Abstract
The (n-3) PUFAs 20:5 (n-3) (EPA) and 22:6 (n-3) (DHA) are thought to benefit human health. The presence of prooxidant compounds in foods, however, renders them susceptible to oxidation during both storage and digestion. The development of oxidation products during digestion and the potential effects on intestinal PUFA uptake are incompletely understood. In the present studies, we examined: 1) the development and bioaccessibility of lipid oxidation products in the gastrointestinal lumen during active digestion of fatty fish using the in vitro digestive tract TNO Intestinal Model-1 (TIM-1); 2) the mucosal cell uptake and metabolism of oxidized compared with unoxidized PUFAs using Caco-2 intestinal cells; and 3) the potential to limit the development of oxidation products in the intestine by incorporating antioxidant polyphenols in food. We found that during digestion, the development of oxidation products occurs in the stomach compartment, and increased amounts of oxidation products became bioaccessible in the jejunal and ileal compartments. Inclusion of a polyphenol-rich grape seed extract (GSE) during the digestion decreased the amounts of oxidation products in the stomach compartment and intestinal dialysates (P < 0.05). In Caco-2 intestinal cells, the uptake of oxidized (n-3) PUFAs was ~10% of the uptake of unoxidized PUFAs (P < 0.05) and addition of GSE or epigallocatechin gallate protected against the development of oxidation products, resulting in increased uptake of PUFAs (P < 0.05). These results suggest that addition of polyphenols during active digestion can limit the development of (n-3) PUFA oxidation products in the small intestine lumen and thereby promote intestinal uptake of the beneficial, unoxidized, (n-3) PUFAs.
Introduction
The (n-3) PUFAs, especially EPA and DHA, have potential benefits for human health (1). However, the presence of compounds such as heme pigments and various trace metals in PUFA-rich foods, particularly fish muscle, leads to a high susceptibility to the development of lipid oxidation products, which are thought to be deleterious to human health. Malondialdehyde has genotoxic properties, inducing DNA damage and mutations (2), and lipid hydroperoxides may promote cell apoptosis (3). In rats, consumption of food with oxidized lipids was shown to increase the concentrations of lipid hydroperoxides in blood (4) and malondialdehyde in serum (5). Oxidized lipid intake has also been suggested to contribute to the development of tumors and atherosclerosis (6, 7).
Lipid hydroperoxides resulting from oxidation of fatty acids (FAs)6 have been suggested to be formed during digestion. The stomach serves as a temporary storage site for newly ingested food and it is possible that the stomach can increase the susceptibility of (n-3) PUFAs to oxidation, thus yielding higher concentrations of peroxides than those present in the ingested food (8, 9). Several studies have observed lipid oxidation using simple in vitro systems with conditions similar to those found in the stomach, where PUFAs may interact with prooxidant compounds present in the food (10). Further, the acid pH of the stomach may increase the prooxidant activity of hemoproteins (8), and saliva secreted from the parotid gland could play a dual role in oxidation in the stomach, increasing lipid peroxidation via the pseudoperoxidase activity of lactoperoxidase but also reducing lipid peroxidation via thiocyanate and nitrate activity (11).
Fish muscle contains prooxidants, such as hemoproteins and low-molecular weight iron (12). Hemoglobin in fish has been shown to be more active in promoting oxidation under acidic conditions, where the release of heme groups by gastric proteolytic activity could act as a catalyst of lipid oxidation as well (13). At present, likely owing to the complexity of ingestion of (n-3) PUFA-rich foods with other foods containing variable pro- and antioxidant compounds, the molecular species of hydroperoxides formed during digestion are not well defined (9). Moreover, there is likely overlap between the oxidation products formed during digestion and those formed during food storage; e.g., in fish, the lipoxygenases, hemoproteins, and PUFA substrates are present, albeit at different ambient conditions, in food storage and during digestion. It is also important to note that although food that develops high amounts of oxidation products during storage may be discarded due to poor odor, common storage conditions may lead to the development of “incipient rancid” odors that can be masked by food flavors (14, 15). Moreover, the further development of oxidation products within the gastrointestinal tract would not be detected.
The use of antioxidants is a common strategy to avoid lipid oxidation. Several studies have shown the inhibition of lipid oxidation in the stomach by wine polyphenols (10, 11, 16), which appear to arrive intact to the small intestine, because they are resistant to gastric acid hydrolysis (17). The catechins, particularly epigallocatechin gallate (EGCG), are not readily absorbed, thereby resulting in high catechin concentrations in the intestinal lumen (18, 19). Thus, antioxidant compounds may act by preventing the formation of lipid oxidation products during digestion and therefore inhibit their damaging effects. No previous studies to our knowledge have examined the gastrointestinal development of lipid oxidation products in PUFA-rich food by using a complete model of the stomach and upper small intestine such as the TNO Intestinal Model-1 (TIM-1) system.
To further understand the development and assimilation of oxidized FAs during gastrointestinal processing, in the present study we examined: 1) the development of lipid oxidation products in the gastrointestinal lumen during active digestion of fatty fish muscle; 2) the bioaccessibility, mucosal cell uptake, and mucosal cell metabolism of oxidized compared with unoxidized PUFAs; and 3) the potential to limit the development of oxidation products at the level of the intestine by incorporating antioxidant polyphenols in food. In particular, a previously characterized grape seed extract (GSE) (20) was examined using the TIM-1 model of the human upper gastrointestinal tract and the Caco-2 human intestinal cell line.
Materials and Methods
Chemicals.
