Abstract
Iron deficiency (ID) is the most common nutrient deficiency worldwide, disproportionally affecting infants, children, and women of childbearing age. Although ID commonly occurs with anemia (IDA), nonanemic ID is 3 times more common than IDA in toddlers and also occurs in infants following gestational complications. Both conditions negatively affect motor, socio-emotional, and cognitive behaviors, suggesting that iron, apart from anemia, has a critical role in neurodevelopment. Here, the specific role of iron in regulation of mammalian target of rapamycin (mTOR) signaling (a kinase pathway that integrates metabolic supply and demand to regulate cell growth and morphology) was examined using 2 hippocampal, pyramidal cell-specific, nonanemic, genetic mouse models of ID: a CAMKIIα cre-loxP permanent knockout of divalent metal transporter-1 (DMT-1 CKO) and a CAMKIIα-tTA–driven reversible, overexpression of nonfunctional, dominant negative transferrin receptor-1 (DN TfR-1). In both models, mTOR activity, assessed by phosphorylation levels of key proteins, was upregulated during development by ID [S6K(Thr389) phosphorylation increased 87 and 57% in the DMT-1 CKO and DN TfR-1 models, respectively; P < 0.05]. This effect was shown to be iron-dependent, because iron repletion at postnatal d 21 normalized mTOR activity in the reversible DN TfR-1 model (62% reduction compared with unrepleted mice; P < 0.05). In the permanent DMT-1 CKO model, suppression of ID-induced mTOR hyperactivity by rapamycin administered during the sensitive period for iron improved Morris water maze performance despite ongoing ID (DMT-1 wild-type and DMT-1 CKO mice reached criterion in 3 d compared with 4 d necessary for vehicle-treated DMT-1 CKO mice; P < 0.05). Together, these findings implicate mTOR dysregulation as a cellular mechanism underlying the acute and persistent neurodevelopmental deficits that accompany early-life ID.
Introduction
Successful construction and maintenance of the hippocampus is a complex process. The hippocampus undergoes a period of rapid structural and functional development in humans between birth and 2 y and in rodents between postnatal (P)8 d 10 and 25. During this critical period, neurons establish complex dendrite arbors and form synaptic connections (1, 2). Supporting the demands of dendritogenesis and synaptogenesis requires regulation of cellular metabolism and, accordingly, utilization of oxygen, glucose, and iron as well as production of ATP and neurotrophins increases in the hippocampus during this period of rapid growth (3–7).
Iron is crucial for all cells to respond to oxygen availability and oxidative stress. It is found in globin oxygen transport proteins and is a cofactor for the activity of prolyl-hydroxylase, which regulates HIF1α stability (8). Mitochondrial enzymes, including cytochromes, NADPH, and flavoproteins, require iron in the form of heme and iron-sulfur clusters (9), which are integral for oxidative phosphorylation and ATP production. Iron status also regulates levels of BDNF and insulin-like growth factors and their receptors, which are primary regulators of neuronal growth and plasticity (6, 10–12).
During development in rodents, rapid hippocampal dendritogenesis is preceded by high rates of local iron import at the dendritic spines via TfR-1 cycling (4, 5, 13). Iron deficiency (ID), ID anemia (IDA), and hypoxia during development result in structural aberrations in the hippocampus that include abnormal dendrite branching, reduced dendrite complexity, and altered spine morphology (14–16). The contribution of cellular metabolism to the integration and regulation of these processes is not well understood. Mammalian target of rapamycin (mTOR) signaling is of particular interest, because it responds to the metabolic state of the cell by integrating metabolic demand mediated through growth factor stimulation of the PI3K signaling pathway and metabolic supply (e.g., oxygen, BCAA, energy) to regulate cell growth and morphology (17, 18).
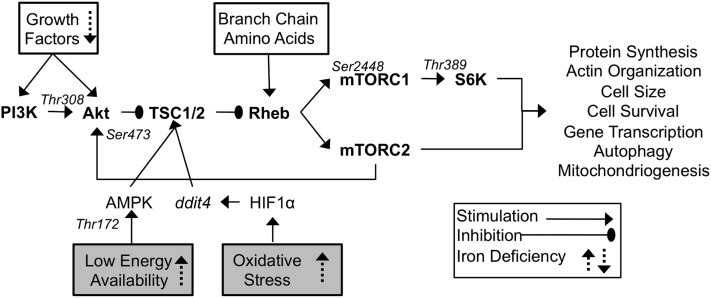
mTOR is a highly conserved Ser/Thr kinase that forms 2 distinct functional complexes (mTORC1 and mTORC2) (Fig. 1). mTORC1’s activity is blocked by rapamycin and its downstream targets regulate protein translation, cell survival, gene transcription, and autophagy (18). Although mTORC2 activity is not acutely sensitive to rapamycin, prolonged rapamycin treatment inhibits mTORC2, which regulates actin organization as well as Akt and PKC activity (19–21). Overall, mTOR activity stimulates protein synthesis and actin organization and also increases metabolic activity by increasing mitochondrial gene expression, oxygen consumption, and iron uptake (22, 23). Through this coordinated regulation, mTOR activity not only promotes neuronal cell growth and differentiation but also modulates the metabolic activity required to support it.
