Summary
Purpose
Removal of areas generating high-frequency oscillations (HFOs) recorded from the intracerebral electroencephalography (iEEG) of patients with medically intractable epilepsy has been found to be correlated with improved surgical outcome. However, whether differences exist according to the type of epilepsy is largely unknown. We performed a comparative assessment of the impact of removing HFO-generating tissue on surgical outcome between temporal lobe epilepsy (TLE) and extratemporal lobe epilepsy (ETLE). We also assessed the relationship between the extent of surgical resection and surgical outcome.
Methods
We studied 30 patients with drug-resistant focal epilepsy, 21 with TLE and 9 with ETLE. Two thirds of the patients were included in a previous report and for these, clinical and imaging data were updated and follow-up was extended. All patients underwent iEEG investigations (500 Hz high-pass filter and 2,000 Hz sampling rate), surgical resection, and postoperative magnetic resonance imaging (MRI). HFOs (ripples, 80–250 Hz; fast ripples, >250 Hz) were identified visually on a 5–10 min interictal iEEG sample. HFO rates inside versus outside the seizure-onset zone (SOZ), in resected versus nonresected tissue, and their association with surgical outcome (ILAE classification) were assessed in the entire cohort, and in the TLE and ETLE subgroups. We also tested the correlation of resected brain hippocampal and amygdala volumes (as measured on postoperative MRIs) with surgical outcome.
Key Findings
HFO rates were significantly higher inside the SOZ than outside in the entire cohort and TLE subgroup, but not in the ETLE subgroup. In all groups, HFO rates did not differ significantly between resected and nonresected tissue. Surgical outcome was better when higher HFO rates were included in the surgical resection in the entire cohort and TLE subgroup, but not in the ETLE subgroup. Resected brain hippocampal and amygdala volumes were not correlated with surgical outcome.
Significance
In TLE, removal of HFO-generating areas may lead to improved surgical outcome. Less consistent findings emerge from ETLE, but these may be related to sample size limitations of this study. Size of resection, a factor that was ignored and that could have affected results of earlier studies did not influence results.
Keywords: High-frequency oscillations, Intracerebral EEG, Epilepsy surgery, Temporal lobe epilepsy, Extra-temporal lobe epilepsy
In epilepsy surgery, an often-debated topic is the extent of cerebral resection required to render a patient seizure free without important postoperative neurologic sequelae (Okonma et al., 2011). Although preserving neurologic function remains a desirable goal, uncontrolled seizures and adverse effects of antiepileptic drugs (AEDs) are the strongest predictors of impaired quality of life after epilepsy surgery (Choi-Kwon et al., 2008). The most critical issue in surgically remediable epilepsies is to define the epileptogenic zone, which involves an accurate assessment of the seizure-onset zone (SOZ) on the electroencephalography (EEG) and the ability to detect a lesion on magnetic resonance imaging (MRI). In EEG and MRI, several advances have been made in recent years potentially allowing for improved surgical resections. High-frequency oscillations (HFOs), namely ripples (80–250 Hz) and fast ripples (FRs, >250 Hz), have been recorded from the intracerebral EEG (iEEG) in experimental models and in patients with pharmacoresistant focal epilepsy undergoing presurgical investigation (Bragin et al., 1999a,b; Jacobs et al., 2008; Engel et al., 2009; Jacobs et al., 2009; Crepon et al., 2010). They are more closely linked to the SOZ than interictal spikes (Jacobs et al., 2008). In addition, removal of HFO-generating areas is associated with a favorable surgical outcome in children and adults with drug-resistant epilepsy (Jacobs et al., 2010; Wu et al., 2010).
Advances in neuroimaging and image processing can assist clinicians in the identification of structural lesions or brain dysfunction. Diffusion tensor imaging and functional MRI, for instance, detect, respectively, white matter abnormalities and blood oxygen level–dependent (BOLD) signal changes that have the potential to provide a better understanding of the concept of epileptogenicity (Gotman & Pittau, 2011; Schmidt & Pohlamnn-Eden, 2011). Image processing, such as voxel-based morphometry, may uncover structural abnormalities that are missed with standard MRI sequences, for example, focal cortical atrophy (Bernasconi & Bernasconi, 2011). At the present time, it is also possible to establish correspondences across brain structures with accurate and rapid registration tools (Klein et al., 2009). These tools allow precise comparison across different imaging modalities and offer additional information in the identification of epileptogenic tissue. In mesial temporal lobe epilepsy (TLE), coregistration techniques have been used to demonstrate that hippocampal fast ripple (FR)–generating sites correspond with local areas of atrophy (Ogren et al., 2009).
In this study, we investigated patients with drug-resistant focal epilepsy of temporal and extratemporal lobe origin. Specifically, our objective was to evaluate whether the impact of removing HFO-generating areas differs on surgical outcome between temporal lobe and extratemporal lobe epilepsy (ETLE). To correlate HFO-generating areas with the extent of surgical resections, we used accurate MRI coregistration techniques. This analysis was complemented by assessing the relationship between the volume of surgical resection and postsurgical seizure outcome.
Patients and Methods
Patients’ selection and recording methods
Of 77 patients with drug-resistant focal epilepsy undergoing iEEG recordings with 500-Hz high-pass filter and 2,000 Hz sampling rate at the Montreal Neurological Institute (MNI) between November 2004 and November 2009, 30 (40%) met study inclusion criteria: (1) use of “MNI” depth electrodes (see description below) for iEEG recordings; (2) a surgical resection; (3) a preoperative and postoperative MRI; and (4) a postoperative follow-up of at least 9 months (Table 1). Of these 30 patients, 21 had TLE and nine had ETLE (six with a seizure focus in the frontal lobe, one in the temporooccipital region, and two in the occipital lobe). Of note, 20 patients were included in a previous report (Jacobs et al., 2010). For these patients, clinical and imaging data were updated (one patient underwent a second surgical resection) and follow-up was extended. The decision to perform an iEEG investigation was based exclusively on clinical reasons. The study was approved by the MNI Research Ethics Committee, and all patients provided written informed consent prior to study enrolment.
Table 1.