α-Amylase, pepsin, bile, BSA (essentially FA free), DMEM, trypsin, lipoxidase from glycine max (soybean), EGCG, EDTA, and Triton-X100 were obtained from Sigma-Aldrich. Pancreatic juice from porcine pancreas (Pancreax V powder) was obtained from Paines and Byrne. Rhizopus lipase (150,000 units/mg) was obtained from Amano Enzyme. Cell culture media and reagents were obtained from GIBCO (Invitrogen). Unlabeled palmitic acid (PA) and DHA were from Nu Check-Prep. Labeled PA and DHA were from NEN. Sodium taurocholate was from Calbiochem and organic solvents were obtained from Fisher Scientific.
Grape proanthocyanidins.
A grape fraction rich in oligomeric catechins (proanthocyanidins) was prepared by fractionation of grape pomace as previously detailed in Torres et al. (21). A set of fractions differing in sizes of monomers and oligomers was obtained and each was characterized for mean molecular weight, mean polymerization degree, and percentage of galloylation (presence of esters with galloyl moieties) by depolymerization with cysteamine (22). An individual fraction having a polymerization degree of 2.4 units and galloylation percentage of 25% was selected for these experiments, because it showed the highest antioxidant effectiveness (12). The polyphenol concentration of this fraction, determined using the Folin Ciocalteau method (23), was 0.717 ± 0.001 mg catechin/mg extract. The major components, expressed in molar percentage, were catechin (29.6%), epicatechin (29.1%), EGCG (23.4%), and procyanidin B2 (7.38%) (20, 21).
Cell culture.
To model the uptake of dietary (n-3) PUFAs across the apical (AP) plasma membrane of the enterocyte, we used a Caco-2 human intestinal cell model grown in culture dishes, with compounds added to the media bathing the cells. As we previously reported, many indexes of Caco-2 cell differentiation, including tight junction formation, sucrose-isomaltase activity, etc., are equivalent for cells grown on plastic and cells grown on filters; for cells grown on plastic, tight junction formation is observed by the presence of domes (24, 25). Caco-2 cell cultures at passage 36–50 were grown using identical conditions to those previously described (26). For experiments, 4 × 104 cells were seeded per well in 6-well plates and used between 14 and 18 d postconfluence.
Preparation of radiolabeled FA uptake media.
16:0 PA or 22:6 (n-3) DHA at a final concentration of 30 μmol/L and 45.8 ppm antioxidants were dried under N2. EGCG at 45.8 ppm is equivalent to 100 μmol/L; 45.8 ppm GSE provides 47 μmol/L catechin, 46 μmol/L epicatechin, 23 μmol/L EGCG, and 6 μmol/L of procyanidin B2. Labeled FA was 5% of the total and the specific activities were 54 mCi/mmol. Dried lipids were then dissolved in ethanol (0.5% volume of the final volume of the solution) and subsequently dispersed in 10 mmol/L taurocholate (27, 28) in PBS (137 mmol/L NaCl, 2.7 mmol/L KCl, 1.5 mmol/L KH2PO4, 8 mmol/L NaHPO4, pH 7.4) and incubated for 1 h at 37°C with shaking, as previously described (29).
FA uptake assay.
Net uptake of FA from a taurocholate-FA mixture was analyzed as previously described (30). At 2 and 6 h of incubation, cells were washed and collected as described (26). Aliquots were removed and solubilized in scintillation cocktail. FA uptake was determined from the specific activity of the incubation medium. Protein was determined by the method of Lowry et al. (31).
Metabolism of FAs in Caco-2 cells.
Total cells lipids were extracted from the sonicated cell preparations using the method of Bligh and Dyer (32). The incorporation of radiolabeled PA and DHA into lipid metabolites was analyzed using TLC separation as previously described (24). The radioactivity in each lipid class was measured using a Storm 840 phosphorimager (Amersham Biosciences). The partitioning of radiolabeled lipid into individual lipid species (e.g., TG, phospholipid, cholesterol ester, etc.) is expressed as a percentage of total label incorporated (25).
Measurement of conjugated dienes.
Conjugated dienes (CDs) were measured in the lipid extracts and DHA solutions to follow the development of lipid oxidation (33). Results are expressed as millimoles linoleic acid hydroperoxides per gram of fat in order to normalize the amounts of lipid oxidation using the same units, as described by Kim and Labella (33).
Oxidation of DHA.
A stock solution of labeled and unlabeled DHA (5 and 95%, respectively) was prepared in absolute ethanol and diluted in PBS. DHA (22:6; 50 μmol/L) was oxidized with soybean lipoxidase (30–120 U/100 nmol of FA, 3 h at 37°C) to produce oxidized DHA. The formation of CDs was spectrophotometrically monitored using PBS as the reference. Under these conditions, conversion of the DHA into oxidized lipid was observed as an increase in absorbance at 234 nm (34). Conversion was reached (63.5 ± 6.3%), expressed as μmol/L of hydroperoxide of linoleic acid. Lipid extraction (32) was used to separate the FAs from the lipoxidase. The use of lipoxygenase is physiologically relevant, because lipoxygenases are present in fish muscle and are an important catalyst of oxidation during storage at low temperatures; they act on fish, producing a range of hydroperoxides derived from DHA and other PUFAs (35).
Chilled minced mullet.
Fish were purchased in a local market, deboned, eviscerated, and white muscle with no skin was separated. The muscle was chopped with a blender to obtain ground fish muscle. The GSE was added as a powder to obtain 200 ppm in the fish muscle, unless otherwise indicated. A total of 200 ppm GSE provides 204 μmol/L catechin, 200 μmol/L epicatechin, 102 μmol/L EGCG, and 26 μmol/L procyanidin B2. Control samples were prepared identically but with no addition of antioxidants.
Lipid extraction.
