Abstract
The accumulation of hepatic TG and development of hepatic steatosis (HS) is a serious complication of the use of parenteral nutrition (PN) formulas containing a high percentage of dextrose. But whether fat emulsions or other nutrients can ameliorate the induction of HS by high-carbohydrate diets is still uncertain. We hypothesized that administration of a lipid emulsion (LE; Intralipid) and/or the vitamin A metabolite retinal (RAL) will reduce hepatic TG accumulation and attenuate indicators of inflammation. C57BL/6 male mice were fed PN formula as their only source of hydration and nutrition for 4–5 wk. In Expt. 1, mice were fed PN only or PN plus treatment with RAL (1 μg/g orally), LE (200 μL i.v.), or both LE and RAL. In Expt. 2, LE was orally administered at 4 and 13.5% of energy to PN-fed mice. All PN mice developed HS compared with mice fed normal chow (NC) and HS was reduced by LE. The liver TG mass was lower in the PN+LE and PN+RAL+LE groups compared with the PN and PN+RAL groups (P < 0.01) and in the 4% and 13.5% PN+LE groups compared with PN alone. Hepatic total retinol was higher in the RAL-fed mice (P < 0.0001), but RAL did not alter TG mass. mRNA transcripts for fatty acid synthase (Fasn) and sterol regulatory element-binding protein-1c (Srebpf1) were higher in the PN compared with the NC mice, but FAS protein and Srebpf1 mRNA were lower in the PN+LE groups compared with PN alone. The inflammation marker serum amyloid P component was also reduced. In summary, LE given either i.v. or orally may be sufficient to reduce the steatotic potential of orally fed high-dextrose formulas and may suppress the early development of HS during PN therapy.
Introduction
Currently, there is great interest in hepatic steatosis (HS)7 caused by the ingestion of high-fat, obesogenic diets. However, very low- or no-fat diets containing dextrose as the major source of energy also have a considerable potential to induce the accumulation of fat in the liver, leading to HS. Parenteral nutrition (PN) formulas comprise a type of high-dextrose diet that is carbohydrate rich (72–86% of total energy from dextrose) compared with the common U.S. diet containing 45–65% of total energy from a mixture of simple and complex carbohydrates. Total PN is a lifesaving therapy in patients unable to absorb enteral nutrients, such as those with intestinal failure (1), and in other patients, i.v. dextrose may be combined with enteral feeding (2). In 2009, ∼390,000 patients in the major markets of the US, EU, and Canada used PN and through 2014, the total number of PN users is expected to rise by 10%/y to 628,000 (3). However, there are several complications with PN. Liver dysfunction is one of the major complications of total PN therapy, which in its more severe form may cause liver failure and death. It has been estimated that 57% of the children who received long-term home total PN developed biochemical liver abnormalities in a mean of 2.9 ± 0.4 y after starting total PN (4). HS frequently develops during PN therapy and is a crucial complication leading to fibrosis after long-term PN therapy. Ways to reduce HS are needed and preventing its development is an important goal for maintaining PN patients in clinical treatment.
Studies in mice have shown that feeding a PN formula as the only source of energy and hydration for 3 wk results in steatotic changes in the liver (5). This animal model presents a convenient and inexpensive way to test treatments that may prevent the development of HS and its progression, which could become applicable for use in patients. Lipid emulsions (LEs) are a common supplement added to PN formulas to prevent essential fatty acid (FA) deficiency, which itself is a cause of HS (6). Javid et al. (5) also tested the effect of an LE, Intralipid, a soybean oil-based emulsion rich in (n-6) FAs, which they administered i.v., and reported exacerbation of HS in PN-fed mice. This form of LE is often given with i.v. PN solutions, but in critically ill patients, lipids are often held because of concerns that (n-6) FAs will fuel inflammatory pathways (7). At present, the usefulness of LEs given orally is still undecided and evaluating their impact on liver fat accumulation is important for deciding how best to use this nutritional supplement in clinical care. Other nutrients may also have an impact on the development of HS. It has been reported that retinal (RAL), a partially oxidized metabolite of vitamin A (retinol), repressed adipogenesis, including development of HS in ob/ob mice (8). Based on these observations, we initially hypothesized that increased lipogenesis and HS would be induced by LE and attenuated by RAL. However, as we report here, we found that LE given i.v. and especially when given orally reduced liver TG accumulation while regulating the lipogenic transcription factor sterol regulatory element-binding protein-1c (SREBP1c) and reducing markers of hepatic inflammation.
Materials and Methods
Animal protocols.
Animal protocols were approved by the Institutional Animal Use and Care Committee of Pennsylvania State University. In Expt. 1, 5- to 6-wk-old male C57/BL6 mice (Taconic, n = 7–9/group) were divided into 5 groups: a group fed a stock rodent chow diet [normal chow (NC), Rodent Diet 5001, Lab Diet] as a reference group for comparison with the PN only group. This diet contained 239 g/kg protein, 50 g/kg fat, 51 g/kg fiber, and 3020 kcal/kg. The other groups were: PN only, PN+RAL (RAL described below), PN+LE (LE described below), and PN+RAL+LE. A sixth group of mice received the PN formula with a PBS injection as a control for the LE injection (below), but because this group did not differ from the PN group for the variables measured, it was not included in the statistical analysis. The PN formula was the only source of nutrition and hydration. PN consisted of CLINIMIX E 5/20 sulfite-free injection (Baxter Healthcare) containing 20% dextrose, 5% amino acids, and electrolytes supplemented with multiple vitamins for infusion (INFUVITE Pediatric, Baxter) and minerals and trace elements (8 μg/L zinc chloride, 6.4 μg/L cupric sulfate, 1.2 μg/L manganous sulfate, 80 μg/L chromium chloride, 176 μg/L sodium selenite, and 1.13 g/L ferrous sulfate). The PN solution was added daily to calibrated bottles fed to 2–3 mice/cage. Body weight (BW) and the volume of PN formula used were recorded daily. For the RAL treatment, all-trans-retinal (Sigma-Aldrich and Santa Cruz Biotechnology) was dissolved in soybean oil (Sigma-Aldrich) and delivered directly into the mouth at a dose of 1 μg/g (BW orally each day); all other groups received an equivalent amount of soybean oil, calculated to provide sufficient essential FAs to prevent essential FA deficiency (9). For the LE treatment, mice in Expt. 1 received Intralipid 20% (Baxter) (10) injected into the retro-orbital sinus, 100 μL/eye to both eyes daily. Mice were briefly anesthetized before injection using isoflurane-oxygen inhalation; a tetracaine analgesic drop was applied to each eye after injection. In Expt. 2, we further tested LE as the more promising of these agents for ameliorating the development of HS in PN-fed mice and we eliminated injections, which could be a source of inflammation. Twenty 5-wk-old male C57/BL6 mice, n = 5/group, were divided into 4 groups: NC, as in Expt. 1; PN solution as described above, which contained 0.5% of total energy as Intralipid for the prevention of essential FA deficiency; PN+4%LE; or PN+13.5%LE, equal to the percent of energy from fat in the NC diet. The PN+LE solutions were mixed daily and fed in calibrated feeding bottles. Intake was measured daily and mice were weighed every 3–4 d.