Clinical data of the 30 patients with drug-resistant focal epilepsy included in the study (21 with TLE, and 9 with ETLE)
| Patients no.-sub group |
Age/gender | Age at epilepsy onset | Duration of epilepsy |
MRI | Electrode location | Seizure-onset zone | Type of surgery | Removed contacts | ILAE class |
Follow-up (month) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1-TLE | 33/M | 19 | 14 | R hipp sclerosis | LA, LH, RA, RC, RH, RPH | RC, RH | R ant Temp | RA, RH | 5 | 15 |
| 2-TLE | 25/M | 11 | 14 | Polymicrogyria | RA, RH, RPH, RAC, RIM, ROT, RPP, RI, RIa, RIp, RIi, | RH1-3, RP1-2 | R post Temp | RH5-9, RPH7-9 | 5 | 11 |
| 3-TLE | 39/M | 25 | 14 | R hipp < L hipp | LA, LH, RA, RH | RA1-3, RH1-2 | R SAH | RA4-9, RH | 1 | 13 |
| 4-TLE | 41/F | 40 | 1 | L mesial temp atrophy | LA, LH, LI, LPH, RA, RH, RI, RPH | LPH1-4 | L SAH | LA7-9 | 1 | 12 |
| 5-TLE | 33/M | 25 | 8 | L hipp < R hipp | LA, LH, LPH, LOF, RA, RH, RPH, ROF | LA, LH1-3 | L SAH | LA | 5 | 10 |
| 6-TLE | 33/M | 12 | 11 | – | LH, LAC, RA, RH, RPH, RSMA, ROF, RAC | RA, RH, RPH | R ant Temp | RA5-9 | 1 | 19 |
| 7-TLE | 30/F | 25 | 5 | Bilat hipp atrophy | LA, LH, LPH, LOF, RA, RH, RPH, ROF | RA3-7, RH3-7 | R ant Temp | RA, RH | 5 | 18 |
| 8-TLE | 30/M | 8 | 22 | – | LA, LH, LPH, LOF, LAC, LPC | LA1-3, LH1-3, LPH1-3 | L ant Temp | LA, LH | 4 | 10 |
| 9-TLE | 50/M | 7 | 43 | Bilat mesial atrophy | LA, LH, LPH, RA, RH, RPH | RA1-3, RH1-3, RPH1-3 | R ant Temp | RA, RH1-4 | 4 | 10 |
| 10-TLE | 28/M | 1.5 | 26.5 | Surgical bed, L post temp cyst | LAC, LP | LPH3-8 | L post lat Temp | 0 | 2 | 40 |
| 11-TLE | 43/F | 12 | 21 | R surgical bed | ROT, RPH | ROT8-9, RPH7-8 | R lat Temp | ROT, RPH | 2 | 40 |
| 12-TLE | 41/F | 8 | 33 | L surgical bed | L MT | LMT1-2 | L post lat Temp | LMT3-7 | 3 | 48 |
| 13-TLE | 29/M | n/a | n/a | L temp polymicrogyria | LA, LH, LPH, RA, RH, RPH | RA1-3, RH1-3, RPH1-3 | R SAH | RA, RH1-3 | 6 | 23 |
| 14-TLE | 54/M | 38 | 16 | R hipp malrotation | LA, LH,RA, RH | RA1-3, RH1-3 | R SAH | RA1-9, RH1-5 | 3 | 46 |
| 15-TLE | 24/M | 13 | 11 | R hipp < L hipp | LH, LPH | LH1-2 | L post lat Temp | 0 | 4 | 27 |
| 16-TLE | 34/M | 9 | 25 | Bilat Fr and ant temp tuber | LA, LH, LPH, LC, LOF, RA, RH, RC, ROF | LOF1-5 | L ant Temp | LA4-9 | 4 | 24 |
| 17-TLE | 43/F | 34 | 9 | L ant temp arachnoid cyst | LA, LH, LPH, RA, RH, RPH | RA1-2, RP1-3 | R SAH | RA1-4, RA7-9, RH1-2 | 3 | 72 |
| 18-TLE | 27/F | 2 | 25 | Bilat PNH | RA, RH, RPH, RC | RA1-3, RH1-3, RC4-8 | R ant Temp | RA, RH1-3 | 4 | 23 |
| 19-TLE | 25/M | 14 | 11 | R hemispheric porencephalic cyst | LA, LH, | RA1-3, RH1-3, RPH 1-2 | R SAH | RA7-9, RH | 5 | 23 |
| 20-TLE | 58/F | 49 | 9 | R hipp atrophy | RA, RH, RPH, ROF, ROT | RA1-3, RH1-3, RPH1-3 | R ant lat Temp | RA, RH, RP, ROT | 4 | 9 |
| 21-TLE | 25/M | 3 | 22 | R PNH | RA, RH, RPH, RP1, RP2 | RA1-2, RH1-3, RPH1-2 | R SAH | RA, RH | 3 | |
| 22-ETLE | 26/F | 4 | 22 | – | LAC, LOF, LSC, LP RAC, ROF, RSC, RP | LSC1-2, LP1-2 | L Fr FCD | LSC | 1 | 11 |
| 23-ETLE | 24/M | 11 | 13 | – | RA, RH, ROT, RSO, RIO, RPP | ROT, RSO, RPP | R Temp-Occ | RSO | 5 | 13 |
| 24-ETLE | 35/F | 24 | 9 | R post-meningioma gliosis | RP, RAC, RSMA | RP | R Occ | RP4-8 | 3 | 10 |
| 25-ETLE | 16/M | 4 | 12 | L Fr FCD | LSC, LSMA, LAC, LOF | LSC1-2 | L sup Fr | LSC | 2 | 12 |
| 26-ETLE | 47/M | 4 | 43 | L Fr FCD | LC, LP1, LP2, LOF, RC, ROF | LP12-6, | L inf Fr | LP1, LP2 | 3 | 12 |
| 27-ETLE | 52/M | 20 | 32 | – | LAC, LPC, LH, LSMAa, LSMAp, LC | LSMAa1-5, LPC5-7 | L SMA | LAC, LSMAa | 5 | 36 |
| 28-ETLE | 40/M | 34 | 6 | Meningiomatosis | RIO, RSO, RA, RH | RIO5-9, RSO1-5 | R lat Occ | RIO, RSO | 3 | 13 |
| 29-ETLE | 33/M | 4 | 29 | – | RA, RAC, RH, ROF, RSC, RIO, RSO | RIO5-7 | R lat Occ | RSO3-7, RSC | 1 | 14 |
| 30-ETLE | 51/M | 4 | 46 | L central FCD | LIM, LIPC, LSM, LSPC | LIM, LSM, LSPC | L inf motor cortex | LIM, LIPC | 6 | 47 |
Ant, anterior; Bilat, bilateral; FCD, focal cortical dysplasia; F, female; Fr, frontal; hipp, hippocampus; inf, inferior; lat, lateral; L, left; M, male; n/a, not available; Occ, occipital; PNH, periventricular nodular heterotopia; post, posterior; temp, temporal; R, right; SAH, selective amygdalohippocampectomy; sup, superior; A, amygdala; AC, anterior cingulate gyrus; C, Heschl gyrus; H, head of hippocampus; I, insula; Ia, anterior insula; Ii, inferior insula; Ip, posterior insula; IM, inferior precentral gyrus; IO, inferior occipital; IPC, inferior postcentral gyrus; MT, middle temporal gyrus; OF, orbitofrontal cortex; OT, occipitotemporal; P, lesion; PC, posterior cingulate gyrus; PH, posterior hippocampus; PP, precuneus; SC, superior cingulate gyrus; SM, superior precentral gyrus; SMA, supplementary motor area; SMAa, anterior SMA; SMAp, posterior SMA; SO, superior occipital; SPC, superior postcentral gyrus; TLE, temporal lobe epilepsy; ETLE, extratemporal lobe epilepsy; MRI, magnetic resonance imaging; ILAE, International League Against Epilepsy; mo, month.