Lipids were extracted from the fish muscle, stomach samples, and small intestinal dialysates (32). Lipid content was gravimetrically determined and expressed on a wet weight basis (36).
TNO in vitro model.
The multicompartmental, dynamic, computer-controlled system developed for TNO Nutrition and Food Research was described in detail by Minekus et al. (37). The TIM-1 model comprises 4 compartments that represent the stomach, duodenum, jejunum, and ileum, and the pH, temperature, peristaltic mixing and transit, salivary, gastric, biliary, and pancreatic secretions are simulated according to physiological data to reflect gastrointestinal conditions of human adults after the intake of a semisolid meal (38). The half-time of stomach emptying was 70 min. Samples were collected each 30 min during 2.5 h from the stomach compartment and at 1-h intervals for 5 h from the dialysates of the jejunal and ileal compartments, which represent the bioaccessible digested products. For the experiments, 100 g minced muscle was mixed with artificial saliva that contained 9600 units amylase, 30 mL citrate buffer (pH 5.5), and 100 mL electrolyte solution (5 g/L NaCl, 0.6 g/L of KCl, and 0.3 g/L CaCl2). Mili Q water was added up to a final volume of 300 mL. This mixture was introduced into the compartment that represents the stomach and the digestion was begun. All samples were stored at –20°C until analysis.
Statistical analysis.
The data were analyzed by 1-way ANOVA and a Scheffé post hoc test was employed to compare different means. Statistical analyses were performed using Statistica 6.0 (StatSoft). Data are reported as mean ± SD, with P ≤ 0.05 considered significant.
Results
PUFA digestion and bioaccessibility of lipid oxidation byproducts in intestinal dialysates.
Prior to TIM-1 digestion, aliquots of each sample type were taken to determine total fat and CD concentrations. The percentage of fat obtained in mullet muscle was 6.78 ± 0.32%. The percent of (n-3) PUFA of this species ranges between 8 and 17%, with DHA and EPA as the main (n-3) PUFAs (39). The CD concentrations in fish stored for 3 d at 4°C without GSE (17.8 ± 2.2 μmol linoleic hydroperoxides/g fat) were greater than those in the fresh mullet (11.8 ± 1.3 μmol linoleic hydroperoxides/g fat) (P < 0.05), whereas the concentration in fish stored in the presence of GSE (12.2 ± 1.0 μmol linoleic hydroperoxides/g fat) did not differ from that in fresh mullet. These results indicate that fish muscle undergoes lipid oxidation during refrigerated storage and that the addition of GSE effectively inhibited the onset of the lipid degradation reactions.
It is important to note that the amount of hydroperoxides that we found in the fish muscle samples following 3 d of refrigerated 4°C storage (11.8 ± 1.3 μmol linoleic hydroperoxide/g fat) is equivalent to a level of off-flavors classified as “incipient rancid” (14, 15). Such subthreshold rancidity is commonly present in many commercial, frozen fish products as well as in fatty food products stored for long periods of time or at insufficiently low temperatures (13). These incipient rancid off-flavors, derived from the presence of hydroperoxides, can be masked by the use of flavorings or by the normal odor of food ingredients; thus, the fish samples used in the current study present a reasonable approximation of what might be consumed in a meal.