FIGURE 1.
mTOR signaling. Central signaling components are in bold, stimulatory regulators are indicated by solid boxes, and inhibitory regulators by shaded boxes. Key phosphorylation sites are noted in italics above each protein. Previously observed effects from in vivo and in vitro studies using iron chelation and IDA are noted with dashed arrows. IDA, iron deficiency anemia; mTOR, mammalian target of rapamycin.
In light of the number of iron-dependent processes occurring during the period of rapid growth and increased metabolic demand that occur during hippocampal development, it stands to reason that iron status may alter mTOR activity and contribute to the developmental deficits caused by early-life ID. Previous studies examined the role of iron in mTOR regulation using relatively broad and severe tools such as iron chelation and total body IDA. In these instances, mTOR activity is suppressed by iron chelation in vitro (24, 25). In vivo, IDA suppresses hippocampal expression of genes in the mTOR pathway and reduces mTOR protein phosphorylation in total brain lysates (24, 26). While these findings indicate that mTOR activity may be responsive to iron status, it is not possible to determine whether the suppression of mTOR is caused by ID or by the confounds of IDA and iron chelation.
In the current study, we examined the specific role of iron in developmental regulation of mTOR signaling using 2 unique genetic mouse models of in vivo neuronal ID that are not confounded by the hypoxia or increased uptake of other divalent metals that occur in dietary IDA (11, 27). One model is generated by permanent, conditional Cre-lox P knockout of Slc11a2, the gene that codes for divalent metal transporter-1 (DMT-1) conditional knockout (DMT-1 CKO) in hippocampal neurons and results in a 40% decrease in the hippocampal iron concentration without affecting the remainder of the brain (27). The other acts through reversible tet-off overexpression of a nonfunctional dominant negative TfR-1 (DN TfR-1) in hippocampal neurons and causes a similar loss of iron in CA1 pyramidal neurons (11). We hypothesized that mTOR activity is altered by neuron-specific ID and contributes to hippocampal structural changes and cognitive deficits that persist following iron repletion.
Materials and Methods
Mice.
All experiments were performed in accordance with the NRC’s Guide for Care and Use of Laboratory Mice and with approval of the Institutional Animal Care and Use Committee of the University of Minnesota. Mice were housed in a 12-h-light/-dark cycle and consumed ad libitum water and standard nonpurified diet (Harlan Teklad 2018 Global 18% Protein Rodent Diet). Mice were killed by i.p. injection of Beuthanasia (10 mg/kg). Hippocampal tissues used for protein analysis were dissected and flash frozen. The DMT-1 CKO model was generated by crossing Slc11a2flox/flox mice (28) with CamKIIa-Cre mice as previously described (27). The resulting offspring positive for Cre are referred to as DMT-1 CKO and Cre negative littermates are referred to as wild type (WT). The tet-off, transgenic DN TfR-1 model was generated as previously described (11). Mice consumed the nonpurified diet ad libitum until weaning (P d 21), after which time they continued to consume that diet or were switched to the same diet but with 0.625mg doxycyline/kg (TD.01306, Harlan-Teklad). The doxycyline diet was identical to the standard diet in all other nutritional aspects.
Rapamycin administration.
A total of 6 mg/kg rapamycin or vehicle was delivered via i.p. injection every other day beginning at P d 10 and continuing through P d 42. Rapamycin was dissolved in 100% ethanol and diluted in 5% PEG and 5% Tween-80 as previously described (29).
Protein analysis.
Protein samples were prepared from individual dissected hippocampal hemispheres or CA1 region by sonication in cytoskeletal buffer. A total of 30 μg of total protein was separated using NuPAGE 4–12% or 12% Bis-Tris gels (Invitrogen) and transferred onto nitrocellulose membrane (Pierce) as previously described (6). Using Rockland Near-Fluorescence blocking solution and the following antibodies, the blots were imaged and analyzed with Odyssey infrared scanning (LiCor Bioscience) as previously described. Anti-β-actin antibody was generated in mice and purchased from Sigma and used at 1:10,000. The following other primary antibodies were generated in rabbit and obtained from Cell Signal Technology and used at 1:1000: anti-S6K(Thr389) no. 9205, anti-S6K no. 9202, anti-Akt(Ser473) no. 9271, anti-Akt(Thr308) no. 9275, anti-Akt no. 9272, anti-mTOR(Ser2448) no. 2971, anti-mTOR no. 2972, anti-AMPKα(Thr172) no. 2531, and anti-AMPKα no. 2532. Secondary antibodies included Alexa Fluor 680 conjugated anti-mouse (Invitrogen) and Infrared Dye 800 conjugated anti-rabbit antibody (Rockland) and were used at 1:12,500.
qPCR.
qPCR was performed as previously described (10). Briefly, mRNA was isolated from individual hippocampal hemispheres using an RNA isolation kit from Applied Biosystems. cDNA was synthesized using a kit from Applied Biosystems. qPCR was performed using MX3000P thermocycler (Stratagene) and TaqMan master mix and probes at one-half the volume of the manufacturer’s recommendation (Applied Biosystems).