A combination of depth and cortical surface electrodes were implanted stereotactically according to clinical criteria using an image-guidance system (SSN Neuronavigation System, Mississauga, ON, Canada). Electrodes were manufactured onsite. A 10/1,000-in. (0.254 mm) wire of stainless steel was used as a central core and wrapped with a 3/1,000-in. (0.076 mm) steel wire. Each electrode had nine contacts, with the deepest contact (contact 1) consisting of the tip of the steel core stripped of insulation. This contact had a length of 1 mm, whereas all other contacts (2–9) were formed from stripped sections of the marginal wire that was tightly wound to create 0.5-mm long coils. The effective surface area for contact 1 was 0.80 and 0.85 mm2 for contacts 2–9.
iEEG were recorded using the Harmonie long-term monitoring system (Stellate, Montreal, QC, Canada), with a low-pass filter of 500 Hz and a sampling rate of 2,000 Hz. We also recorded the electrooculogram (EOG) and electro-myogram (EMG) to facilitate sleep staging. We analyzed interictal samples of slow-wave sleep lasting 5–10 min, selected as previously described (Bagshaw et al., 2009). The SOZ was defined by the same experienced neurophysiologist (FD) as the contacts involved in the first definite ictal activity. We used the International League Against Epilepsy (ILAE) classification (Wieser et al., 2001) to classify patient’s outcome at the time of the last follow-up visit. A favorable outcome was defined as ILAE class 1–3, whereas a poor outcome as ILAE class 4–6.
Marking of spikes and HFOs
HFOs (ripples, 80–250 Hz; FRs, 250–500 Hz) were marked visually on a 5–10 min slow-wave sleep interictal iEEG sample that was separated by at least 4 h from any seizure, according to a method used in several studies (Zelmann et al., 2009). Spikes were marked independently of HFOs. Spikes were marked in the unfiltered iEEG, and markings were then made invisible before applying settings for HFO identification. To mark HFOs, which are usually undetectable in the unfiltered iEEG, traces were displayed with the maximum time resolution of the computer monitor (0.6 s, 1,200 samples). The display was split vertically with an 80-Hz high-pass filter on one side and a 250-Hz high-pass filter on the other side. A ripple was marked if an event was only visible on the 80-Hz filter and not on the 250-Hz filter. An FR was marked if an event was visible on the 250-Hz filter and not on the 80-Hz filter. Because filtering of sharp transients, such as spikes, sometimes results in “false” HFOs consisting in most cases of three consecutive oscillations or less (Bénar et al., 2010), only events containing at least four consecutive oscillations were regarded as HFOs. In addition, two events were considered as distinct if they were separated by at least two non-HFO oscillations. After marking HFOs and spikes, a program was run on MATLAB (The Mathworks Inc., Natick, MA, U.S.A.) to calculate the frequency of HFO and spike occurrence per contact.
Imaging processing
All patients had preoperative (preop) and postoperative (postop) MRIs. Postop MRIs were acquired 5–46 months after surgery in the TLE subgroup, and 7–36 months after surgery in the ETLE subgroup. Seven patients (five with TLE and two with ETLE) also had an MRI immediately after depth electrodes explantation (expl MRI) and prior to surgery, which enabled precise assessment of electrode localization by visualization of their tracts. MRIs cannot be obtained during iEEG study because of electrode-MRI incompatibility. MRI acquisition protocols varied among patients. In 25 preop, 20 postop, and 7 expl MRIs, T1-weighted sequences were acquired using the following parameters: 180 slices of 1-mm thickness, echo time (TE) = 8 msec, repetition time (TR) = 23 msec, flip angle 20 degrees, 0.5 × 0.5 mm2 in-plane pixel size on 1.5 Tesla GE Signa EXCITE (General Electric, Milwaukee, WI, U.S.A.). The remaining 5 preop and 10 postop MRIs included gadolinium injection, whereas images were acquired using analogous sequence parameters.
The following preprocessing steps were applied to all MRI scans. First, the program N3 (Sled et al., 1998) was used to correct for image intensity nonuniformity. Second, linear histogram scaling to the International Consortium Brain Mapping (ICBM152) template (Fonov et al., 2011), based on the technique of Nyul et al. (2003), was used to obtain intersubject linear image intensity normalization. Third, a linear (nine parameter) transformation estimated with the mritotal tool from the MINC mni_autoreg software package (Collins et al., 1994) was applied to achieve inter-subject spatial alignment to the Talairach-like ICBM152 stereotaxic space. Fourth, a patient-specific brain mask was created by applying Brain Extraction Tool (BET) from the Oxford Centre of Functional MRI of the brain Software Package (FSL) to the MRI data (Smith, 1998). At the end of this four-step procedure, MRI data were intensity normalized, skull stripped, and spatially aligned within the ICBM152 stereotaxic space.
All preop and expl MRIs were transformed into the postop MRIs space using the Automatic Nonlinear Image Matching and Anatomical Labelling (ANIMAL) registration method (Collins et al., 1994), which aligned preop and postop MRI data. This allowed for an accurate assessment on the expl and postop MRI of whether a contact was inside a tissue subsequently resected (Table 1 and Fig. 1).
Figure 1.