After 4 wk (Expt. 1) or 5 wk (Expt. 2), mice were individually killed with CO2. BW, liver, and epididymal fat pad weights were recorded. Blood for hematocrit and plasma preparation was collected in heparinized syringes. Portions of liver and epididymal adipose tissue were snap-frozen in liquid nitrogen and other portions were fixed in formalin or snap-frozen with Optimal Cutting Temperature compound (Sakura Finetek Tissue-Tek, Fisher) and stored at −80°C.
Histology.
Hematoxylin and eosin (H-E) staining was performed on 5-μm sections of formalin-fixed, paraffin-embedded liver and epididymal adipose tissue. The adipocyte size in the image of HE staining was quantified using Image-J software (NIH). Oil Red O staining was used to detect hepatic lipid droplets (11). Briefly, 8-μm-thick, frozen sections of liver were air-dried for 15 min and fixed with 3.7% formaldehyde for 1 h. An Oil Red O working solution was applied to each section and incubated for 30 min. To prepare the Oil Red O working solution, 6 mL of a stock solution consisting of 250 mg Oil Red O dye, 30 mL triethyl phosphate, and 20 mL distilled water was mixed with 4 mL distilled water and filtered. Slides were counterstained with hematoxylin.
Immunohistochemistry was performed to detect macrophage infiltration in the liver using 5-μm-thick, formalin-fixed, paraffin-embedded sections that were first incubated with blocking buffer containing 5% normal goat serum and 0.3% TritonX-100 in PBS, then incubated with a 1:100 dilution of rat monoclonal anti-mouse F4/80 antibody (eBioscience) in blocking buffer overnight at 4°C. Alkaline phosphatase-conjugated goat anti-rat IgG antibody (Sigma) was applied to each section as a secondary antibody and incubated for 1 h at room temperature. Nitroblue-tetrazolium/5-bromo-4-chloro-3′-indolyphosphate dye was used for color development. To suppress endogenous phosphatase activity and reduce background, 1.2 mmol/L levamisole was applied with the nitroblue-tetrazolium/5-bromo-4-chloro-3′-indolyphosphate solution.
Lipid assays.
Liver total lipid was extracted from 100 mg of liver (12). After solvent evaporation, the total weight was measured gravimetrically. The plasma TG concentration was quantified by a TG quantification kit (BioVision) and liver TG was assayed by a modification of the method of Sardesi and Manning (13). The lipid extract from 50 mg liver tissue was applied to a column of 3% water-deactivated aluminum oxide and the TG fraction was eluted with 25% diethyl ether in hexane (12). Then 100-μL aliquots of the eluate were used in duplicate for the TG assay, measured at 415 nm with triolein as a standard.
Liver retinol analysis.
The concentrations of retinol in plasma and liver were quantified by ultra performance liquid chromatography using an Acquity (Waters) system and a reverse-phase C-18 column. Plasma (20 μL) or lipids extracted from 100 mg liver (12) were saponified in 5% (wt:v) KOH in ethanol containing 1% pyrogallol (14) and then analyzed by ultra performance liquid chromatography.
qRT-PCR.
Total RNA was extracted from 100 mg liver tissue using Trizol reagent (Life Technologies), homogenized until the mixture became smooth, and the RNA was isolated. cDNA synthesized from 1 μg total RNA in 20 μL of the reaction mixture using Moloney murine leukemia virus reverse transcriptase (Promega) was used for qRT-PCR using 400 ng of primers (Supplemental Table 1) for each reaction and either iQ SYBR Green Supermix (Bio-Rad) or Fast SYBR Green Master mix (Applied Biosystems).
Statistical analysis.
Data are reported as the mean ± SEM. Student’s t test was used to compare the PN group with the NC group only. One-way ANOVA with Tukey’s post hoc test was used to compare the groups that were fed PN-based diets by using Prism5 software (GraphPad Software). P < 0.05 was considered significant.
Results
Growth and liver weight were not altered by PN diet or treatment with RAL or LE.
Expts. 1 and 2 each included mice fed NC as a reference group for histology and to establish usual concentrations for liver and plasma TG and markers of inflammation. In Expt. 2, we selected 13.5% LE, because this matches the percent of fat in the NC diet. Four percent LE was selected to represent a lower intake that was still compatible with clinical usage. The NC and PN groups did not differ in growth and final BW (Table 1). The intake of the PN diets [11.6 ± 0.3 mL/(mouse · d)] did not differ among any of the PN-fed groups and was calculated to provide 10.6 ± 0.2 kcal/d. Liver weight as a percent of BW did not differ among groups in Expt. 1 but was higher in the PN than in the NC mice in Expt. 2 and lower in the LE-supplemented mice (Table 1). Hematocrit (not shown) did not differ among groups in either experiment.
TABLE 1.