Enabled Morris water maze.
An enabled Morris water maze (EMWM) was utilized as previously described with these genetic mutant strains (27). Males and females were tested separately. Briefly, on the first day, the mice were habituated to handling and swimming. On each of the next 5 d, the mice were given six 90-s training trials followed by a single, 30-s probe trial. During training trials, the platform location remained fixed in the center of a predetermined target quadrant. During the first 2 d of training (training trials 1–12), trials 1–4 used a 20-cm diameter platform, trials 5–8 used a 15-cm platform, and trials 9–12 used a 10-cm diameter platform. For the remaining 3 d of training, the first 2 daily trials used the 20-cm platform, followed by two 15-cm platform trials, and finally two 10-cm platform trials. During probe trials, the platform was removed from the water and the mouse was allowed to swim for 30 s before being removed by the experimenter. Video was captured and analyzed using Topscan (Clever Systems).
Data analysis.
Most data were analyzed by 2-factor ANOVA with post hoc Bonferroni comparisons. For developmental protein phosphorylation, the main effects compared were postnatal age and genotype (2 × 2). For EMWM performance, the main effects of training day and genotype/drug treatment were compared (5 × 3). To compare individual proteins at a single age, 1-factor ANOVA with Bonferroni post hoc tests or Student‘s t tests were used with 3 or 2 experimental groups respectively. Differences were considered significant at P < 0.05. When variances were unequal, nonparametric Kruskal-Wallis tests were used. For protein and PCR experiments, a minimum of 4 samples was used and a minimum of 9 for behavior unless noted in the legend.
Results
Neuronal ID increases S6K activity over time.
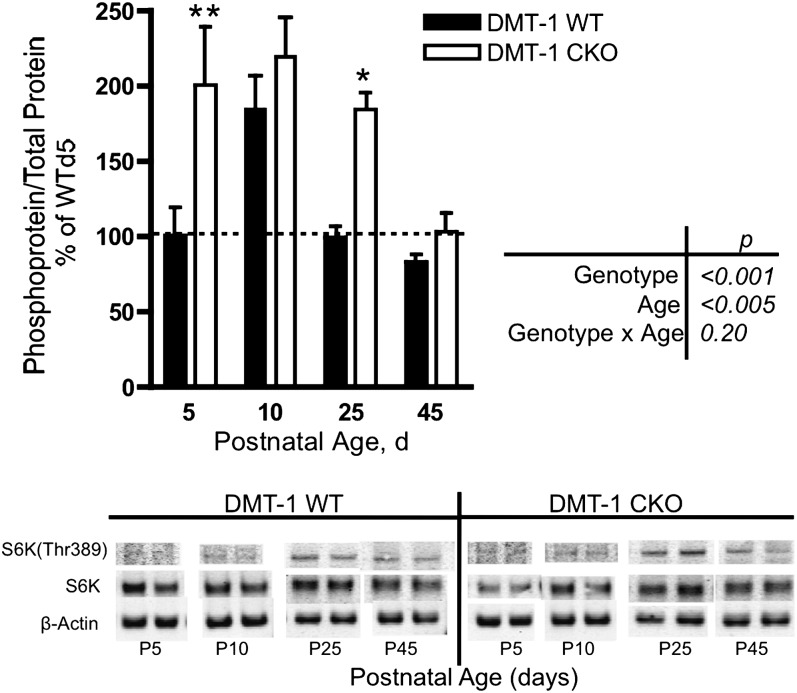
mTORC1 activity was measured by S6K(Thr389) phosphorylation, a direct mTORC1 target (30) (Fig. 1). In WT mice, S6K phosphorylation increased nearly 1-fold between P d 5 and 10 and decreased to P d 5 levels by P d 25 (Fig. 2), coincident with the increase in hippocampal metabolic activity that occurs during this period of rapid growth and differentiation. Hippocampal pyramidal cell ID generated through a timed (embryonic d 18.5) conditional Cre-loxP deletion of Slc11a2, the gene encoding DMT-1 (27), resulted in greater hippocampal S6K(Thr389) phosphorylation at P d 5 compared with WT. Increased activation of hippocampal mTORC1 output in the DMT-1 CKO mice was sustained through P d 25 and decreased to WT levels only at P d 45 (Fig. 2).
FIGURE 2.
S6K(Thr389) phosphorylation during postnatal development in DMT-1 WT and DMT-1 CKO mice. Representative Western blots are shown. Values are mean ± SEM, n = 2–5. Asterisks indicate different from WT: *P < 0.05, **P < 0.01. CKO, conditional knockout; P, postnatal; WT, wild type.