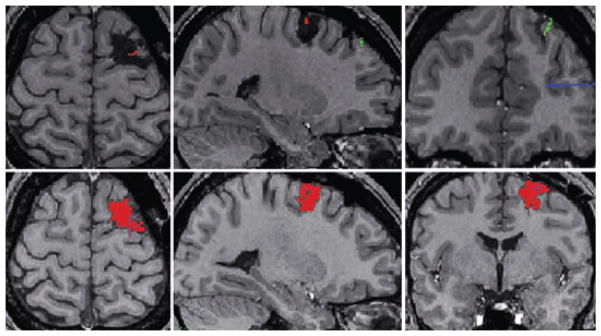
Axial (left), sagittal (middle), and coronal (right) MRI views of a patient with extratemporal lobe epilepsy. The first row shows the postoperative MRI with the right frontal surgical cavity and the registered intracerebral electrodes. The electrodes were implanted in the right supplementary motor area (green), through the lesion (red), and in the anterior cingulate gyrus (blue). The views below show only part of the electrodes. The second row shows in red the right frontal surgical cavity painted with the Display software (MNI, Montreal, Canada).
Epilepsia © ILAE
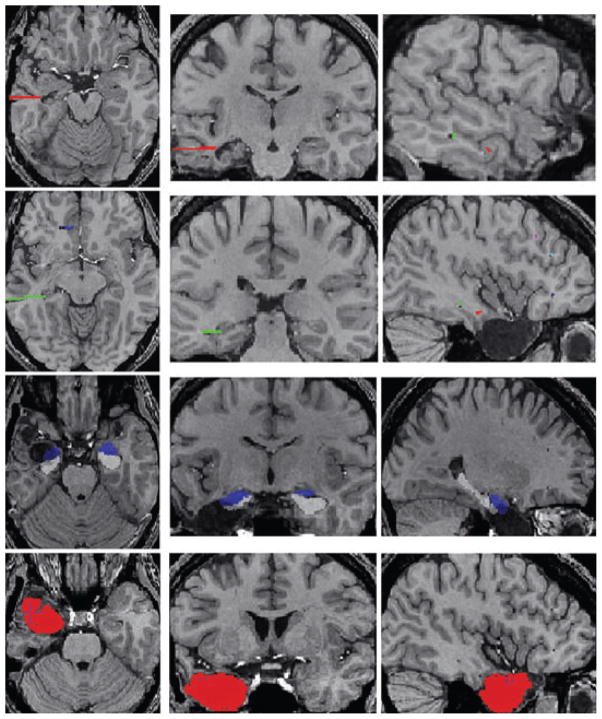
Using the MNI Display software (www.bic.mni.mcgill.ca/ServicesSoftware), we segmented the surgical cavity to measure the volume of the resected brain tissue on the postop MRI. We could assess accurately the resected brain tissue because postoperative MRIs were acquired at least 5 months after surgery, which is enough time for any postoperative brain alteration to heal. To compute the volume of the resected hippocampal and amygdala tissue, the segmentation of the surgical cavity was then mapped through each inversed transformation onto the preop MRI. We used a fully automated method with a template library and label fusion (Collins & Pruessner, 2010) to segment the amygdala and hippocampus of the preop MRI in the TLE subgroup. In brief, this method is based on the same preprocessing steps described above and on label fusion techniques to combine segmentations from the most similar templates to a given MRI (Fig. 2). Finally, combining the hippocampus-amygdala segmentations and the segmentation of the surgical cavity allows enabled estimation of the volume of the resected hippocampus and amygdala.
Figure 2.
Axial (left), coronal (middle), and sagittal (right) brain MRI views in a patient with temporal lobe epilepsy. The two upper rows of the figure illustrate the trajectories of the depth electrodes on the postexplantation MRI. The electrodes targeted the head of the hippocampus (red), the posterior hippocampus (green), and the orbitofrontal cortex (blue). The third row shows the automatic segmentation of the amygdala and hippocampus obtained after registration of the preoperative MRI with the postoperative MRI. The bottom row shows in red the left anterior temporal surgical cavity painted with Display software.
Epilepsia © ILAE
Statistical analysis
In all patient groups, the Wilcoxon rank sum test was used to assess HFO and spike rates in contacts inside versus outside the SOZ, and in contacts inside versus outside the resected tissue. We also calculated the ratio between HFO rates in removed (Rem) contacts and those in nonremoved (nonRem) contacts, using the following formula:
where R̄ is the mean rate of an ev or event (ripple, FR). A ratio of +1 indicates that HFO-generating areas have been removed entirely. On the other hand, a ratio of −1 indicates that HFO-generating areas have remained entirely in brain. Similar ratios were also computed for spikes and for the number of contacts inside/outside the SOZ.
In the TLE subgroup, a similar equation for HFO rates was applied only to the electrodes targeting the amygdala, the head of the hippocampus, and the posterior hippocampus on the side of the surgical resection. This secondary analysis was undertaken because we aimed to assess the importance of HFO removal in mesial temporal structures in the TLE subgroup. If the ratio for the three electrodes targeting the mesial temporal structures had a value close to +1, it would mean that the majority of HFOs have been removed in the mesial temporal structures.
The Wilcoxon rank-sum test was also used to compare event ratios between patients with a favorable surgical outcome (ILAE classes 1–3) and those with a poor surgical outcome (ILAE classes 4–6). We hypothesized that ratios would be close to +1 in patients with a favorable outcome, and close to −1 in patients with a poor outcome.
The Spearman rho was used to explore correlations between the surgical outcome and volume of resection. Significance was set at p < 0.05.
Results
Clinical characteristics and imaging data
Demographic, clinical, and imaging data for the entire cohort and the two subgroups are shown in Tables 1 and 2.
Table 2.
