A. 5-HETE was found to be released into the medium and then taken up again by the cells. To determine its metabolic fate, [14C]5-HETE was prepared biosynthetically, purified, and incubated with stimulated, unlabeled neutrophils. Most of the radioactivity entered the cells and was esterified into phospholipids and triglycerides. The radiolabeled complex lipids were saponified, and the released fatty acids cochromatographed with authentic 5-HETE. The esterification of 5-HETE, a hydroxylated fatty acid, into membrane phospholipids may be an example of a more generalized mechanism for altering membrane characteristics.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Michell R. H. Accumulation of 1,2-diacylglycerol in the plasma membrane may lead to echinocyte transformation of erythrocytes. Nature. 1975 Nov 27;258(5533):348–349. doi: 10.1038/258348a0. [DOI] [PubMed] [Google Scholar]
- Bills T. K., Smith J. B., Silver M. J. Metabolism of [14C]arachidonic acid by human platelets. Biochim Biophys Acta. 1976 Feb 23;424(2):303–314. doi: 10.1016/0005-2760(76)90198-3. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Hamberg M., Samuelsson B. Transformation of arachidonic acid and homo-gamma-linolenic acid by rabbit polymorphonuclear leukocytes. Monohydroxy acids from novel lipoxygenases. J Biol Chem. 1976 Dec 25;251(24):7816–7820. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Flower R. J., Blackwell G. J. The importance of phospholipase-A2 in prostaglandin biosynthesis. Biochem Pharmacol. 1976 Feb 1;25(3):285–291. doi: 10.1016/0006-2952(76)90216-1. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Malmsten C. L., Kindahl H., Kaplan H. B., Rådmark O., Samuelsson B., Weissmann G. Thromboxane generation by human peripheral blood polymorphonuclear leukocytes. J Exp Med. 1978 Sep 1;148(3):787–792. doi: 10.1084/jem.148.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. On the specificity of the oxygenation of unsaturated fatty acids catalyzed by soybean lipoxidase. J Biol Chem. 1967 Nov 25;242(22):5329–5335. [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Park B. H., Malawista S. E., Quie P. G., Nelson D. L., Good R. A. Chronic granulomatous disease in females. N Engl J Med. 1970 Jul 30;283(5):217–221. doi: 10.1056/NEJM197007302830501. [DOI] [PubMed] [Google Scholar]
- Hong S. L., Levine L. Inhibition of arachidonic acid release from cells as the biochemical action of anti-inflammatory corticosteroids. Proc Natl Acad Sci U S A. 1976 May;73(5):1730–1734. doi: 10.1073/pnas.73.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakschik B. A., Kulczycki A., Jr, MacDonald H. H., Parker C. W. Release of slow reacting substance (SRS) from rat basophilic leukemia (RBL-1) cells. J Immunol. 1977 Aug;119(2):618–622. [PubMed] [Google Scholar]
- Lapetina E. G., Chandrabose K. A., Cuatrecasas P. Ionophore A-23187- and thrombin-induced platelet aggregation: independence from cycloxygenase products. Proc Natl Acad Sci U S A. 1978 Feb;75(2):818–822. doi: 10.1073/pnas.75.2.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W. Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem. 1969 May 10;244(9):2459–2464. [PubMed] [Google Scholar]
- Romeo D., Zabucchi G., Miani N., Rossi F. Ion movement across leukocyte plasma membrane and excitation of their metabolism. Nature. 1975 Feb 13;253(5492):542–544. doi: 10.1038/253542a0. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G. Quantitative liquid column and thin layer chromatography of lipids and other water insoluble substances, elution selectivity principles, and a graphic method for pattern analysis of chromatographic data. J Chromatogr Sci. 1973 Feb;11(2):60–76. doi: 10.1093/chromsci/11.2.60. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Shohet S. B. Remodeling of granulocyte membrane fatty acids during phagocytosis. J Clin Invest. 1974 Mar;53(3):726–734. doi: 10.1172/JCI107611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzell B., Epand R. M. Stimulation of the release of prostaglandins from polymorphonuclear leukocytes by the calcium inophore A23187. FEBS Lett. 1978 Feb 15;86(2):255–258. doi: 10.1016/0014-5793(78)80574-2. [DOI] [PubMed] [Google Scholar]
- Zurier R. B. Prostaglandin release from human polymorphonuclear leukocytes. Adv Prostaglandin Thromboxane Res. 1976;2:815–818. [PubMed] [Google Scholar]