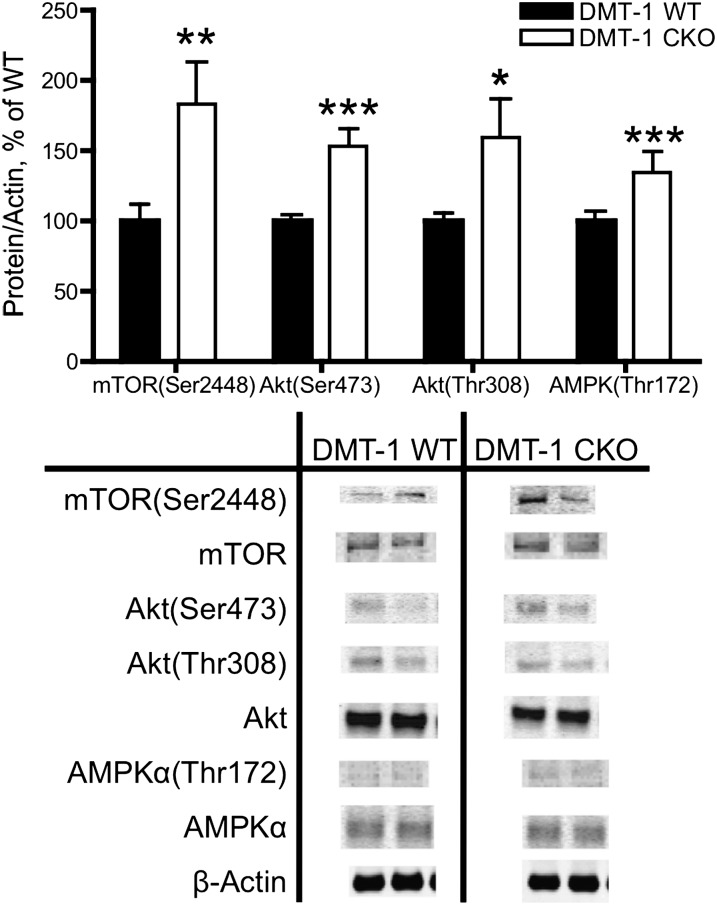
mTORC1 and mTORC2 activity are increased by neuronal ID at P d 25.
Upstream of S6K(Thr389), phosphorylation of central components of mTOR signaling were also increased by ID at P d 25 in the DMT-1 CKO. These included mTOR(Ser2448) phosphorylation and Akt(Ser473) phosphorylation, which reflect increased mTORC1 and mTORC2 activity, respectively (31) (Figs. 1 and 3).
FIGURE 3.
Activity of mTOR and upstream regulators is upregulated at P d 25 in DMT-1 CKO mice. mTORC1 [mTOR(Ser2448)], mTORC2 [Akt(Ser473)], Akt(Thr308), and AMPK(Thr172) phosphorylation is increased in DMT-1 CKO compared with WT. Representative Western blots are shown. Values are mean ± SEM, n = 4. Asterisks indicate different from WT: *P < 0.05, **P < 0.01, ***P < 0.001. CKO, conditional knockout; DMT-1 WT, mTOR, mammalian target of rapamycin; P, postnatal; WT, wild type.
Positive and negative mTOR regulatory signaling is stimulated by ID at P d 25.
Three potential iron-dependent inputs into the mTOR pathway were assessed: PI3K, HIF1α, and AMPK (Fig. 1). Signaling through the PI3K pathway, as indexed by phosphorylation of Akt(Thr308) (30), was greater in DMT-1 CKO mice than in WT mice (Fig. 3). At P d 25, relative mRNA expression of ddit4, a direct HIF1α target that inhibits mTOR signaling (32), was elevated by 54% (100 ± 9.8 vs. 154 ± 15.2; P < 0.01; n = 4–8). Phosphorylation of AMPK(Thr172) is increased by reduced ATP availability and acts to inhibit mTOR activity (33). Consistent with lower metabolic activity and reduced ATP availability in ID models (3, 27, 34, 35), DMT-1 CKO mice had higher levels of AMPK(Thr172) phosphorylation than WT mice at P d 25 (Fig. 3).
mTORC1 activity is normalized by iron repletion in the inducible DN TfR-1 model of hippocampal ID.
The transgenic DN TfR-1 model induces ID with the same timing (embryonic d 18.5), tissue specificity (hippocampal CA1 pyramidal cell neurons), and biochemical and behavioral phenotype as the DMT-1 CKO but can be reversed by doxycyline through a tet-off mechanism (11, 27). Similar to the whole hippocampal findings in the DMT-1 CKO mouse, S6K(Thr389), Akt(Thr473), and Akt(Thr308) phosphorylation was greater in hippocampal area CA1 of ID DN TfR-1 mice relative to WT at P d 21 (Fig. 4A). Doxycyline treatment beginning at P d 21 and subsequent iron repletion [which prevents long-term learning, memory, and structural deficits (11)] reduced S6K phosphorylation to WT levels at P d 30 and 42, the window of highest activation in the DMT-1 CKO model (Fig. 4B). Consistent with normalization of S6K, iron repletion also normalized phosphorylation of Akt(Ser473) and Akt(308) (Fig. 4C).