Preoperative and resected volumes for the 30 patients included in the study
| Patients no.-subgroup | Resected brain volume (cm3) | Preoperative hippocampal volume (cm3) | Resected hippocampal volume [cm3 (% of preop vol)] | Preoperative amygdala volume (cm3) | Resected amygdala volume [cm3 (% of preop vol)] |
|---|---|---|---|---|---|
| 1-TLE | 35.93 | 3.4 | 0 (0) | 1.4 | 0 (0) |
| 2-TLE | 11.4 | 4.3 | 0 (0) | 1.64 | 0 (0) |
| 3-TLE | 9.98 | 3.03 | 1.17 (38.6) | 1.45 | 0.14 (9.6) |
| 4-TLE | 2.57 | 3.74 | 0 (0) | 1.7 | 0 (0) |
| 5-TLE | 4.41 | 4.88 | 0.92 (18.8) | 2.2 | 0.03 (1.4) |
| 6-TLE | 12.54 | 3.76 | 0 (0) | 1.51 | 0 (0) |
| 7-TLE | 6.99 | 3.23 | 1.01 (31.2) | 1.74 | 0.27 (15.5) |
| 8-TLE | 25.18 | 4.63 | 0.25 (5.39) | 2.05 | 0.48 (23.4) |
| 9-TLE | 12.66 | 2.2 | 0.54 (24.5) | 1.03 | 0.5 (48.5) |
| 10-TLE | 20.1 | 1.65 | 0.007 (0.4) | 0.41 | 0.01 (2.4) |
| 11-TLE | 58.94 | 0 | 0 (0) | 0 | 0 (0) |
| 12-TLE | 49.2 | 0 | 0 (0) | 0 | 0 (0) |
| 13-TLE | 14.2 | 4.9 | 1.51 (30.8) | 1.84 | 0.4 (21.7) |
| 14-TLE | 5.7 | 3.6 | 1.48 (41.1) | 1.66 | 0.2 (12) |
| 15-TLE | 36.7 | 2.17 | 0.47 (21.6) | 0.66 | 0.13 (19.7) |
| 16-TLE | 18.9 | 4.68 | 0 (0) | 1.76 | 0 (0) |
| 17-TLE | 8.3 | 3.77 | 1.07 (28.3) | 1.43 | 0.47 (32.8) |
| 18-TLE | 6.9 | 3.38 | 0.25 (7.4) | 1.2 | 0.21 (17.5) |
| 19-TLE | 7.04 | 4.06 | 1.67 (41.1) | 2.2 | 0.1 4.5) |
| 20-TLE | 25.01 | 4.2 | 0.24 (5.7) | 1.37 | 0.14 (10.2) |
| 21-TLE | 4.98 | 3.16 | 1.16 (36.7) | 1.35 | 0.51 (37.7) |
| 22-ETLE | 8.89 | – | – | – | – |
| 23-ETLE | 38.84 | – | – | – | – |
| 24-ETLE | 4.05 | – | – | – | – |
| 25-ETLE | 24.14 | – | – | – | – |
| 26-ETLE | 1.2 | – | – | – | – |
| 27-ETLE | 52.75 | – | – | – | – |
| 28-ETLE | 23.48 | – | – | – | – |
| 29-ETLE | 10.45 | – | – | – | – |
| 30-ETLE | 0.1 | – | – | – | – |
| Mean | 18.05 | 3.27 | 0.56 (17.1) | 1.36 | 0.17 (12.5) |
| SD | 16.03 | 1.39 | 0.59 | 0.63 | 0.19 |
TLE, temporal lobe epilepsy; ETLE, extratemporal lobe epilepsy; % of preop vol, percentage of preoperative volume; SD, standard deviation.
In the entire cohort, there was a higher representation of men compared to women (21 vs. 9). Age (mean [range]: 36.1 [16–58] years), age at onset of epilepsy (16.5 [1.5–49] years), and duration of epilepsy (18.7 [1–46] years) were representative of samples from tertiary-care epilepsy centers. The median number of implanted depth electrodes per patient was 6 and the mean duration of postoperative follow-up was 23.7 (9–72) months.
In the 21 patients with TLE (14 men, 7 women), the mean age was 35.4 (25–58) years and the mean duration of postoperative follow-up was 26.6 (9–72) months. Nine patients (43%) had a favorable surgical outcome (ILAE classes 1–3). In the nine patients with ETLE (seven men, two women), the mean age was 36 (16–52) years and the mean duration of follow-up was 18.5 (10–49) months. Six patients (67%) had a favorable outcome (ILAE classes 1–3).
Mean resected brain volume was 18.05 cm3 (0.1–58.9) in the entire cohort, 18.4 cm3 (2.6–58.9) in the TLE subgroup, and 22.91 cm3 (0.1–52.7) in the ETLE subgroup. In one patient with MRI-evidence of focal cortical dysplasia in the precentral gyrus, a very limited resection (0.1 cm3 corresponding to a volume of 5 × 5 × 5 mm) was performed to spare the motor cortex. In the TLE subgroup, the resected hippocampal volumes were substantially smaller than the preoperative volumes (0.56 cm3 [0–1.7] vs. 3.27 cm3 [0–4.9]). The resected amygdala volumes were substantially smaller than the preoperative volumes (0.17 cm3 [0–0.5] vs. 1.36 cm3 [0–2.2]). One case (patient 10) previously underwent a partial amygdalohippocampectomy, which explains the small preoperative hippocampal and amygdala volumes (Table 2). Two other patients (11 and 12) did not have measurable preoperative hippocampal and amygdala volumes because they had previously undergone a standard anterior temporal lobectomy (Table 2).
HFOs, spikes, and the SOZ
In the entire cohort, ripple rates were significantly higher inside the SOZ than outside the SOZ (mean [range] ripples/min: 41.8 [0.4–132.4] vs. 10.9 [0.3–46], p = 0.004), but no significant differences were found for FR rates between contacts inside the SOZ and those outside the SOZ (mean [range] FRs/min: 8.8 [0–31.4] vs. 1.7 [0–12.5]). Spike rates were also significantly higher inside the SOZ than outside the SOZ (mean [range] spikes/min: 14.3 [0.2–52.9] vs. 3.7 [0 –3.1], p = 0.023).
In the TLE subgroup, ripple and FR rates were significantly higher inside the SOZ than outside the SOZ (ripples: 38 [0–132.4] vs. 6.7 [0.3–64.8], p = 0.015; FRs: 7.4 [0–31.4] vs. 1.1 [0–4], p = 0.018). Spike rates were also significantly higher inside the SOZ than outside the SOZ (spikes: 25.4 [0–28.7] vs. 3.3 [0–4.2], p = 0.012).
In the ETLE group, no significant differences were found between contacts inside the SOZ and those outside the SOZ in HFO (ripples: 20.8 [0.6–87.7] vs. 16.8 [1.3–20.9]; FRs: 0.06 [0–74.3] vs. 0.9 [0–12.5]) and spike rates (9.8 [0–52.9] vs. 17.8 [0–49.5]). When excluding electrode contacts located in the amygdala and hippocampus, in which physiologic HFOs are more commonly recorded, there was still no significant difference between contacts inside the SOZ and those outside the SOZ in HFO and spike rates (data not shown).