PN formula composition and formula intake, BW, and liver weight of mice administered PN with or without RAL and LE treatments (Expts. 1 and 2)1
| Expt. 1 |
Expt. 2 |
||||||||
| NC | PN | PN+RAL | PN+LE | PN+RAL+LE | NC | PN | PN+4%LE | PN+13.5%LE | |
| Macronutrient, % | |||||||||
| Carbohydrate | 58 | 77 | 77 | 74 | 74 | 58 | 77 | 74 | 67 |
| Protein | 28.5 | 22 | 22 | 22 | 22 | 28.5 | 23 | 22 | 20 |
| Fat | 13.5 | 0.5 | 0.5 | 4.0 | 4.0 | 13.5 | 0.5 | 4.0 | 13.5 |
| PN intake, mL/d | 12.2 ± 0.29 | 11.1 ± 0.60 | 11.3 ± 0.09 | 11.5 ± 0.34 | –2 | – | – | – | |
| BW, g | 23.7 ± 0.6 | 22.7 ± 0.6 | 22.4 ± 0.5 | 21.5 ± 0.4 | 22.1 ± 0.8 | 23.8 ± 0.8 | 22.1 ± 1.2 | 22.0 ± 0.8 | 22.3 ± 0.8 |
| Relative liver weight, mg/g BW | 59.2 ± 4.2 | 55.3 ± 1.4* | 54.3 ± 0.9 | 52.9 ± 1.3 | 50.4 ± 1.8 | 56.8 ± 0.5 | 64.8 ± 2.0a* | 55.2 ± 1.1b | 54.7 ± 1.2b |
Values are percentage or mean ± SEM, = 9 (Expt. 1 except NC, n = 7) or 5 (Expt. 2). *Different from NC, P < 0.05. Within an experiment, means for PN groups in a row with superscripts without a common letter differ, P < 0.05. BW, body weight; LE, lipid emulsion; NC, normal chow (nonpurified rodent diet); PN, parenteral nutrition; RAL, retinal.
PN intake data were similar to Expt. 1 and are not reported.
PN-induced liver TG accumulation is reduced by LE.
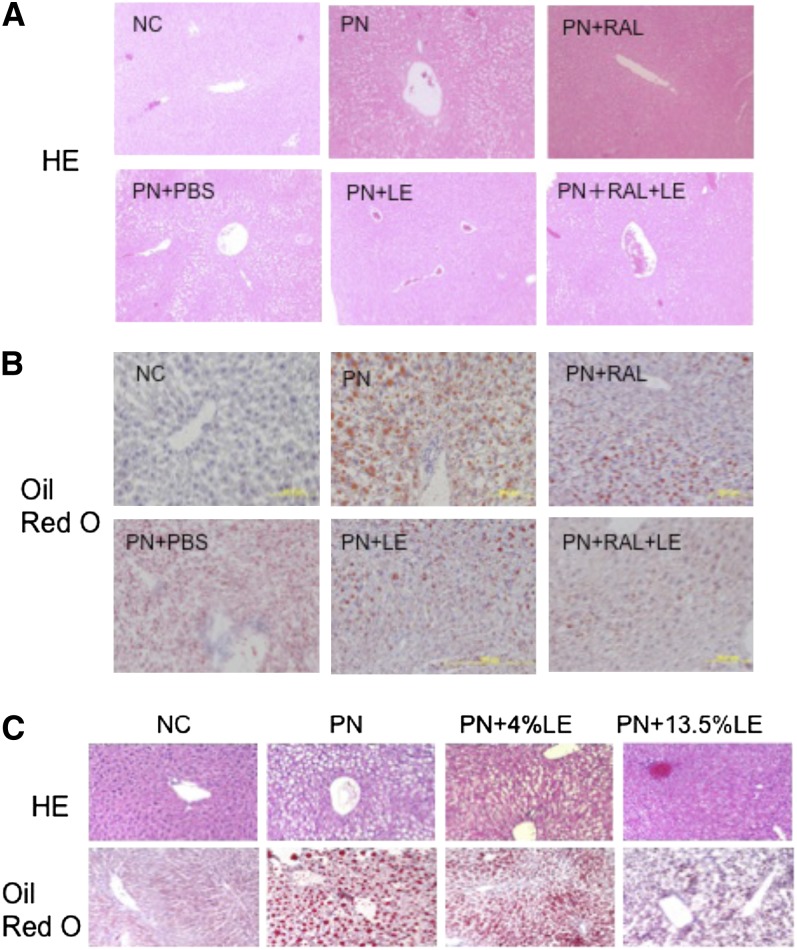
In Expt. 1, NC mice had no evidence of HS, whereas the livers of all PN mice showed macrovesicular fatty changes characteristic of HS, evaluated by staining with HE and Oil Red O. Hepatocytes had a characteristic signet ring appearance in H-E–stained sections, which was most prominent surrounding the periportal area and less so surrounding the central vein (Fig. 1A). By comparison, in PN-fed mice treated with RAL, LE, and RAL+LE, macrovesicular fatty change was reduced and hardly observed in the PN+LE group (Fig. 1A). Oil Red O staining of the PN liver revealed numerous large and small lipid droplets within the hepatocytes in the periportal area (Fig. 1B), which were apparently smaller in LE- and RAL+LE-treated mice (Fig. 1B). Fibrosis or inflammatory changes were scarce in all of the groups, which may be related to the relatively short (4 wk) duration of this study. Hepatocyte cord formation appeared to be conserved.
FIGURE 1.
Histology of liver from mice administered PN formula with or without RAL and LE treatments. (A) H-E staining of formalin-fixed, paraffin-embedded sections and (B) Oil Red O staining of frozen liver sections with hematoxylin counter staining (Expt. 1). Sections from liver of mice fed NC serves as a reference for normal histology and Oil Red O staining. (C) H-E and Oil Red O staining of liver sections (Expt. 2). Red: lipid; purple: nuclei. Original magnification ×100. Representative images from each group (n = 6–9) are shown. H-E, hematoxylin and eosin; LE, lipid emulsion; NC, normal chow; PN, parenteral nutrition diet; RAL, retinal.
In Expt. 2, PN-fed mice were supplemented daily for 5 wk with LE at 2 levels, PN+4%LE and PN+13.5%LE, admixed daily with the PN formula. The calculated caloric intake did not differ among the 3 PN groups in Expt. 2. As in Expt. 1, mice fed PN alone developed HS (Fig. 1C). LE dose dependently inhibited hepatic lipid accumulation. Not only the number but also the size of lipid droplets in the liver were visibly smaller in the PN+4%LE and PN+13.5%LE groups compared with the PN mice.