FIGURE 4.
Iron repletion reduces mTOR activity in the CA1 region of DN TfR-1 mice. (A) At P d 21, phosphorylation levels of several mTOR signaling proteins in CA1 of WT and DN TfR-1 mice. (B) S6K(Thr389) phosphorylation in area CA1 across development in iron deficient DN TfR-1, iron repleted DN TfR-1 relative to WT. (C) At P d 42, phosphorylation of signaling proteins in CA1 of iron-deficient DN TfR-1 and iron repleted DN TfR-1. Values are mean ± SEM, n = 4–9. Asterisks indicate different than WT: *P < 0.05, labeled means at a time without a common letter differ, P < 0.05. DN, dominant negative; P, postnatal; WT, wild type.
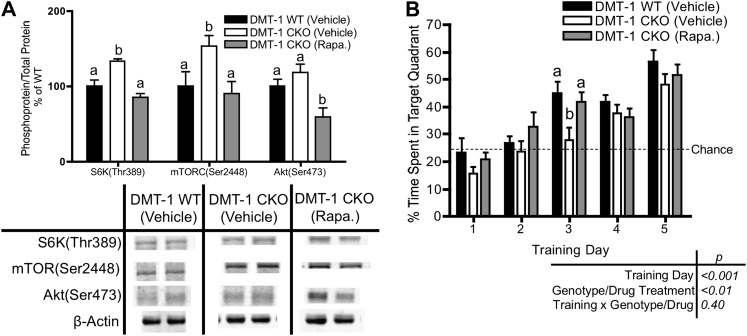
Rapamycin administration between P d 10 and 42 normalized mTOR activity at P d 25 and improved learning and memory behavior at 2–3 mo in DMT-1 CKO mice.
Rapamycin administration beginning at P d 10 resulted in normalization of S6K(Thr389), Akt(Ser473), and mTOR(Ser2448) phosphorylation at P d 25 in DMT-1 CKO mice compared with vehicle-treated DMT-1 CKO mice despite ongoing ID (Fig. 5A). We previously demonstrated that DMT-1 CKO mice are able to learn an EMWM task but have delayed acquisition compared with DMT-1 WT mice (27). Consistent with these observations, adult DMT-1 WT mice treated with vehicle between P d 10 and 42 mice spent >40% of time in the target quadrant during a probe trial after 3 d of training (Fig. 5B). Vehicle-treated DMT-1 CKO mice, however, required 4 d of training to perform better than chance (25%) levels (Fig. 5B). Despite ongoing ID, DMT-1 CKO mice treated with rapamycin between P d 10 and 42 performed similarly to vehicle-treated DMT-1 WT mice and spent significantly more time in the target quadrant than vehicle-treated DMT-1 CKO mice after 3 d of training (Fig. 5B).
FIGURE 5.
Rapamycin treatment normalizes mTOR activity and improves spatial learning in DMT-1 CKO mice. (A) Phosphorylation of mTOR components in hippocampus at P d 25, representative Western blots are shown. (B) Percentage time spent in the target quadrant during EMWM probe trials, dotted line represents chance performance (25% time spent in target quadrant). Values are mean ± SEM, n = 9–13. Labeled means at a time without a common letter differ, P < 0.05. CKO, conditional knockout; EMWM, enabled Morris water maze; mTOR, mammalian target of rapamycin; P, postnatal; Rapa, rapamycin; WT, wild type.
Discussion
Developmental activation of mTOR activity in the hippocampus occurs between P d 5 and 25 in WT mice and completely overlaps the period of peak hippocampal iron uptake, growth factor expression, and energy production (4–7, 35–39). This co-occurrence in developmental activation patterns is consistent with mTOR’s role in modulating rapid cellular growth and differentiation (18, 40). mTOR activity increases cellular energy production by modulating mitochondrial activity through retrograde signaling (41–43). Furthermore, cellular iron uptake via TfR-1 is modulated by mTOR activity-induced TfR-1 surface expression in HeLa cells (23). The simultaneous developmental activation of mTOR alongside growth factors, energy production, and iron uptake suggests that mTOR activity is a central integrator of metabolic signaling during rapid hippocampal differentiation.
We hypothesized that there are at least 3 direct points in the mTOR pathway through which iron is likely to affect signaling: 1) BDNF stimulation of Akt activity (44); 2) AMPK activity determined by ATP availability (45); and 3) HIF1α transcription of ddit4 (32). Iron, therefore, likely exerts a complex effect on mTOR signaling, which has multiple feedback and feed-forward loops (18).