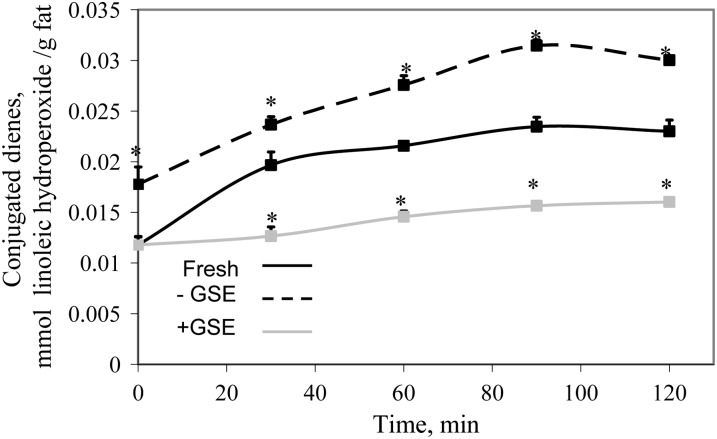
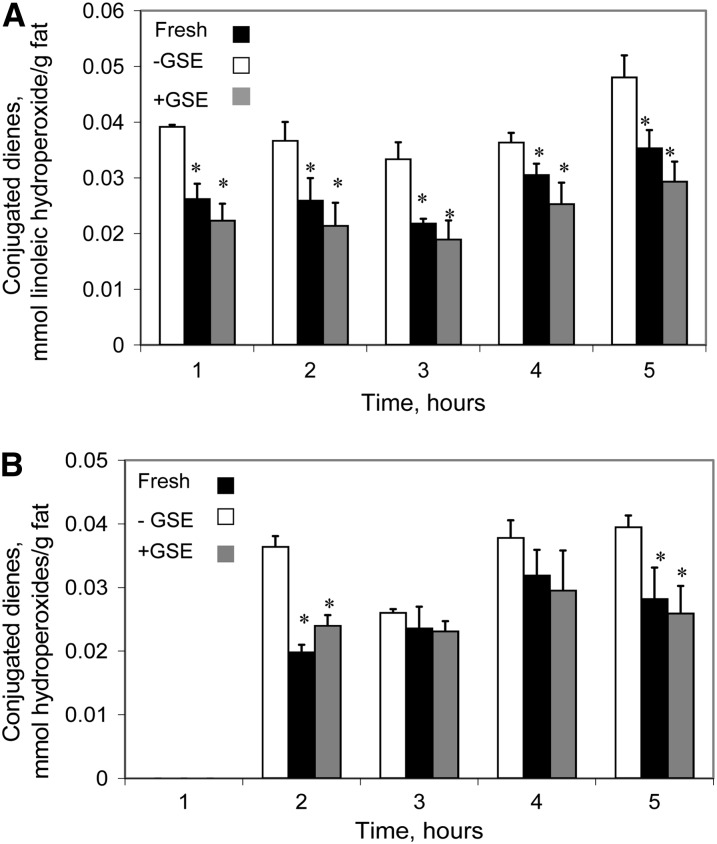
The kinetics of fish muscle lipid oxidation were followed in the TIM-1 stomach luminal compartment and in the jejunal and ileal dialysates, which represent the bioaccessible products of digestion (40). Figure 1 shows that CD concentrations, indicative of primary lipid oxidation degradation products, increased in the fresh fish and in the fish stored for 3 d at 4°C, both without antioxidant added, during digestion in the stomach, whereas the concentration of primary lipid oxidation products in samples stored with GSE was significantly lower than in fresh fish at all sampling times (P < 0.05). These results indicate that the fish muscle undergoes lipid oxidation during refrigerated storage and that the addition of GSE to the fish effectively inhibited the onset of lipid oxidation reactions. It is noteworthy that the increase in hydroperoxide during digestion was essentially parallel for the 2 fish samples digested in the absence of GSE, despite their different initial hydroperoxide concentrations. In the jejunal (Fig. 2A) and ileal (Fig. 2B) dialysates, the concentration of CD from fish stored without GSE was significantly higher than that of the other 2 groups. Fresh fish and fish stored with GSE did not differ, despite the higher amounts of lipid oxidation products found in the stomach compartment with the fresh fish. Experiments using 1000 ppm of GSE (which provides 1.02 mmol/L catechin, 1.00 mmol/L epicatechin, 0.51 mmol/L EGCG, and 0.13 mmol/L procyanidin B2) showed that the concentration of lipid hydroperoxides in the jejunal and ileal dialysates was lower than in fresh, unstored mullet at 3, 4, and 5 h (data not shown), indicating that higher antioxidant concentrations are also able to inhibit oxidation in jejunal and ileal compartments as well as in the stomach.
FIGURE 1.
Concentrations of CD hydroperoxides in fresh fish and fish stored for 3 d at 4°C in the absence (-) or presence (+) of GSE over time in the TIM-1 stomach compartment. Results are mean ± SD, n = 3. *Different from fresh fish at that time, P < 0.05. CD, conjugated diene; GSE, grape seed extract; TIM-1, TNO Intestinal Model-1.
FIGURE 2.
Concentrations of CD hydroperoxides in fresh fish and fish stored for 3 d at 4°C in the absence (-) or presence (+) of GSE over time in the TIM-1 jejunal (A) and ileal (B) dialysates. Results are mean ± SD, n = 3. *Different from fish stored without antioxidant, P < 0.05. CD, conjugated diene; GSE, grape seed extract; TIM-1, TNO Intestinal Model-1.
Assimilation and metabolism of FAs by Caco-2 cells.
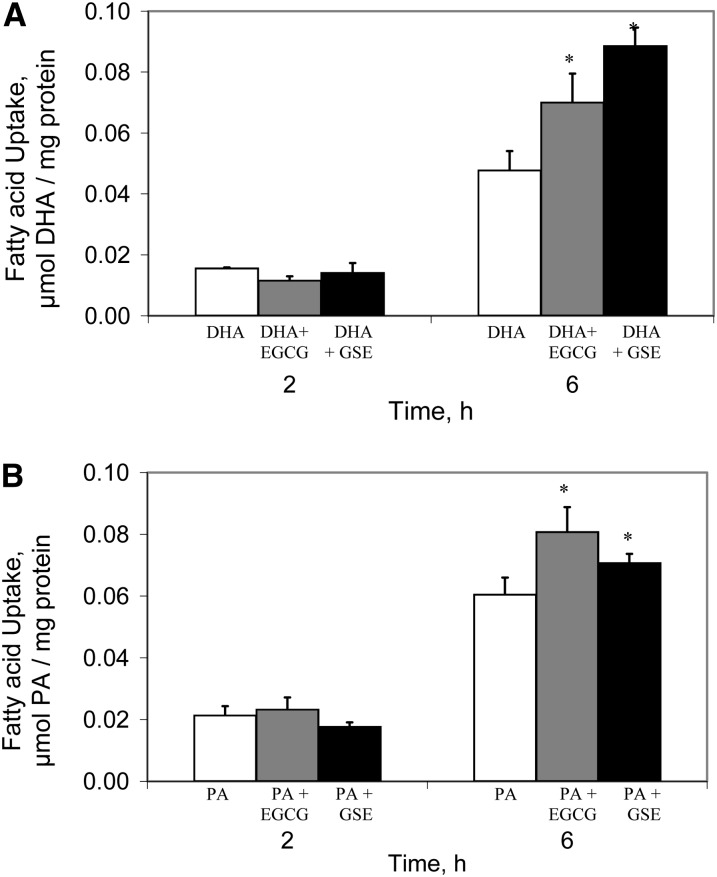
We measured the uptake of DHA or PA in the presence or absence of polyphenolic antioxidants (GSE and EGCG) using conditions that mimic the physiological presentation of FAs in the intestinal lumen (24, 41). Figure 3A shows that after 2 h, there were no effects of either GSE or EGCG on the DHA uptake. However, after 6 h of DHA incubation, DHA uptake increased almost 50% by co-incubation with EGCG and almost 2-fold by co-incubation with GSE. Although similar results were obtained for 16:0 uptake, the effects were considerably less: only ∼25% for EGCG and 15% for GSE (Fig. 3B).