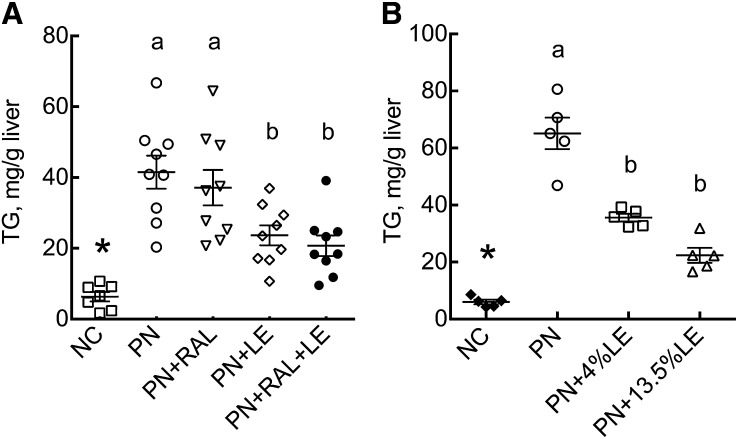
The liver TG concentration was significantly higher in PN than in NC mice (Fig. 2A,B). In Expt. 1, only those treatments that included LE reduced the liver TG mass in PN-fed mice (Fig. 2A). The liver total lipid weight (not shown) followed the same pattern as TG. The plasma TG concentration did not differ among groups (not shown). In Expt. 2, hepatic TG was significantly lower in PN-fed mice given oral LE supplementation at both 4 and 13.5% of energy (Fig. 2B).
FIGURE 2.
Liver TG concentration in mice administered PN formula with or without RAL and LE treatments in Expt. 1 (A) and 2 (B). Data shown are for individual mice with mean (wide bar) and SEM (narrow bars) for each group. Mean ± SEM, n = 7–9 (Expt. 1) or 5 (Expt. 2). *Different from PN, P < 0.001. PN-fed groups without a common letter differ, P < 0.05. LE, lipid emulsion; NC, normal chow; PN, parenteral nutrition diet; RAL, retinal.
Hepatic retinoid status in RAL-treated mice.
Liver total retinol, as retinyl esters and retinol, measured in Expt. 1 was lower in PN compared with NC mice (P < 0.01) (Supplemental Fig. 1), which may reflect the higher provitamin A content of the NC diet. Total retinol was higher in mice that received RAL regardless of LE (P < 0.0001). Plasma retinol (not shown) did not differ among any of the groups.
The relative expression levels of several genes related to retinoid homeostasis were quantified by qRT-PCR, including cytochrome P-450 (Cyp) Cyp26A1, which catalyzes retinoic acid (RA) oxidation, lecithin:retinol acyltransferase (Lrat), which esterifies retinol, retinol dehydrogenase (Rdh)1, and 3 retinal dehydrogenase genes, Aldh1a1, Aldh1a2, and Aldh1a3, which are implicated in RA biosynthesis. Of these, only Aldh1a3 (not shown) was higher in PN compared with NC mice (P < 0.05), but it did not differ among the PN treatment groups.
Transcription factors related to FA synthesis, oxidation, and lipid mobilization.
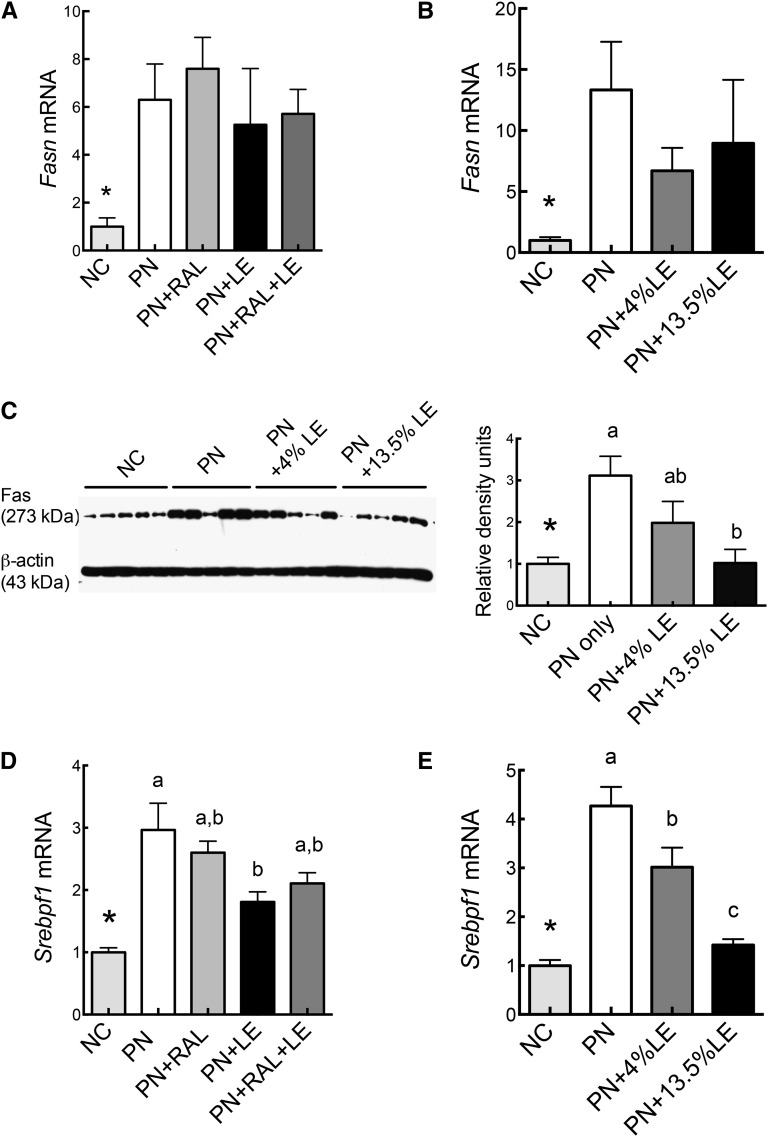
The relative mRNA transcript levels for FAS (Fasn), a major lipogenic gene, and for SREBP1c (Srebpf1), known to be involved in the development of HS, were determined in both experiments (Fig. 3). Fasn transcripts were higher in PN compared with NC mice (Fig. 3A,B), as was FAS protein (Fig. 3C). Whereas Fasn mRNA was not significantly reduced by LE or RAL, FAS protein was lower in mice treated with LE (Fig. 3C). Srebpf1 followed a similar pattern to FAS protein (Fig. 3E). We also measured the transcripts for carbohydrate response element binding protein (Chrebp) (15) carnitine palmitoyltransferase 1 (Cpt1), steroyl-CoA desaturase (Scd1), apolipoprotein (Apo)AI, ApoB, adiponectin receptor 2 (AdipoR2), uncoupling protein-2 (Ucp2), and the reference gene tubulin (Tub); these mRNAs did not differ among groups (not shown).