Stimulation of mTOR activity by BCAA, growth factors, and oxygen (which are all substrates for cellular metabolism) is well established (18). Here, we demonstrate for the first time to our knowledge that iron is an additional important substrate for support of neuronal metabolism through this pathway. We previously showed that the DMT-1 CKO model has abnormal hippocampal dendrite structure, suggestive of disordered actin, and altered expression of synaptic plasticity genes (27). In the current study, neither neuronal soma size nor LCIII protein levels, an indicator of autophagy activity, were altered by ID in any hippocampal region at P d 25 (data not shown), suggesting that the major effect of mTOR dysregulation by neuronal ID is more likely to be on cytoskeletal structure and gene transcription.
Previous observations in non-neuronal cells and in hippocampal cells from anemic rats appear to be at odds with our current study in which nonanemic, hippocampal, neuron-specific ID increases mTOR activity. For example, iron chelation studies in mature cell lines such as COS and myeloid leukemia cells show that reduced intracellular iron availability inhibits overall mTOR activity (24, 25). Our group has also demonstrated downregulation of mTOR pathway genes and S6K phosphorylation in the hippocampus of P15 rats with severe dietary IDA, where tissue hypoxia is a prominent feature (12, 26). mTOR downregulation in severe IDA is not surprising, because the condition causes reduced BDNF and insulin-like growth factor concentrations and lower cytochrome c oxidase activity and HIF1α activation, which can all act to suppress mTOR activity by modulating activity of upstream regulators Akt, ddit4, and AMPK (6, 8, 12, 34). These would inhibit dendritogenesis and reduce electrophysiologic output (14, 46–49).
In humans and rodent models, dietary IDA not only renders the whole brain iron deficient, it also induces tissue hypoxia via anemia, which can influence mTOR signaling, increases uptake of other potentially toxic divalent metals (e.g., Mn, Pb), and induces a stress response. These multiple effects during IDA have made it difficult to distinguish the specific role of iron in neuronal development, structure, and signaling pathways, including mTOR. Whereas IDA commonly occurs during pregnancy and in toddlers, nonanemic ID is common in newborn infants, particularly those born prematurely and following common gestational complications such as diabetes mellitus and intrauterine growth restriction (50–53). Moreover, nonanemic ID is 3 times more common than IDA in toddlers and has important negative effects on motor, socio-emotional, and cognitive behaviors (54). Therefore, isolating the role of iron in neuronal development and function is clinically relevant. By using the DMT-1 CKO and reversible DN TfR-1 mouse models of hippocampal neuronal ID, we were able to begin examining the specific effects of iron on mTOR signaling in the hippocampus.
The observed upregulation of mTOR by neuronal ID in the current study presents the question of how tissue-level ID without anemia influences mTOR signaling. Iron is a necessary substrate for energy production and in iron-sufficient conditions, mTOR signaling facilitates energy production necessary for rapid developmental growth by increasing metabolic output, mitochondriogenesis, and stimulating iron uptake (22, 23, 42). In IDA, mTOR activity is likely suppressed by oxidative stress, increased HIF1α activity, and decreased energy production, which accompany anemia and total body ID (55, 56). Consistent with the role of iron-containing proteins in regulating HIF-1α and ATP production, neuron-specific ID increased ddit4 transcription and AMPK activation. However, in the absence of anemia and total body ID, neuronal ID alone was not sufficient to suppress the developmental peak in mTOR activity. ID cells are not able to adequately synthesize cytochromes and other iron-containing proteins necessary to support mTOR activity and thus meet the increased energy needs of the rapidly differentiating cell. This disruption of metabolic supply and demand created by the lack of iron substrate concurrent with exuberant mTOR activity, likely mediated by continued growth factor signaling through Akt, could impair mTOR-dependent neuronal differentiation and dendrite arborization. Together, these findings present a complex interrelationship between cell growth, metabolic demand, iron availability, and mTOR signaling during periods of rapid neuronal development.
Ultimately, overactivation of the mTOR signaling pathway may be as detrimental to the developing nervous system as hypo-activity observed in dietary IDA studies. Genetic and pharmacologic manipulations of mTOR in mice demonstrate the importance of optimal hippocampal neuronal mTOR signaling for morphology, electrophysiology, and spatial learning (48, 57, 58). Overactivity of mTOR in some of these models causes abnormal dendrite morphology and abnormal learning and memory behavior. The structural and behavioral phenotypes match those of the DMT-1 CKO and DN TfR-1 mice (11, 27). This is not surprising, because mTOR interacts with genes that mediate synaptic plasticity (e.g., bdnf) and structural guidance/actin polymerization (e.g., profilins and cofilin).
Normalization of mTOR activity following iron repletion in the DN TfR-1 model and through rapamycin treatment in the DMT-1 CKO model, coincident with prevention of long-term spatial learning deficits in adulthood, highlights the interaction between iron availability, developmental mTOR regulation, and lifelong hippocampal function. In other non-ID mouse models characterized by excessive mTOR activity, structural abnormalities, and behavioral deficits, rapamycin administrations normalizes these outcome variables in a similar manner seen in the current study (29, 48, 58, 59).