FIGURE 3.
Net uptake of FAs in Caco-2 cells incubated with DHA (A) or PA (B) alone (30 μmol/L) or with antioxidants for 2 or 6 h (100 μmol/L EGCG or 45.8 ppm GSE that contained 47 μmol/L catechin, 46 μmol/L epicatechin, 23 μmol/L EGCG, and 6 μmol/L of procyanidin B2). Results are mean ± SD, n = 3. *Different from FA alone at that time, P < 0.05. EGCG, epigallocatechin gallate; FA, fatty acid; GSE, grape seed extract; PA, palmitic acid.
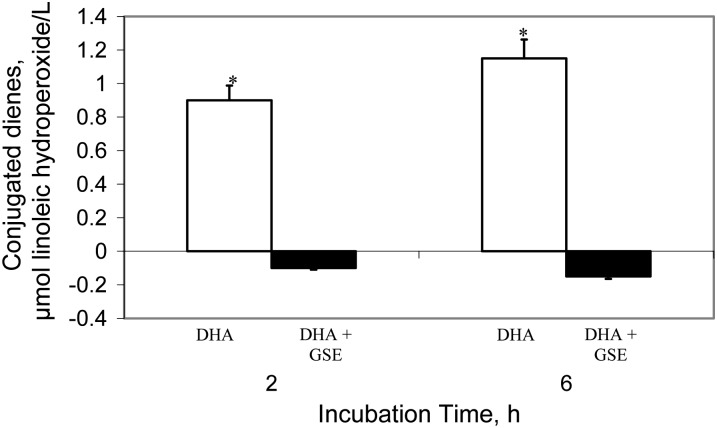
The possibility that the FA became oxidized, at least in part during Caco-2 cell incubation in samples with no addition of polyphenol, was tested using DHA. Under identical incubation conditions to those used with the cells, there was an increase (P < 0.05) in the concentrations of lipid hydroperoxides, measured as CD, in samples of DHA incubated in the absence of polyphenols (Fig. 4). In contrast, no increase in oxidation products was detected in the medium when DHA was incubated in the presence of GSE extract. Thus, DHA oxidation occurs during the Caco-2 incubation period and this can be successfully inhibited by GSE.
FIGURE 4.
Formation of CD hydroperoxides in the incubation medium of Caco-2 Cells with DHA (30 μmol/L) and GSE (45.8 ppm that contained 47 μmol/L catechin, 46 μmol/L epicatechin, 23 μmol/L EGCG, and 6 μmol/L procyanidin B2) for 2 or 6 h. Results are mean ± SD, n = 3. *Different from DHA+GSE at that time, P < 0.05. CD, conjugated diene; EGCG, epigallocatechin gallate; GSE, grape seed extract.
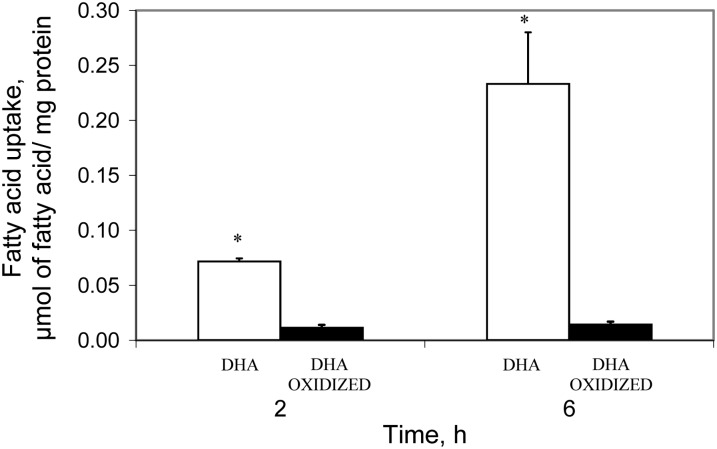
We then compared the uptake of oxidized DHA with unoxidized DHA in the Caco-2 cell model. Remarkably, as shown in Figure 5, the amount of oxidized DHA taken up by the cells was considerably lower than the uptake of unoxidized DHA.
FIGURE 5.
Net uptake of DHA and oxidized DHA by Caco-2 cells incubated with 30 μmol/L FA for 2 or 6 h. Results are mean ± SD, n = 3. *Different from oxidized DHA, P < 0.05. FA, fatty acid.
The metabolic fate of FAs added to Caco-2 cells was also analyzed to determine whether the presence of the antioxidants might modify the partitioning of DHA or PA into the different lipid classes. No significant differences were found in the percentage of any metabolite for either FA with either of the antioxidants (Supplemental Tables 1 and 2). Thus, although differences in the absolute amounts of FA taken up into Caco-2 cells were found, the subsequent metabolic fate of the FAs was not affected.
Discussion
Although it is generally thought that PUFA intake confers health benefits, the ingestion of increased amounts of oxidizable substrates, such as long-chain (n-3) PUFAs, has been related to increased production of several markers of lipid peroxidation (43, 44). Increased amounts of oxidizable substrate could lead to increased utilization of endogenous defense antioxidants and consequently an in vivo oxidative stress as well (44). Accordingly, there has been concern that marine (n-3) PUFAs may increase lipid peroxidation, causing a consumption of endogenous antioxidants and provoking an oxidative unbalance. On the other hand, others have not found an increase, or have even reported a decrease, in lipid oxidation markers, like isoprostanes, after dietary fish oil interventions (45). Additionally, fish oil supplementation has been suggested to protect against oxidative stress-induced DNA damage (46). Thus, although the results in vivo are somewhat inconclusive, it is certainly agreed that long-chain PUFAs present in fish muscle and fish oils are highly susceptible to oxidation. The present results with GSE or EGCG would suggest that these exogenous antioxidants would in fact mitigate against increased oxidative markers that might occur in their absence subsequent to (n-3) PUFA ingestion.