FIGURE 3.
Relative mRNA levels for FAS (Fasn) transcripts (A,B) and protein (C) and SREBP1c (Srebpf1) transcripts (D,E) in the liver of mice administered PN formula with or without RAL and LE treatments in Expt. 1 (A,D) and 2 (B,C,E). The mean of the NC group was set to 1.0. Values are mean ± SEM, n = 7–9 (Expt. 1) or 5 (Expt. 2). *Different from PN, P < 0.05. PN-fed groups without a common letter differ, P < 0.05. LE, lipid emulsion; NC, normal chow; PN, parenteral nutrition diet; RAL, retinal.
Reduction in hepatic inflammatory markers by LE.
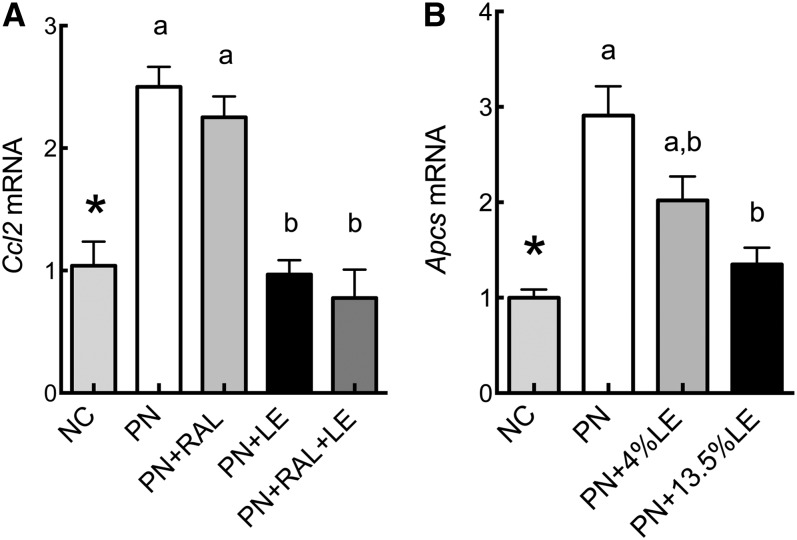
As indicators of hepatic inflammation, we determined the mRNA transcript levels for serum amyloid P component (Apcs), an acute-phase protein produced in liver, and chemokine C-C motif ligand-2 (Ccl2). Ccl2 and Apcs transcripts were higher in PN than in NC mice, whereas LE-treated groups had lower levels of these transcripts compared with PN only (Fig. 4A,B). Other genes that were measured, Tnf and Il1β, did not significantly differ (data not shown), whereas Il6 mRNA was below the limit of detection by qRT-PCR. Additionally, immunohistochemistry of liver sections, using F4/80 antibody as a marker of macrophages, showed no noticeable evidence of infiltration (Supplemental Fig. 2).
FIGURE 4.
Relative mRNA levels for inflammatory markers Ccl2 and Apcs in the liver of PN-fed mice administered PN formula with or without RAL and LE treatments in Expt. 1 (A) and 2 (B). Values are mean ± SEM, n = 7–9 (Expt. 1) or 5 (Expt. 2). *Different from PN, P < 0.005. PN-fed groups without a common letter differ, P < 0.05. LE, lipid emulsion; NC, normal chow; PN, parenteral nutrition diet; RAL, retinal.
Adipose tissue weight and adipocyte size.
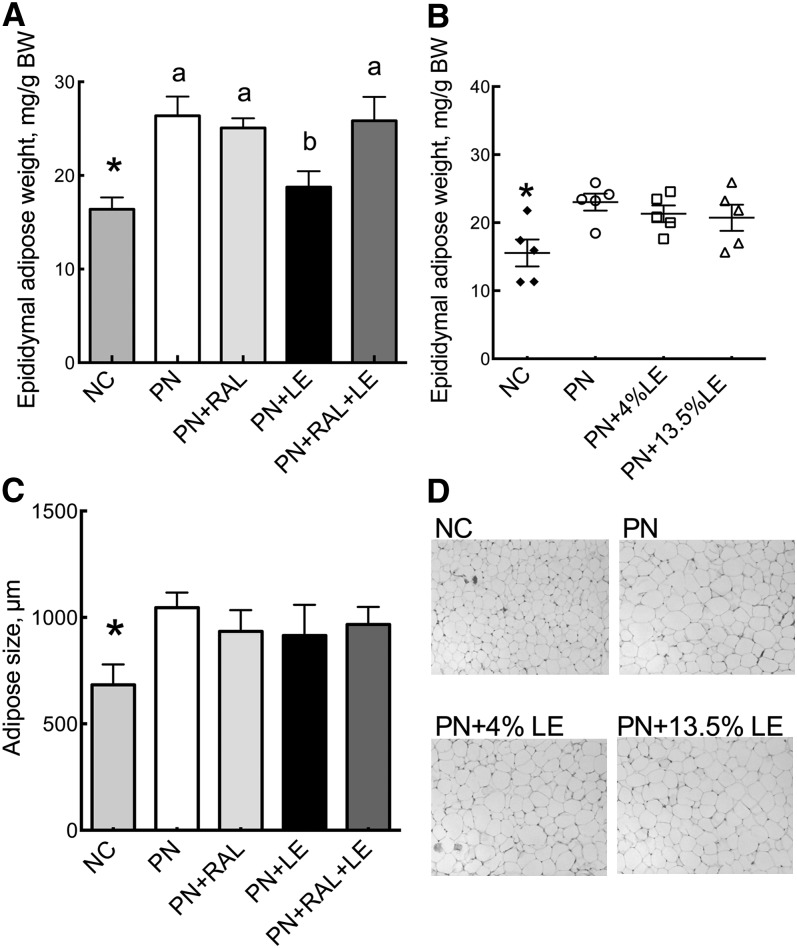
Epididymal adipose tissue weight was greater in the PN than in the NC groups (Fig. 5A,B). Whereas RAL had no effect, weight was lower in the LE group in Expt. 1 compared with PN only (Fig. 5A), but not in Expt. 1 in which LE was given orally (Fig. 5B). Epididymal adipocytes were larger in PN compared with NC mice in Expt. 1 (Fig. 5C) and as illustrated for Expt. 2 (Fig. 5D), but treatments with RAL or LE did not alter levels compared with the PN group.