Together, these findings suggest mTOR dysregulation as a cellular mechanism underlying the acute and persistent neurodevelopmental deficits that accompany early-life ID. Our studies support the hypothesis that maintaining optimal mTOR activity is critical for normal hippocampal growth and differentiation and that iron is a necessary substrate for this process. Although the observed alterations in phosphorylation of key mTOR signaling proteins suggest that ID disrupts mTOR homeostasis, further, more mechanistic studies will be necessary to elucidate how iron contributes to regulation of the mTOR pathway.
Acknowledgments
S.J.B.F. designed and conducted research, analyzed data, and wrote the manuscript; E.S.C. contributed essential materials and assisted in research design; and M.K.G. wrote the manuscript and had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: DMT-1, divalent metal transporter-1; DMT-1 CKO, divalent metal transporter-1 conditional knockout; DN TfR-1, dominant negative transferrin receptor-1; EMWM, enabled Morris water maze; ID, iron deficiency; IDA, iron deficiency anemia; mTOR, mammalian target of rapamycin; P, postnatal; WT, wild type.
Literature Cited
- 1.Pokorný J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. II. Development of ultrastructure in stratum lacunosum and moleculare. Brain Res Bull. 1981;7:121–30 [DOI] [PubMed] [Google Scholar]
- 2.Pokorný J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. I. Development of dendritic arborisation in pyramidal neurons. Brain Res Bull. 1981;7:113–20 [DOI] [PubMed] [Google Scholar]
- 3.Dallman PR. Biochemical basis for the manifestations of iron deficiency. Annu Rev Nutr. 1986;6:13–40 [DOI] [PubMed] [Google Scholar]
- 4.Siddappa AJ, Rao RB, Wobken JD, Leibold EA, Connor JR, Georgieff MK. Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J Neurosci Res. 2002;68:761–75 [DOI] [PubMed] [Google Scholar]
- 5.Taylor EM, Morgan EH. Developmental changes in transferrin and iron uptake by the brain in the rat. Brain Res Dev Brain Res. 1990;55:35–42 [DOI] [PubMed] [Google Scholar]
- 6.Tran PV, Carlson ES, Fretham SJ, Georgieff MK. Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J Nutr. 2008;138:2495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erecinska M, Cherian S, Silver IA. Energy metabolism in mammalian brain during development. Prog Neurobiol. 2004;73:397–445 [DOI] [PubMed] [Google Scholar]
- 8.Siddiq A, Aminova LR, Ratan RR. Prolyl 4-hydroxylase activity-responsive transcription factors: from hydroxylation to gene expression and neuroprotection. Front Biosci. 2008;13:2875–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maguire JJ, Davies KJ, Dallman PR, Packer L. Effects of dietary iron deficiency of iron-sulfur proteins and bioenergetic functions of skeletal muscle mitochondria. Biochim Biophys Acta. 1982;679:210–20 [DOI] [PubMed] [Google Scholar]
- 10.Tran PV, Fretham SJ, Carlson ES, Georgieff MK. Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr Res. 2009;65:493–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fretham SJ, Carlson ES, Wobken J, Tran PV, Petryk A, Georgieff MK. Temporal manipulation of transferrin-receptor-1-dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment. Hippocampus. 2012;22:1691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran PV, Fretham SJ, Wobken J, Miller BS, Georgieff MK. Gestational-neonatal iron deficiency suppresses and iron treatment reactivates IGF signaling in developing rat hippocampus. Am J Physiol Endocrinol Metab. 2012;302:E316–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanpied TA, Scott DB, Ehlers MD. Age-related regulation of dendritic endocytosis associated with altered clathrin dynamics. Neurobiol Aging. 2003;24:1095–104 [DOI] [PubMed] [Google Scholar]
- 14.Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;25:412–20 [DOI] [PubMed] [Google Scholar]
- 15.Pokorný J, Trojan S. The development of hippocampal structure and how it is influenced by hypoxia. Acta Univ Carol Med Monogr. 1986;113:1–79 [PubMed] [Google Scholar]
- 16.Brunette KE, Tran PV, Wobken JD, Carlson ES, Georgieff MK. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev Neurosci. 2010;32:238–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieberthal W, Levine JS. Mammalian target of rapamycin and the kidney. I. The signaling pathway. Am J Physiol Renal Physiol. 2012;303:F1–10 [DOI] [PubMed] [Google Scholar]
- 18.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84 [DOI] [PubMed] [Google Scholar]
- 19.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–8 [DOI] [PubMed] [Google Scholar]
- 20.Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–40 [DOI] [PubMed] [Google Scholar]