We first examined the bioaccesibility of lipid oxidation by-products from minced mullet muscle systems under different initial conditions. The initial concentrations of CD in the minced fish muscle systems were measured to obtain their oxidative status. The stability of many fish species has been tested during various refrigerated (20) and frozen (47) storage periods. The observed inhibition of lipid oxidation using the GSE in minced mullet during chilled storage is in agreement with results found in liposomes and mackerel muscle (20, 48).
Studies that have mimicked stomach conditions in vitro to examine the evolution of lipid oxidation (10, 49) have demonstrated the development of lipid oxidation catalyzed by prooxidant compounds such as hemoproteins and iron present in fish muscle. Kanner et al. (16) suggested that the stomach may act as a bioreactor and accelerate the generation of lipid hydroperoxides and cooxidation of dietary constituents due to the physiological temperature and the presence of large amounts of endogenous catalysts, such as iron ions and hemeproteins (50). Furthermore, acid pH increases the prooxidant activity of hemoproteins (8). Preexisting lipid hydroperoxides also may catalyze further lipid oxidation. The decomposition of these primary oxidation products generates new free radicals that may catalyze the production of new lipid hydroperoxides (51). Partially oxidized food undergoes further oxidation under simulated stomach conditions, yielding deleterious compounds such as hydroperoxides (9, 16, 52).
In contrast to storage in the absence of polyphenols, the minced fish system stored in the presence of GSE did not show any increment of lipid hydroperoxides in the stomach compartment. The same GSE extract has shown high effectiveness against the development of lipid oxidation catalyzed by endogenous prooxidants, like hemoglobin (20, 48), in fish muscle. Similarly, red wine polyphenols had an antioxidant effect on heated red muscle tissue homogenate in simulated in vitro gastric conditions (11, 16). Importantly, the amounts of polyphenolic antioxidants used in this work have been found in foods such as fruits and vegetables, which may be consumed with the fish (53), underscoring the fact that physiologic, rather than pharmacologic, doses of antioxidant compounds are effective. Additionally, although these studies used raw fish, it has been reported that the temperatures typically used in cooking do not substantially affect polyphenol content (54).
The differences in the concentration of CD in the stomach compartment between fresh fish muscle and fish stored with GSE were not found in the small intestinal dialysates. Due to the high amount of fat (6.78 ± 0.32%) and the antioxidant protection obtained during the storage period and in the stomach with prooxidant conditions, it is possible that the GSE polyphenols were completely consumed. Indeed, when GSE was used at higher amounts, we did observe lower CD concentrations not only in the stomach but also in the duodenal and jejuna dialysates, suggesting that higher amounts of plant polyphenols can be protective during the entire digestive and absorptive process.
When grown in culture, Caco-2 cells spontaneously develop many functions characteristics of mature villus cells of the small intestinal epithelium (55), including formation of a polarized monolayer of columnar epithelium with intercellular tight junctions (56), an AP membrane with a brush border of organized microvilli, and the secretion of lipoprotein particles (41, 57). Thus, Caco-2 cells are considered a reasonable model with which to approximate dietary lipid assimilation by intestinal enterocytes (26).
When we directly compared the uptake of nonoxidized and oxidized DHA in Caco-2 cells, a markedly greater uptake of the native FA was observed. This is in contrast with a previous study by Penumetcha, et al. (6), who reported no differences in Caco-2 cell uptake of native and oxidized linoleic acid. The reason for the discordant findings are not clear; however, the studies used different PUFA species and different uptake media, with no FA carrier used in the prior study (6) and bile salt micelles used in the present study to simulate the native luminal milieu (24). Co-incubation of DHA with either GSE or EGCG resulted in a large increase in net uptake relative to incubation without plant polyphenols. Based on the differential uptake of oxidized and unoxidized DHA, we hypothesize that the higher DHA uptake in cells incubated with GSE or EGCG can be attributed at least in part to their direct inhibition of oxidative degradation of the (n-3) PUFAs. Interestingly, however, we also found that co-incubation of the SFA palmitate with the plant polyphenols increased uptake. This suggests that the GSE and EGCG may be exerting an indirect effect on FA uptake in addition to their direct protection of PUFA against oxidative damage.
The redox status of the enterocyte plays a key role in the control of intestinal growth and function (58). Thus, the presence of the antioxidants may help to preserve the endogenous redox balance of Caco-2 cells, promoting greater uptake of all FAs, including SFA. Indeed, oleic acid hydroperoxides have been shown to cause Caco-2 cell membrane damage (59) and lipid hydroperoxides have been demonstrated to induce apoptosis in Caco-2 cells, indicative of dysregulated homeostasis of the intestinal epithelium (60). The presence of lipid hydroperoxides has also been found to impair mucosal detoxification pathways and cause enterocyte dysfunction. When the concentration of lipid hydroperoxides is higher than the detoxification capacity of intestinal cells, increased FA excretion in feces may occur (61) due to mucosal cell injury (62). Further, oxidative degradation produced by increased oxidative stress can provoke loss of membrane integrity, dysregulation of membrane transport mechanisms, and changes in permeability. The differential uptake of long-chain FAs across the AP and basolateral membranes of Caco-2 cells could be related to differences in plasma membrane fluidity, because the AP membrane of the enterocyte has lower fluidity than basolateral membranes (59). Both EGCG alone and the GSE used in this work have been shown to decrease the fluidity of model membranes (20, 63). This increase of membrane lipid order decreases the penetration of free radicals into the membrane hydrocarbon chain interior and also inhibits the propagation of lipid radicals within the membrane. Additionally, oxidative stress has been reported to cause alterations in protein structure, including cross-linking, amino acid side-chain modifications, and protein fragmentation (64). Thus, it is possible that as a consequence of the protection of the enterocyte plasma membrane attributed to the presence of GSE or EGCG, both the passive diffusion of FFAs through the lipid bilayer as well as protein-mediated FA transport are maintained.