FIGURE 5.
Epididymal adipose tissue weight, cell number, and cellularity in PN-fed mice. Adipose tissue weight per BW in Expt. 1 (A) and Expt. 2 (B). Values are mean ± SEM, n = 7–9 (Expt. 1) or 5 (Expt. 2). *Different from PN, P < 0.05. PN-fed groups without a common letter differ, P < 0.05. (C) Adipocyte size (Expt. 1). (D) Representative H-E–stained adipose sections (Expt. 2). BW, body weight; H-E, hematoxylin and eosin; LE, lipid emulsion; NC, normal chow; PN, parenteral nutrition diet; RAL, retinal.
Discussion
In the present study, 4–5 wk of enterally feeding a PN diet as the only source of nutrition and hydration resulted in a moderate but significantly greater hepatic TG concentration, compared with NC, and liver morphology typical of HS. Our experiments of a 4- and 5-wk duration focused on the inductive phase of HS and were relatively short compared with studies of 12–16 wk that have addressed the hepatic complications of a high-fat diet (16). This short-term scenario is relevant to prevention of HS during short-term management using high-dextrose formulas, but it could also be relevant to long-time use of total PN in which HS is an important problem that, over time, may progress to liver fibrosis and liver failure. Hepatic inflammation was modest in the PN mice in our model, but nonetheless, the inflammatory markers Ccl2 and serum amyloid P component (gene Apcs) were significantly reduced by LE. This suggests both that hepatic inflammation was in the beginning stages in this model and that LE may prevent this initiation phase.
The model we used was selected based on previous studies in which LE was given by venous injection to mice orally fed PN solution (5). In those studies, PN feeding was reported to result in HS in 3 wk, thus providing a convenient model for testing the effects of other nutrients as a means to prevent or slow the development of HS. Although many aspects of the previous study and our current study were similar, including use of the same strain, sex, and age of mice, similar PN formulas, and the same LE, we nevertheless observed different results. Mice in the previous study were treated with 120 mg/mouse of Intralipid every other day, injected into the tail vein, whereas mice in our first experiment received 40 mg/mouse of Intralipid every day by retro-orbital injection. It is possible that the amount of LE injected is critical, but the route of administration could also be a factor. We chose retro-orbital injection because, in our hands, injection into the tail vein is much more difficult. We had no known problems with injection and this is supported by the relatively low levels of expression of inflammatory markers in all groups, which were not higher in the LE-treated mice. This route of injection does, however, require brief anesthesia. To eliminate anesthesia and injection entirely, we designed Expt. 2 to deliver a designated amount of Intralipid, which we added directly into the PN solution each day. In both experiments, we took precautions to eliminate essential FA deficiency as a confounding factor by either orally supplementing mice with soybean oil in Expt. 1 or by including 0.5% of LE in the PN group in Expt. 2. Expt. 2 showed that LE is very effective in reducing HS when given orally, because morphological indicators of HS and the elevation of hepatic TG in the PN group, compared with NC, were dose-dependently lowered by 4 and 13.5% LE. This is relevant, because it supports a transition to enteral feeding as soon as feasible in the clinical setting and also raises a rationale for dual modality feeding when full enteral feeding is not possible. Concomitantly, LE also reduced Apcs expression, a marker of hepatic inflammation. Overall, our results suggest that LE delivered either i.v. or orally can substantially reduce the lipogenic potential of a high-dextrose PN solution.
We found that PN mice grew normally, but they did not gain more weight than NC mice. Although the PN-fed mice were nonobese, their epididymal adipose tissue weight was slightly greater and their epididymal adipocyte size was greater, although less so than in mice fed a high-fat diet for longer times (16). LE, although not RAL+LE, reduced adipose tissue weight in Expt. 1 but not in Expt. 2. Overall, these results suggest that the antilipogenic effect of LE was focused on the liver. Future studies are needed to assess the optimal composition of the LE, which could help to optimize the use of PN therapy in clinical care (17). It also is noteworthy that LEs containing (n-3) FAs have produced favorable results in pediatric total PN feeding studies (18–21) and in adults (1). Our mouse model may provide a convenient approach to rapidly and efficiently testing different formulations for their impact on the prevention of HS.
Retinal treatment has been described to reduce lipid accumulation in white adipose tissue and liver, although it should be noted that RAL was previously injected (8). In our Expt. 1 in which RAL was given daily as an oral supplement, there was no discernable effect on liver weight, lipid or TG mass, or inflammatory markers. The elevation of liver total retinol in RAL-treated mice was still within the normal range and the expression of retinoid homeostasis genes did not differ. It is likely that most orally administered RAL was reduced to retinol and esterified to form retinyl esters prior to absorption (22), but it could also be oxidized to form RA. Recently, studies using Aldh1a1 knockout mice have indicated that RA produced from RAL is the likely regulator of glucose homeostasis and adipose tissue lipid accumulation (23). The ability of RAL to suppress lipogenesis may be limited to routes that bypass the intestine. Other investigators have reported a hyperdynamic state of retinoid metabolism in which mRNA transcripts for several enzymes involved in retinoid metabolism, including LRAT, RDH, retinal dehydrogenases 1 and 3, and Cyp26A1, retinoid-binding proteins, and nuclear retinoid receptors, were upregulated (24). The role of RA, a more oxidized retinoid produced from RAL, in lipid metabolism is controversial. RA used clinically is known to induce hypertriglyceridemia (25, 26). Two lipogenic genes, SREBP-1 and FAS, were induced in hepatic cells by all-trans-RA in vitro (27), whereas they were dramatically suppressed in vivo (28). Further studies are needed to understand the impact of retinoids on the development of HS.
