Abstract
The natriuresis and concomitant decline in absolute proximal reabsorption (APR) that occur in rats in response to saline loading are blunted markedly when renal perfusion pressure is reduced immediately before, but not after, the volume load. To ascertain the mechanism responsible for these differences between early clamp (EC) vs. late clamp (LC), intracapillary and interstitial determinants of peritubular capillary uptake of APR were measured in seven LC and seven EC Munich-Wistar rats before and after isotonic saline loading (80% body wt). With volume expansion in LC animals, we observed a marked decline in APR (averaging 11±1 nl/min), associated with large increases in urinary sodium excretion rate, which averaged 8±2 μeq/min. In EC, the changes in urinary sodium excretion rate (+1±0 μeq/min) and APR (−3±1 nl/min) with volume expansion were smaller in magnitude. Since peritubular capillary reabsorption coefficient and mean peritubular transcapillary hydraulic pressure difference did not change with saline loading in LC, the marked fall in APR was attributed primarily to a measured large decline in mean peritubular transcapillary oncotic pressure difference (δ̄π̄). Despite an equivalent mean fall in δ̄π̄ with volume expansion in EC, near-constancy of APR was found to be associated with a simultaneous and equivalent decline in mean peritubular transcapillary hydraulic pressure difference (a consequence of decreased mean peritubular capillary hydraulic pressure), which effectively offset the fall in δ̄π̄. These results demonstrate the importance of hydraulic pressure patterns of the peritubular capillaries in modulating APR and are consistent with the view that Starling forces across the postglomerular microcirculation play a fundamental role in determining APR.
Full text
PDF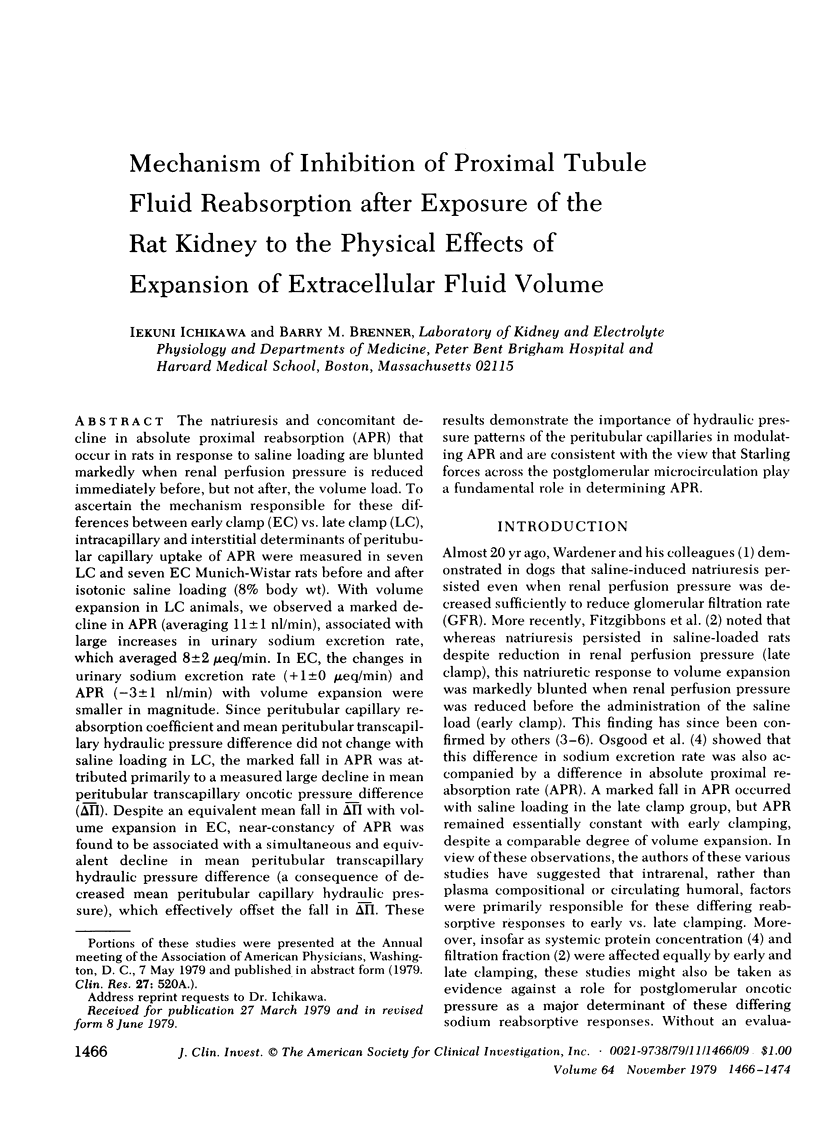




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aperia A. C., Broberger C. G., Söderlund S. Relationship between renal artery perfusion pressure and tubular sodium reabsorption. Am J Physiol. 1971 May;220(5):1205–1212. doi: 10.1152/ajplegacy.1971.220.5.1205. [DOI] [PubMed] [Google Scholar]
- Bank N., Aynedjian H. S., Wada T. Effect of peritubular capillary perfusion rate on proximal sodium reabsorption. Kidney Int. 1972 Jun;1(6):397–405. doi: 10.1038/ki.1972.52. [DOI] [PubMed] [Google Scholar]
- Bell R. D., Parry W. L., Grundy W. G. Renal lymph sodium and potassium concentrations following renal vasodilation. Proc Soc Exp Biol Med. 1973 Jun;143(2):499–501. doi: 10.3181/00379727-143-37352. [DOI] [PubMed] [Google Scholar]
- Blantz R. C., Tucker B. J. Determinants of peritubular capillary fluid uptake in hydropenia and saline and plasma expansion. Am J Physiol. 1975 Jun;228(6):1927–1935. doi: 10.1152/ajplegacy.1975.228.6.1927. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M. On the mechanism of inhibition in fluid reabsorption by the renal proximal tubule of the volume-expanded rat. J Clin Invest. 1971 Aug;50(8):1596–1602. doi: 10.1172/JCI106647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE WARDENER H. E., MILLS I. H., CLAPHAM W. F., HAYTER C. J. Studies on the efferent mechanism of the sodium diuresis which follows the administration of intravenous saline in the dog. Clin Sci. 1961 Oct;21:249–258. [PubMed] [Google Scholar]