Polyphenols may alter intracellular lipid processing and packaging into lipoproteins. Several studies have reported that plant polyphenols were associated with decreased cholesterol esterification, inhibition of diacylglycerol acyltransferase, and decreased microsomal TG transfer protein activity, all of which could lead to decreases in postprandial lipemia (65, 66). In the present studies, we found no differences in intracellular FA metabolism in EGCG or GSE-treated Caco-2 cells, suggesting that enzymatic conversion of the labeled lipids was not saturated so as to change the metabolic fate of the FAs. It is possible that greater amounts of polyphenols may lead to decreased TG and cholesterol ester synthesis and decreased lipoprotein formation.
In summary, we show using a detailed in vitro model of the stomach and small intestine that PUFA-rich fish undergoes oxidation within the lumen of the stomach and that more lipid hydroperoxides become available for uptake in the proximal small intestine. The addition of GSE during storage and active digestion limits the development of lipid oxidation products in fatty fish. We further show that in Caco-2 cells, enterocyte uptake of oxidized DHA is markedly diminished relative to the uptake of unoxidized PUFA. The addition of GSE or EGCG alone leads to greater FA uptake into the Caco-2 enterocyte model. This increased uptake is likely related both to a direct effect of the polyphenols in preventing oxidation of PUFA and an indirect effect of maintaining the redox status and homeostasis of the intestinal epithelium and the integrity of the plasma membrane. Studies are underway to determine whether the markedly decreased uptake of oxidized FAs is secondary to alterations in diffusion- or protein-mediated PUFA transport (26) or both.
Supplementary Material
Acknowledgments
The authors thank Professors Maria Jose Nuñez and Xurxo Sineiro for providing the GSE. The authors also thank Dr. David Ribnicky, Dr. Rob Havenaar, and Andrew Oren for invaluable assistance with the TIM-1 instrument. R.M., I.M., and J.S. designed the research; R.M., J.D.D., and S.K. conducted the research; and R.M., I.M., and J.S. wrote the paper. All the authors read and approved the final manuscript.
Footnotes
Abbreviations used: AP, apical; CD, conjugated diene; DG, diacylglycerol; EGCG, epigallocatechin gallate; FA, fatty acid; GSE, grape seed extract; PA, palmitic acid; TIM-1, TNO Intestinal Model-1.
Literature Cited
- 1.Lee KW, Lip GY. The role of omega-3 fatty acids in the secondary prevention of cardiovascular disease. QJM. 2003;96:465–80 [DOI] [PubMed] [Google Scholar]
- 2.Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 2003;278:31426–33 [DOI] [PubMed] [Google Scholar]
- 3.Wang T-G, Gotoh Y, Jennings MH, Rhoads CA, Aw TY. Lipid hydroperoxide-induced apoptosis in human colonic Caco-2 cells associated with an early loss of cellular redox balance. FASEBJ 2000;14:1567–76 [DOI] [PubMed] [Google Scholar]
- 4.Staprans I, Rapp JH, Pan XM, Feingold KR. The effect of oxidized lipids in the diet on serum lipoprotein peroxides in control and diabetic rats. J Clin Invest. 1993;92:638–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adam SK, Soelaiman I, Umar N, Jaarin K. Effects of repeatedly heated palm oil on serum lipid profile, lipid peroxidation and homocysteine levels in a post-menopausal rat model. McGill J Med. 2008;11:145–51 [PMC free article] [PubMed] [Google Scholar]
- 6.Penumetcha M, Khan N, Parthasarathy S. Dietary oxidized fatty acids: an atherogenic risk? J Lipid Res. 2000;41:1473–80 [PubMed] [Google Scholar]
- 7.Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am J Clin Nutr. 1993;57:S779–89 [DOI] [PubMed] [Google Scholar]
- 8.Lapidot T, Granit R, Kanner J. Lipid hydroperoxidase activity of myoglobin and phenolic antioxidants in simulated gastric fluid. J Agric Food Chem. 2005;53:3391–6 [DOI] [PubMed] [Google Scholar]
- 9.Gorelik S, Lapidot T, Shaham I, Granit R, Ligumsky M, Kohen R, Kanner J. Lipid peroxidation and coupled vitamin oxidation in simulated and human gastric fluid inhibited by dietary polyphenols: health implications. J Agric Food Chem. 2005;53:3397–402 [DOI] [PubMed] [Google Scholar]
- 10.Gorelik S, Ligumsky M, Kohen R, Kanner J. The stomach as a bioreactor: when red meat meets red wine. J Agric Food Chem. 2008;56:5002–7 [DOI] [PubMed] [Google Scholar]
- 11.Gorelik S, Kohen R, Ligumsky M, Kanner J. Saliva plays a dual role in oxidation process in stomach medium. Arch Biochem Biophys. 2007;458:236–43 [DOI] [PubMed] [Google Scholar]
- 12.Pazos M, Lois S, Torres JL, Medina I. Inhibition of hemoglobin and iron-promoted oxidation in fish microsomes by natural phenolics. J Agric Food Chem. 2006;54:4417–23 [DOI] [PubMed] [Google Scholar]