HFOs, spikes, and the surgical resection
In the entire cohort, no significant differences were found in HFO and spike rates between contacts that were included in the surgical resection and those that were not part of the surgical resection (ripples: 18.3 [0–80.1] vs. 14.9 [0–59.8]; FRs: 3.5 [0–34] vs. 2.2 [0–13.5]; spikes: 8.3 [0–35.4] vs. 2.9 [0–5.8]).
In the TLE subgroup, no significant differences were found in HFO and spike rates between contacts that were included in the surgical resection and those that were not part of the surgical resection (ripples: 16.7 [0–58.2] vs. 14.8 [0–43.4]; FRs: 2.9 [0–14.5] vs. 1.6 [0–4.3]; spikes: 6.2 [0–14.9] vs. 2.9 [0–5.2]).
In the ETLE subgroup, no significant differences were found in HFO and spike rates between contacts that were included in the surgical resection and those that were not part of the surgical resection (ripples: 22 [0.6–80.1] vs. 15.3 [0.7–59.8]; FRs: 4.9 [0–34] vs. 3.6 [0–13.5]; spikes: 12.9 [0–35.4] vs. 2.9 [0–5.8]).
HFOs, spikes, and the surgical outcome
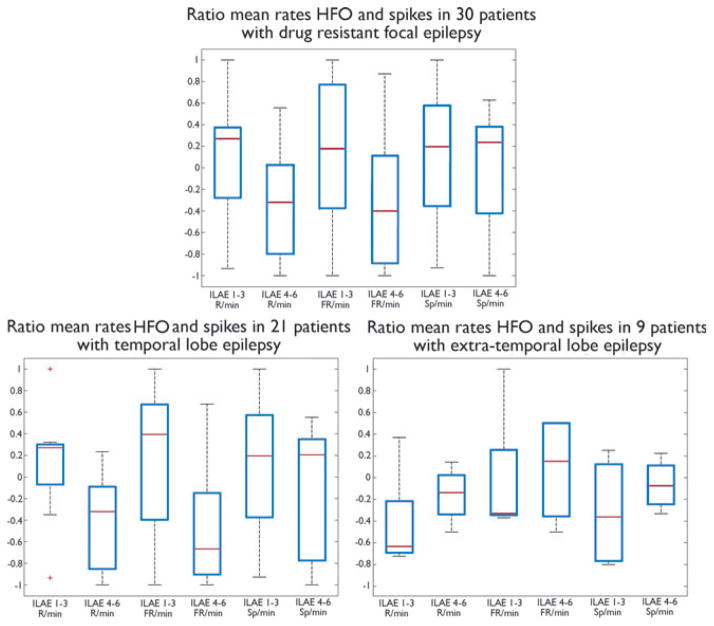
In the entire cohort, the ratio between ripple rates in removed and nonremoved contacts was significantly higher in patients with a favorable outcome (ILAE classes 1–3) compared to patients with a poor outcome (ILAE classes 4–6) (0.06 [range, −1 to 1] vs. −0.44 [−1 to 0.5] respectively, p = 0.02; Fig. 3). No significant between-group differences were found for FRs and spikes (FRs: 0.08 [range, −1 to 1] vs. −0.4 [−1 to 0.86]; spikes: −0.05 [range, −1 to 1] vs. −0.08 [−1 to 0.6]).
Figure 3.
Boxplots illustrating ratios between HFOs in removed and nonremoved contacts for patients with a favorable surgical outcome (ILAE classes 1–3) and those with a poor outcome (ILAE classes 4–6), in the entire cohort (graph at the top) and in the TLE and ETLE subgroups (graphs at the bottom). Note that the results in the entire cohort are influenced mainly by the TLE subgroup, which is more than twice as large as the ETLE subgroup (21 vs. 9 patients). Symbol (*) indicates statistically significant differences. R, ripples; FR, fast ripples.
Epilepsia © ILAE
In the TLE subgroup, the ratio between HFOs in removed and nonremoved contacts was significantly higher in patients with a favorable outcome (ILAE classes 1–3) compared to patients with a poor outcome (ILAE classes 4–6) (ripples: 0.13 [range, −0.9 to 0.3] vs. −0.4 [−1 to 0.2], p = 0.018; FRs: 0.16 [range, −1 to 0.7] vs. −0.5 [−1 to 0.7], p = 0.049; Fig. 3). No significant between-group differences were found for spikes (0.1 [range, −0.2 to 0.5] vs. −0.06 [−1 to 0.5]).
In the ETLE subgroup, ratios of HFOs and spikes did not differ between the two outcome groups (ripples: 0.1 [range, −0.6 to 0.9] vs. −0.2 [−0.8 to 0.5]; FRs: 0.2 [range, −0.9 to 1] vs. 0.3 [−0.9 to 0.8]; spikes: 0.16 [range, −0.4 to 0.8] vs. 0.3 [−0.3 to 0.6]; Fig. 3). HFO ratios did not differ between the two outcome groups even after excluding from the analysis contacts located in the amygdala and hippocampus, in which, as previously mentioned, physiologic HFOs are more commonly recorded (data not shown).
When analyzing only the number of channels within the SOZ, the ratios were not significantly different. When limiting the analysis to the electrodes targeting the amygdala and the hippocampus in TLE subgroup, HFO ratios did not differ between the outcome groups.
Extent of surgical resection and surgical outcome
The mean volume of resection (18.05 cm3 [0.1–58.9] in the entire cohort, 18.4 cm3 [2.6–58.9] in the TLE subgroup, and 22.91 cm3 [0.1–52.7] in the ETLE subgroup) did not correlate with the surgical outcome in any of the patient groups. Similarly, in the TLE subgroup, no correlation was found between the resected amygdala and hippocampal volumes (0.17 cm3 [0–0.5] and 0.56 cm3 [0–1.7]) and the surgical outcome.
Discussion
In line with previous studies (Jacobs et al., 2008; Wu et al., 2010), we found that HFOs are intimately associated with the SOZ and that removing HFO-generating tissue may lead to improved surgical outcome. In our cohort, these findings were likely attributable to the sizeable subgroup with TLE, in which rates of HFOs were higher inside the SOZ than outside and removal of HFO-generating tissue was correlated with a favorable surgical outcome. These findings were not replicated in the smaller group of patients with ETLE. We also found that rates of HFOs did not differ between removed and nonremoved contacts, independently of the type of epilepsy. Furthermore, we found no correlations between the volume of surgical resection (including the volume of resected amygdala and hippocampal tissue in the TLE subgroup) and the surgical outcome. We will now discuss these different aspects of our findings.