- Daugharty T. M., Ueki I. F., Nicholas D. P., Brenner B. M. Comparative renal effects of isoncotic and colloid-free volume expansion in the rat. Am J Physiol. 1972 Jan;222(1):225–235. doi: 10.1152/ajplegacy.1972.222.1.225. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Robertson C. R., Brenner B. M. A model of peritubular capillary control of isotonic fluid reabsorption by the renal proximal tubule. Biophys J. 1973 Apr;13(4):340–358. doi: 10.1016/S0006-3495(73)85989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Ueki I. F., Brenner B. M. Permeability of renal peritubular capillaries to neutral dextrans dextrans and endogenous albumin. Am J Physiol. 1976 Aug;231(2):283–291. doi: 10.1152/ajplegacy.1976.231.2.283. [DOI] [PubMed] [Google Scholar]
- Earley L. E., Friedler R. M. The effects of combined renal vasodilatation and pressor agents on renal hemodynamics and the tubular reabsorption of sodium. J Clin Invest. 1966 Apr;45(4):542–551. doi: 10.1172/JCI105368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Falchuk K. H., Brenner B. M., Tadokoro M., Berliner R. W. Oncotic and hydrostatic pressures in peritubular capillaries and fluid reabsorption by proximal tubule. Am J Physiol. 1971 May;220(5):1427–1433. doi: 10.1152/ajplegacy.1971.220.5.1427. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons J. P., Gennari F. J., Garfinkel H. B., Cortell S. Dependence of saline-induced natriuresis upon exposure of the kidney to the physical effects of extracellular fluid volume expansion. J Clin Invest. 1974 Dec;54(6):1428–1436. doi: 10.1172/JCI107890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennari F. J., Lefavour G. S., Caflisch C. R., Spevack S., Cortell S. Identification of two components in the natriuretic response to saline loading in the rat. Am J Physiol. 1978 Aug;235(2):F126–F130. doi: 10.1152/ajprenal.1978.235.2.F126. [DOI] [PubMed] [Google Scholar]
- Grandchamp A., Boulpaep E. L. Pressure control of sodium reabsorption and intercellular backflux across proximal kidney tubule. J Clin Invest. 1974 Jul;54(1):69–82. doi: 10.1172/JCI107751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargens A. R., Tucker B. J., Blantz R. C. Renal lymph protein in the rat. Am J Physiol. 1977 Oct;233(4):F269–F273. doi: 10.1152/ajprenal.1977.233.4.F269. [DOI] [PubMed] [Google Scholar]
- Källskog O., Wolgast M. Driving forces over the peritubular capillary membrane in the rat kidney during antidiuresis and saline expansion. Acta Physiol Scand. 1973 Sep;89(1):116–125. doi: 10.1111/j.1748-1716.1973.tb05502.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewy J. E., Windhager E. E. Peritubular control of proximal tubular fluid reabsorption in the rat kidney. Am J Physiol. 1968 May;214(5):943–954. doi: 10.1152/ajplegacy.1968.214.5.943. [DOI] [PubMed] [Google Scholar]