- 23.Galvez T, Teruel MN, Heo WD, Jones JT, Kim ML, Liou J, Myers JW, Meyer T. siRNA screen of the human signaling proteome identifies the PtdIns(3,4,5)P3-mTOR signaling pathway as a primary regulator of transferrin uptake. Genome Biol. 2007;8:R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ndong M, Kazami M, Suzuki T, Uehara M, Katsumata S, Inoue H, Kobayashi K, Tadokoro T, Suzuki K, Yamamoto Y. Iron deficiency down-regulates the Akt/TSC1–TSC2/mammalian Target of Rapamycin signaling pathway in rats and in COS-1 cells. Nutr Res. 2009;29:640–7 [DOI] [PubMed] [Google Scholar]
- 25.Ohyashiki JH, Kobayashi C, Hamamura R, Okabe S, Tauchi T, Ohyashiki K. The oral iron chelator deferasirox represses signaling through the mTOR in myeloid leukemia cells by enhancing expression of REDD1. Cancer Sci. 2009;100:970–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus. 2007;17:679–91 [DOI] [PubMed] [Google Scholar]
- 27.Carlson ES, Tkac I, Magid R, O'Connor MB, Andrews NC, Schallert T, Gunshin H, Georgieff MK, Petryk A. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr. 2009;139:672–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–8 [DOI] [PubMed] [Google Scholar]
- 31.Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle. 2011;10:2305–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest. 2007;117:1926–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Deungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–76 [DOI] [PubMed] [Google Scholar]
- 35.Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–21 [DOI] [PubMed] [Google Scholar]
- 36.Fretham SJ, Carlson ES, Georgieff MK. The role of iron in learning and memory. Adv Nutr. 2011;2:112–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dallman PR, Spirito RA. Brain iron in the rat: extremely slow turnover in normal rats may explain long-lasting effects of early iron deficiency. J Nutr. 1977;107:1075–81 [DOI] [PubMed] [Google Scholar]
- 38.Moos T, Morgan EH. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol Neurobiol. 2000;20:77–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dallman PR, Schwartz HC, Ctyochrome C. Concentrations during rat and guinea pig development. Pediatrics. 1964;33:106–10 [PubMed] [Google Scholar]
- 40.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–93 [DOI] [PubMed] [Google Scholar]
- 41.Schieke SM, Phillips D, McCoy JP, Jr, Aponte AM, Shen RF, Balaban RS, Finkel T. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281:27643–52 [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Butow RA. Mitochondrial retrograde signaling. Annu Rev Genet. 2006;40:159–85 [DOI] [PubMed] [Google Scholar]
- 43.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75 [DOI] [PubMed] [Google Scholar]
- 44.Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–80 [DOI] [PubMed] [Google Scholar]
- 45.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–85 [DOI] [PubMed] [Google Scholar]
- 46.Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. Control of dendritic arborization by the phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci. 2005;25:11300–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci. 2005;25:11288–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jorgenson LA, Sun M, O'Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–102 [DOI] [PubMed] [Google Scholar]
- 50.Chockalingam UM, Murphy E, Ophoven JC, Weisdorf SA, Georgieff MK. Cord transferrin and ferritin values in newborn infants at risk for prenatal uteroplacental insufficiency and chronic hypoxia. J Pediatr. 1987;111:283–6 [DOI] [PubMed] [Google Scholar]
- 51.Georgieff MK, Landon MB, Mills MM, Hedlund BE, Faassen AE, Schmidt RL, Ophoven JJ, Widness JA. Abnormal iron distribution in infants of diabetic mothers: spectrum and maternal antecedents. J Pediatr. 1990;117:455–61 [DOI] [PubMed] [Google Scholar]
- 52.Georgieff MK. Mills MM, Gordon K, Wobken JD. Reduced neonatal liver iron concentrations after uteroplacental insufficiency. J Pediatr. 1995;127:308–4 [DOI] [PubMed] [Google Scholar]
- 53.Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J Pediatr. 1992;121:109–14 [DOI] [PubMed] [Google Scholar]
- 54.Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobson SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr. 2008;152:696–702, 31–3. [DOI] [PMC free article] [PubMed]
- 55.Lee DW, Andersen JK. Role of HIF-1 in iron regulation: potential therapeutic strategy for neurodegenerative disorders. Curr Mol Med. 2006;6:883–93 [DOI] [PubMed] [Google Scholar]
- 56.Bianchi L, Tacchini L, Cairo G. HIF-1-mediated activation of transferrin receptor gene transcription by iron chelation. Nucleic Acids Res. 1999;27:4223–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knox S, Ge H, Dimitroff BD, Ren Y, Howe KA, Arsham AM, Easterday MC, Neufeld TP, O'Connor MB, Selleck SB. Mechanisms of TSC-mediated control of synapse assembly and axon guidance. PLoS ONE. 2007;2:e375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeng Z, Sarbassov dos D, Samudio IJ, Yee KW, Munsell MF, Ellen Jackson C, Giles FJ, Sabatini DM, Andreeff M, Konopleva M. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–12 [DOI] [PMC free article] [PubMed] [Google Scholar]