Surgical outcome and HFOs
In this study, postoperative seizure outcome was worse than in other series (Cohen-Gadol et al., 2006; Ochi et al., 2007; Cossu et al., 2008; Devaux et al., 2008; Wu et al., 2010). Approximately half of the entire cohort (9 of 21 patients with TLE and 6 of 9 patients with ETLE) had a favorable surgical outcome. Our results are similar to an earlier report from our group (Jacobs et al., 2010). This is not completely surprising, as 20 of 30 patients in our analysis were also included in the previous study, although for these patients clinical and imaging data were updated and follow-up extended. In this study, 8 patients had nonlesional epilepsy and 21 had one or multiple epileptogenic lesions, consistent with a patient group in whom accurate localization of the epileptogenic zone is particularly difficult.
Surgical outcome was correlated with the resection of HFO-generating tissue in the entire cohort and in the TLE subgroup. This is consistent with several recent studies (Ochi et al., 2007; Jacobs et al., 2010; Wu et al., 2010; Akiyama et al., 2011) indicating that HFOs may be a good marker for the epileptogenic region. This correlation was absent from the ETLE subgroup. This is surprising in view of the previously cited studies, which included both TLE and ETLE patients. Three of these studies dealt with neocortical epilepsy (Ochi et al., 2007; Wu et al., 2010; Akiyama et al., 2011) but they took place in children, and the possible differences between children and adults have not been systematically evaluated. Another difference is that patients included in these three studies were explored with grids and not with implanted electrodes.
Although available evidence suggests that HFOs may be reliable biomarkers of epileptogenicity and that removing HFO-generating tissue may result in improved surgical outcome, similar considerations may not apply to spikes. In fact, spikes are a marker of epileptogenicity, but our results and those of Jacobs et al. (2010) do not support their unequivocal value in guiding effective surgical resections. These data could be interpreted within the context of the simulation study of Dermont-Guignard et al. (2012), who suggested that fast ripples might constitute a local marker of the epileptogenicity of underlying neuronal circuits, whereas spikes may be generated in epileptogenic areas, but may also result from a transient highly synchronous excitatory input in less epileptogenic areas. These less epileptogenic areas are also referred to as irritative areas, in which interictal epileptiform discharges are recorded but which are sometimes excluded from surgical resection if they are outside the SOZ (de Curtis & Avanzini, 2001).
HFOs in removed and nonremoved areas
It is noteworthy that HFO rates were not higher in removed contacts compared to nonremoved contacts. If we hypothesize that HFOs represent the epileptogenic area, this result is not surprising, given that close to one half of our patients had a poor outcome. If we had the opposite result (HFOs significantly more frequent in removed areas) we would expect that most of our patients would do well. We did find, as indicated above, that HFOs were more frequent in the removed areas of patients with a good outcome. This is compensated by the fact that HFOs are also frequent in the nonremoved areas of patients who do poorly, resulting in an overall lack of relationship between HFO rates and removed contacts.
Surgical outcome and volume of resection
The studies discussed above, which have shown that a better outcome occurs when more sites with HFOs are removed, have not taken into account the following possible confounding factor: if HFOs were distributed equally throughout the brain and were therefore not specific to the epileptogenic region, and if the success of the outcome was a function of the amount of tissue removed, then these studies would have erroneously concluded that removing HFO-containing channels is a determinant of the quality of the outcome. We therefore decided to study the relationship between the volume of resection and the success of surgery. We used very accurate tools to delineate the area of surgical resection (Collins et al., 1994; Collins & Pruessner, 2010), but we did not find any correlation between the volume of resection and the postoperative seizure outcome in the whole group. Previous studies have investigated the impact of the extent of surgical resection on postoperative outcome (Jehi et al., 2009; Sarkis et al., 2010; Okonma et al., 2011). In most cases, larger resections result in more favorable outcomes: optimal results, however, can also be obtained when limited resections are necessary to preserve eloquent cortex. In addition, we did not find a correlation between the volume of resected amygdala/hippocampus, assessed with a validated tool, and the postoperative outcome. This reinforces the conclusion that HFOs are indeed a marker of the epileptogenic zone, since their removal predicts outcome independently of the volume of resection.
Limitations
In this study, the lack of correlation between HFO-generating areas and surgical outcome in the ETLE subgroup may be attributable to the small sample size and heterogeneity of this group (four had a normal MRI, three had focal cortical dysplasia [FCD], and two had other types of lesion). It is possible that the correlation “HFO-removal/surgical outcome” does not hold for some etiologies, and this could be sufficient to render the results of the small ETLE group nonsignificant. It indicates that larger groups of patients with various etiologies need to be analyzed. Moreover, our patients were included in the ETLE subgroup because their SOZ was in extratemporal neocortical regions. Among the nine patients with ETLE, four also had electrodes in the amygdala and/or the head of the hippocampus. The baseline rates of HFOs in the mesial temporal structures are often higher than in other structures, especially during slow-wave sleep (Le Van Quyen et al., 2010), a factor that could have contributed to the lack of correlation between HFOs and surgical outcome in this subgroup. We reanalyzed data for the ETLE subgroup after excluding electrode contacts that targeted the amygdala and the hippocampus. This second analysis did not change our results for the ETLE subgroup. Finally, the assessment of HFOs was performed visually, the accuracy of which is dependent on the reader’s level of experience. Semiautomated and automated methods have been used in other studies (Staba et al., 2002, 2004; Ogren et al., 2009; Akiyama et al., 2011), although clinical neurophysiologists often validated their performance.
Conclusions
Our study indicates that, at least in TLE, removing HFO-generating areas may lead to improved surgical outcome. Less-consistent findings emerge from ETLE, but these may be related to sample size limitations and to the heterogeneity of epilepsies. Size of surgical resection, a potential confounder not taken into account in earlier studies, did not affect the results, independent of the type of epilepsy.
Acknowledgments
This work was supported by grant MOP 102710 from the Canadian Institutes of Health Research. Dr Piero Perucca is funded by the “Susan S Spencer clinical research training fellowship in epilepsy” from the American Brain Foundation, the American Epilepsy Society, and the Epilepsy Foundation.