- Maddox D. A., Bennett C. M., Deen W. M., Glassock R. J., Knutson D., Brenner B. M. Control of proximal tubule fluid reabsorption in experimental glomerulonephritis. J Clin Invest. 1975 Jun;55(6):1315–1325. doi: 10.1172/JCI108051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox D. A., Price D. C., Rector F. C., Jr Effects of surgery on plasma volume and salt and water excretion in rats. Am J Physiol. 1977 Dec;233(6):F600–F606. doi: 10.1152/ajprenal.1977.233.6.F600. [DOI] [PubMed] [Google Scholar]
- Marchand G. R. Interstitial pressure during volume expansion at reduced renal artery pressure. Am J Physiol. 1978 Sep;235(3):F209–F212. doi: 10.1152/ajprenal.1978.235.3.F209. [DOI] [PubMed] [Google Scholar]
- Nizet A. Quantitative influence of non-hormonal blood factors on the control of sodium excretion by the isolated dog kidney. Kidney Int. 1972;1(1):27–37. doi: 10.1038/ki.1972.5. [DOI] [PubMed] [Google Scholar]
- O'morchoe C. C., Omorchoe P. J., Donati E. J. Comparison of hilar and capsular renal lymph. Am J Physiol. 1975 Aug;229(2):416–421. doi: 10.1152/ajplegacy.1975.229.2.416. [DOI] [PubMed] [Google Scholar]
- Osgood R. W., Lameire N. H., Sorkin M. I., Stein J. H. Effect of aortic clamping on proximal reabsorption and sodium excretion in the rat. Am J Physiol. 1977 Feb;232(2):F92–F96. doi: 10.1152/ajprenal.1977.232.2.F92. [DOI] [PubMed] [Google Scholar]
- Ott C. E., Haas J. A., Cuche J. L., Knox F. G. Effect of increased peritubule protein concentration on proximal tubule reabsorption in the presence and absence of extracellular volume expansion. J Clin Invest. 1975 Mar;55(3):612–620. doi: 10.1172/JCI107969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer A., Windhager E. E. Effect of peritubular oncotic pressure changes on proximal tubular fluid reabsorption. Am J Physiol. 1970 Apr;218(4):1188–1193. doi: 10.1152/ajplegacy.1970.218.4.1188. [DOI] [PubMed] [Google Scholar]
- Tucker B. J., Blantz R. C. Determinants of proximal tubular reabsorption as mechanisms of glomerulotubular balance. Am J Physiol. 1978 Aug;235(2):F142–F150. doi: 10.1152/ajprenal.1978.235.2.F142. [DOI] [PubMed] [Google Scholar]
- Viets J. W., Deen W. M., Troy J. L., Brenner B. M. Determination of serum protein concentration in nanoliter blood samples using fluorescamine or 9-phthalaldehyde. Anal Biochem. 1978 Aug 1;88(2):513–521. doi: 10.1016/0003-2697(78)90451-7. [DOI] [PubMed] [Google Scholar]
- Weinman E. J., Kashgarian M., Hayslett J. P. Role of peritubular protein concentration in sodium reabsorption. Am J Physiol. 1971 Nov;221(5):1521–1528. doi: 10.1152/ajplegacy.1971.221.5.1521. [DOI] [PubMed] [Google Scholar]
- Wolgast M., Persson E., Schnermann J., Ulfendahl H., Wunderlich P. Colloid osmotic pressure of the subcapsular interstitial fluid of rat kidneys during hydropenia and volume expansion. Pflugers Arch. 1973 May 18;340(2):123–131. doi: 10.1007/BF00588171. [DOI] [PubMed] [Google Scholar]