Various mechanisms have been suggested to be involved in the development HS by PN feeding. An increase in hepatic TG production driven by high carbohydrate intake and insulin secretion may be especially important. Insulin regulates hepatic lipogenesis by activation of the transcription of SREBP1-c, which in turn can activate the transcription of many genes required for lipogenesis (29) including acetyl-CoA carboxylase-2 (Acc2), which produces malonyl-CoA at the mitochondrial membrane. SREBP1-c also activates the transcription of FAS (Fasn) and stearoyl-CoA desaturase-1 (Scd-1). FAS protein expression was reduced as shown in Expt. 2. Our data for Srebpf1 suggest that the PN-induced elevation of expression can be nearly completely attenuated by LE. The LE used in our study contains ∼53% of linoleic acid (10, 20). The provision of a sufficient amount of preformed unsaturated FAs and/or the supply of PUFAs that mammals cannot synthesize may be crucial for reducing the requirement for de novo FA production in the face of an obligatory high glucose intake. A limitation of our study is that plasma glucose and insulin were not determined. Srepbf1c mRNA was significantly elevated by PN feeding, which may have promoted sufficient insulin release to increase the de novo synthesis of FA in the liver. The reduction in Srebpf1c mRNA by including LE in the diet could be a plausible mechanism linking LE intake to a reduction in hepatic TG accumulation. Additional studies are needed to compare (n-6)-FA–rich LE, such as the one used here, which may also support the production of inflammatory lipid mediators, and (n-3) or (n-3)+(n-6) FA LE mixtures that may have a more favorable profile regarding inflammation and related outcomes in patients (30). However, the reduction in Ccl2 and Apcs expression in our studies suggests that a (n-6) FA-rich LE is not necessarily proinflammatory. Nevertheless, further studies are needed with additional biomarkers of inflammation and in models with preexisting inflammation.
The impact of PN therapy and high-carbohydrate diets on the immune system is not fully understood. Kanuri et al. (31) reported that a high-fructose diet caused a 5-fold greater hepatic TG accumulation, a doubling in CCL2 and CCL19 production, and neutrophil infiltration, whereas these factors were attenuated in mice lacking TNFα receptor-1. An activation or increase in the number of Kupffer cells/macrophages has been reported in experimental nonalcoholic fatty liver disease (24, 32). Reimund et al. (33) found that long-term home PN patients had higher circulating TNFα, which correlated with the circulating level of alkaline phosphatase as a measure of liver dysfunction and with the amount of infused energy. IL-6, which is known to induce TNFα production, was reported to be significantly higher among home PN patients, relative to controls, in blood and urine (34). It has been reported that visceral adiposity tissue in patients with nonalcoholic fatty liver disease produced numerous proinflammatory cytokines, including TNFα and IL-6, which promote the recruitment and activation of Kupffer cells (35). LEs are known to be modulators of immune function in a composition-dependent manner (36). In our study using an (n-6) FA-rich LE, Tnf expression was not affected and Il6 mRNA was below the limit of detection; however, LE downregulated the PN-induced elevation in Ccl2 and Apcs. We observed little evidence of macrophage infiltration by microscopy. The increase in epididymal adipose, although significant in PN compared with NC mice, was still modest, suggesting that PN feeding over a short course may not be a strong inducer of inflammation. Therefore, the lipogenic response to glucose may be the main factor in early HS. Further studies are needed to clarify the specific mechanisms through which different types of LE, administered either i.v. or enterally, can prevent the hepatic complications of short- and long-term PN therapy.
Supplementary Material
Acknowledgments
The authors thank Baxter Healthcare for a postdoctoral fellowship to the Department of Nutritional Sciences, which enabled Dr. Ito’s work on this project, and for providing the Clinimix, Intralipid, and vitamin mix used in this study. The authors also thank Dr. Gordon L. Jensen for his advice and encouragement and Natalie M. Mueller and Moksha Atluri for excellent technical assistance. K.I. and A.C.R. designed research; K.I., L.H., A.E.W., and A.C.R. conducted research; K.I., L.H., and A.E.W. analyzed data; K.I., L.H., and A.C.R. wrote the paper; and A.C.R. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BW, body weight; FA, fatty acid; H-E, hematoxylin and eosin; HS, hepatic steatosis; LE, lipid emulsion; NC, normal chow (nonpurified rodent diet); PN, parenteral nutrition; RA, retinoic acid; RAL, retinal.
Literature Cited
- 1.Raman M, Allard JP. Parenteral nutrition related hepato-biliary disease in adults. Appl Physiol Nutr Metab. 2007;32:646–54 [DOI] [PubMed] [Google Scholar]
- 2.Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr. 2001;74:534–42 [DOI] [PubMed] [Google Scholar]
- 3.The Future of parenteral nutrition: key players, disease specific opportunities and market forecasts. New York: PR Newswire; 2011
- 4.Peyret B, Collardeau S, Touzet S, Loras-Duclaux I, Yantren H, Michalski MC, Chaix J, Restier-Miron L, Bouvier R, Lachaux A, et al. Prevalence of liver complications in children receiving long-term parenteral nutrition. Eur J Clin Nutr. 2011;65:743–9 [DOI] [PubMed] [Google Scholar]
- 5.Javid PJ, Greene AK, Garza J, Gura K, Alwayn IP, Voss S, Nose V, Satchi-Fainaro R, Zausche B, Mulkern RV, et al. The route of lipid administration affects parenteral nutrition-induced hepatic steatosis in a mouse model. J Pediatr Surg. 2005;40:1446–53 [DOI] [PubMed] [Google Scholar]
- 6.Adolph M, Heller AR, Koch T, Koletzko B, Kreymann KG, Krohn K, Pscheidl E, Senkal M. Working group for developing the guidelines for parenteral nutrition of The German Association for Nutritional Medicine. LEs: guidelines on parenteral nutrition. Ger Med Sci. 2009;7:Doc2220049078 [Google Scholar]
- 7.Vanek VW, Seidner DL, Allen P, Bistrian B, Collier S, Gura K, Miles JM, Valentine CJ, Kochevar M, Novel Nutrient Task Force Intravenous Fat Emulsions Workgroup, and American Society for Parenteral and Enteral Nutrition Board of Directors A.S.P.E.N. position paper: clinical role for alternative intravenous fat emulsions. Nutr Clin Pract. 2012;27:150–92 [DOI] [PubMed] [Google Scholar]
- 8.Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Vogel S, et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. 2007;13:695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerecedo LR, Panzarella FP, Vasta AB, de Renzo EC. Studies on essential fatty acid deficiency in 3 strains of mice. J Nutr. 1952;48:41–7 [DOI] [PubMed] [Google Scholar]
- 10.Daily Med Current Medication Information. Intralipid [cited 2012 Sep 9]. Available from: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=67182.