Footnotes
Disclosure
None of the other authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Akiyama T, McCoy B, Go CY, Ochi A, Elliott IM, Akiyama M, Donner EJ, Weiss SK, Snead OC, III, Rutka JT, Drake JM, Otsubo H. Focal resection of fast ripples on extraoperative intracranial EEG improves seizure outcome in pediatric epilepsy. Epilepsia. 2011;52:1802–1811. doi: 10.1111/j.1528-1167.2011.03199.x. [DOI] [PubMed] [Google Scholar]
- Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2009;50:617–628. doi: 10.1111/j.1528-1167.2008.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénar CG, Chauvière L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on “false” ripples. Clin Neurophysiol. 2010;121:301–310. doi: 10.1016/j.clinph.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Bernasconi A, Bernasconi N. Unveiling epileptogenic lesions: the contribution of image processing. Epilepsia. 2011;52(Suppl 4):20–24. doi: 10.1111/j.1528-1167.2011.03146.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999a;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999b;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Choi-Kwon S, Chung CK, Lee SK, Choi J, Han K, Lee EH. Quality of life after epilepsy surgery in Korea. J Clin Neurol. 2008;4:116–122. doi: 10.3988/jcn.2008.4.3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Gadol AA, Wilhelmi BG, Collignon F, White JB, Britton JW, Cambier DM, Christianson TJ, Marsh WR, Meyer FB, Cascino GD. Long-term outcome of epilepsy surgery among 399 patients with nonlesional seizure foci including mesial temporal lobe sclerosis. J Neurosurg. 2006;104:513–524. doi: 10.3171/jns.2006.104.4.513. [DOI] [PubMed] [Google Scholar]
- Collins DL, Pruessner JC. Towards accurate, automatic segmentation of the hippocampus and amygdala from MRI by augmenting ANIMAL with a template library and label fusion. Neuroimage. 2010;52:1355–1366. doi: 10.1016/j.neuroimage.2010.04.193. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Cossu M, Chabrades S, Hoffmann D, Lo Russo G. Presurgical evaluation of intractable epilepsy using stereo-electro-encephalography methodology: principles, technique and morbidity. Neurochirurgie. 2008;54:367–373. doi: 10.1016/j.neuchi.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Crepon B, Navarro V, Hasboun D, Clemenceau S, Martinerie J, Baulac M, Adam C, Le Van Duyen M. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain. 2010;133:33–45. doi: 10.1093/brain/awp277. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–567. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Dermont-Guignard S, Benquet P, Gerber U, Biraben A, Martin B, Wendling F. Distinct hyperexcitability mechanisms underlie fast ripples and epileptic spikes. Ann Neurol. 2012;71:342–352. doi: 10.1002/ana.22610. [DOI] [PubMed] [Google Scholar]
- Devaux B, Chassoux F, Guenot M, Haegelen C, Bartolomei F, Rougier A, Bourgeois M, Colnat-Coulbois S, Bulteau C, Sol JC, Geffredo S, Reyns N, Vinchon M, Proust F, Masnou P, Dupont S, Chabardes S, Coubes P. Epilepsy surgery in France. Neurochirurgie. 2008;54:453–465. doi: 10.1016/j.neuchi.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL Brain Development Cooperative Group. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J, Pittau F. Combining EEG and fMRI in the study of epileptic discharges. Epilepsia. 2011;52(Suppl 4):38–42. doi: 10.1111/j.1528-1167.2011.03151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Châtillon CE, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132:1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, Dubeau F, Gotman J. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–220. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehi LE, O’Dwyer R, Najm I, Alexopoulos A, Bingaman W. A longitudinal study of surgical outcome and its determinants following posterior cortex epilepsy surgery. Epilepsia. 2009;50:2040–2052. doi: 10.1111/j.1528-1167.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chianq MC, Christensen GE, Collins DL, Gee L, Hellier P, Sonq JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Quyen M, Staba R, Bragin A, Dickson C, Valderrama M, Fried I, Engel J. Large-scale microelectrode recordings of high-frequency gamma oscillations in human cortex during sleep. J Neurosci. 2010;30:7770–7782. doi: 10.1523/JNEUROSCI.5049-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyul LG, Udupa JK, Saha PK. Incorporating a measure of local scale in voxel-based 3-D image registration. IEEE Trans Med Imaging. 2003;22:228–237. doi: 10.1109/TMI.2002.808358. [DOI] [PubMed] [Google Scholar]
- Ochi A, Otsubo H, Donner EJ, Elliott I, Iwata R, Funaki T, Akizuki Y, Akiyama T, Imai K, Rutka JT, Snead OC., 3rd Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 2007;48:286–296. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- Ogren JA, Wilson CL, Bragin A, Lin JJ, Salamon N, Dutton RA, Luders E, Fields TA, Fried I, Toga AW, Thompson PM, Engel J, Jr, Staba RJ. Three-dimensional surface maps link local atrophy and fast ripples in human epileptic hippocampus. Ann Neurol. 2009;66:789–791. doi: 10.1002/ana.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonma SV, Blount JP, Gross RE. Planning extent of resection in epilepsy: limited versus large resections. Epilepsy Behav. 2011;20:233–240. doi: 10.1016/j.yebeh.2010.09.036. [DOI] [PubMed] [Google Scholar]
- Sarkis RA, Jehi LE, Bingaman WE, Najm IM. Surgical outcome following resection of rolandic focal cortical dysplasia. Epilepsy Res. 2010;90:240–247. doi: 10.1016/j.eplepsyres.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Schmidt MH, Pohlamnn-Eden B. Neuroimaging in epilepsy: the state of art. Epilepsia. 2011;52(Suppl 4):49–51. doi: 10.1111/j.1528-1167.2011.03154.x. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 1998;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J., Jr High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, Sperling MR, Luders H, Pedley TA Commission on Neurosurgery of the International League Against Epilepsy (ILAE) ILAE Commission Report; Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42:282–286. [PubMed] [Google Scholar]
- Wu JY, Sankar R, Lerner JT, Matsumoto JH, Vinters HV, Mathern GW. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology. 2010;75:1686–1694. doi: 10.1212/WNL.0b013e3181fc27d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelmann R, Zilfmans M, Jacobs J, Châtillon CE, Gotman J. Improving the identification of high-frequency oscillations. Clin Neurophysiol. 2009;120:1457–1464. doi: 10.1016/j.clinph.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]