- 11.Koopman R, Schaart G, Hesselink MK. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol. 2001;116:63–8 [DOI] [PubMed] [Google Scholar]
- 12.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509 [PubMed] [Google Scholar]
- 13.Sardesi VM, Manning JA. The determination of triglycerides in plasma and tissues. Clin Chem. 1968;114:147–52 [Google Scholar]
- 14.Ross AC. Separation and quantitation of retinyl esters and retinol by high-performance liquid chromatography. Methods Enzymol. 1986;123:68–74 [DOI] [PubMed] [Google Scholar]
- 15.Postic C, Dentin R, Denechaud PD, Girard J. ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annu Rev Nutr. 2007;27:179–92 [DOI] [PubMed] [Google Scholar]
- 16.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, II, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–8 [DOI] [PubMed] [Google Scholar]
- 17.Koletzko B. Intravenous lipid emulsions for infants: when and which? Am J Clin Nutr. 2012;96:225–6 [DOI] [PubMed] [Google Scholar]
- 18.Gura KM, Duggan CP, Collier SB, Folkman J, Bistrian RB, Puder M. Reversal of parenteral nutrition–associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118:e197–201 [DOI] [PubMed] [Google Scholar]
- 19.Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, Arsenault DK, Strijbosch RA, Lopes S, Duggan C, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121:e678–86 [DOI] [PubMed] [Google Scholar]
- 20.Vlaardingerbroek H, Veldhorst MAB, Spronk S, van den Akker CHP, van Goudoever JB. Parenteral lipid administration to very-low-birth- weight infants: early introduction of lipids and use of new lipid emulsions: a systematic review and meta-analysis. Am J Clin Nutr. 2012;96:255–68 [DOI] [PubMed] [Google Scholar]
- 21.Rollins MD, Scaife ER, Jackson WD, Meyers RL, Mulroy CW, Book LS. Elimination of soybean lipid emulsion in parenteral nutrition and supplementation with enteral fish oil improve cholestasis in infants with short bowel syndrome. Nutr Clin Pract. 2010;25:199–204 [DOI] [PubMed] [Google Scholar]
- 22.Ross AC, Harrison EH. Vitamin A: nutritional aspects of retinoids and carotenoids. In: Zempleni J, Rucker, RB, McCormick D, Suttie JW, editors. Handbook of vitamins. 4th ed. Boca Raton: Taylor & Francis Group; 2007. p. 1–40.
- 23.Kiefer FW, Orasanu G, Nallamshetty S, Brown JD, Wang H, Luger P, Qi NR, Burant CF, Duester G, Plutzky J. Retinaldehyde dehydrogenase 1 coordinates hepatic gluconeogenesis and lipid metabolism. Endocrinology. 2012;153:3089–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashla AA, Hoshikawa Y, Tsuchiya H, Hashiguchi K, Enjoji M, Nakamuta M, Taketomi A, Maehara Y, Shomori K, Kurimasa A, et al. Genetic analysis of expression profile involved in retinoid metabolism in non-alcoholic fatty liver disease. Hepatol Res. 2010;40:594–604 [DOI] [PubMed] [Google Scholar]
- 25.Krupková M, Janků M, Liska F, Sedová L, Kazdová L, Krenová D, Kren V, Seda O. Pharmacogenetic model of retinoic acid-induced dyslipidemia and insulin resistance. Pharmacogenomics. 2009;10:1915–27 [DOI] [PubMed] [Google Scholar]
- 26.Gerber LE, Erdman JWJ. Comparative effects of all-trans and 13-cis retinoic acid administration on serum and liver lipids in rats. J Nutr. 1980;110:343–51 [DOI] [PubMed] [Google Scholar]
- 27.Roder K, Zhang L, Schweizer M. SREBP-1c mediates the retinoid-dependent increase in fatty acid synthase promoter activity in HepG2. FEBS Lett. 2007;581:2715–20 [DOI] [PubMed] [Google Scholar]
- 28.Amengual J, Ribot J, Bonet ML, Palou A. Retinoic acid treatment enhances lipid oxidation and inhibits lipid biosynthesis capacities in the liver of mice. Cell Physiol Biochem. 2010;25:657–66 [DOI] [PubMed] [Google Scholar]
- 29.Wong RH, Sul HS. Insulin signaling in fatty acid and fat synthesis: a transcriptional perspective. Curr Opin Pharmacol. 2010;10:684–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei C, Hua J, Bin C, Klassen K. Impact of lipid emulsion containing fish oil on outcomes of surgical patients: systematic review of randomized controlled trials from Europe and Asia. Nutrition. 2010;26:474–81 [DOI] [PubMed] [Google Scholar]
- 31.Kanuri G, Spruss A, Wagnerberger S, Bischoff SC, Bergheim I. Role of tumor necrosis factor-α (TNFα) in the onset of fructose-induced nonalcoholic fatty liver disease in mice. J Nutr Biochem. 2011;22:527–34 [DOI] [PubMed] [Google Scholar]
- 32.Shimamura M, Karasawa H, Sakakibara S, Shinagawa A. Raldh3 expression in diabetic islets reciprocally regulates secretion of insulin and glucagon from pancreatic islets. Biochem Biophys Res Commun. 2010;401:79–84 [DOI] [PubMed] [Google Scholar]
- 33.Reimund JM, Duclos B, Arondel Y, Baumann R. Persistent inflammation and immune activation contribute to cholestasis in patients receiving home parenteral nutrition. Nutrition. 2001;17:300–4 [DOI] [PubMed] [Google Scholar]
- 34.Ling PR, Khaodhiar L, Bistrian BR, Keane-Ellison M, Thibault A, Tawa N. Inflammatory mediators in patients receiving long-term home parenteral nutrition. Dig Dis Sci. 2001;46:2484–9 [DOI] [PubMed] [Google Scholar]
- 35.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–60 [PubMed] [Google Scholar]
- 36.Wanten GJA, Calder PC. Immune modulation by parenteral lipid emulsions. Am J Clin Nutr. 2007;85:1171–84 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.