- 13.Richards MP, Dettmann MA, Grunwald EW. Pro-oxidative characteristics of trout hemoglobin and myoglobin: a role for released heme in oxidation of lipids. J Agric Food Chem. 2005;53:10231–8 [DOI] [PubMed] [Google Scholar]
- 14.Özalp Özen B, Eren M, Pala A, Özmen İ, Soyer A. Effect of plant extracts on lipid oxidation during frozen storage of minced fish muscle. Int J Food Sci Technol. 2011;46:724–31 [Google Scholar]
- 15.Gray DA, Payne G, McClements DJ, Decker EA, Lad M. Oxidative stability of Echium plantagineum seed oil bodies. Eur J Lipid Sci Technol. 2010;112:741–9 [Google Scholar]
- 16.Kanner J, Lapidot T. The stomach as a bioreactor: dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic Biol Med. 2001;31:1388–95 [DOI] [PubMed] [Google Scholar]
- 17.Manach C, Scalbert A, Morand C, Ramasy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47 [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Lee M-J, Li H, Yang CS. Absorption, distribution, and elimination of tea polyphenols in rats. Drug Metab Dispos. 1997;25:1045–50 [PubMed] [Google Scholar]
- 19.Warden BA, Smith LS, Beecher GR, Balentine DA, Clevidence BA. Catechins are bioavailable in men and women drinking black tea throughout the day. J Nutr. 2001;131:1731–7 [DOI] [PubMed] [Google Scholar]
- 20.Maestre R, Micol V, Funes L, Medina I. Incorporation and interaction of grape seed extract in membranes and relation with efficacy in muscle foods. J Agric Food Chem. 2010;58:8365–74 [DOI] [PubMed] [Google Scholar]
- 21.Torres JL, Varela B, García MT, Carilla J, Matito C, Centelles JJ, Cascante C, Sort X, Bobet R. Valorization of grape (Vitis vinifera) byproducts. Antioxidant and biological properties of polyphenolic fractions differing in procyanidin composition and flavonol content. J Agric Food Chem. 2002;50:7548–55 [DOI] [PubMed] [Google Scholar]
- 22.Torres J, Lozano C. Chromatographic characterization of proanthocyanidins after thiolysis with cysteamine. Chromatographia. 2001;54:523–6 [Google Scholar]
- 23.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58 [Google Scholar]
- 24.Trotter PJ, Storch J. Fatty acid esterification during differentiation of the human intestinal cell line Caco-2. J Biol Chem. 1993;268:10017–23 [PubMed] [Google Scholar]
- 25.Trotter PJ, Storch J. Nutritional control of fatty acid esterification in differentiating Caco-2 intestinal cells is mediated by cellular diacylglycerol concentrations. J Nutr. 1993;123:728–36 [DOI] [PubMed] [Google Scholar]
- 26.Ho S-Y, Storch J. Common mechanisms of monoacylglycerol and fatty acid uptake by human intestinal Caco-2 cells. Am J Physiol Cell Physiol. 2001;281:C1106–17 [DOI] [PubMed] [Google Scholar]
- 27.Dietschy JM. Mechanisms for the intestinal absorption of bile acids. J Lipid Res. 1968;9:297–309 [PubMed] [Google Scholar]
- 28.Dakka T, Dumoulin V, Chayvialle JA, Cuber JC. Luminal bile salts and neurotensin release in the isolated vascularly perfused rat jejuno-ileum. Endocrinology. 1994;134:603–7 [DOI] [PubMed] [Google Scholar]
- 29.Murota K, Storch J. Uptake of micellar long-chain fatty acid and sn-2-monoacylglycerol into human intestinal Caco-2 cells exhibits characteristics of protein-mediated transport. J Nutr. 2005;135:1626–30 [DOI] [PubMed] [Google Scholar]
- 30.Trotter PJ, Ho SY, Storch J. Fatty acid uptake by Caco-2 human intestinal cells. J Lipid Res. 1996;37:336–46 [PubMed] [Google Scholar]
- 31.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75 [PubMed] [Google Scholar]
- 32.Bligh EG, Dyer WJ. A rapid method of total lipidextraction and purification. J Biochem Physiol. 1959;37:911–7 [DOI] [PubMed] [Google Scholar]
- 33.Kim RS, LaBella FS. Comparison of analytical methods for monitoring autoxidation profiles of authentic lipids. J Lipid Res. 1987;28:1110–7 [PubMed] [Google Scholar]
- 34.Chan SH. Autoxidation of unsaturated lipids. London: Academic Press; 1987.
- 35.Medina I, Saeed S, Howell N. Enzymatic oxidative activity in sardine (Sardina pilchardus) and herring (Clupea harengus) during chilling and correlation with quality. Eur Food Res Technol. 1999;210:34–8 [Google Scholar]
- 36.Herbes SE, Allen CP. Lipid quantification of freshwater invertebrates: method modification for microquantitation. Can J Fish Aquat Sci. 1983;40:1315–7 [Google Scholar]
- 37.Minekus M, Marteau P, Havenaar R, Huis in 't Veld JHJ. A multicompartimental dynamic computer-controlled model simulating the stomach and small intestine. ATLA. 1995;23:197–209 [Google Scholar]
- 38.Larsson M, Minekus M, Havenaar R. Estimation of the bioavailability of iron and phosphorus in cereals using a dynamic in vitro gastrointestinal model. J Sci Food Agric. 1997;74:99–106 [Google Scholar]
- 39.Köse S, Koral S, Özoğul Y, Tufan B. Fatty acid profile and proximate composition of Pacific mullet (Mugil so-iuy) caught in the Black Sea. Int J Food Sci Technol. 45, 1594–602 [Google Scholar]
- 40.Haraldsson A-K, Rimsten L, Alminger M, Andersson R, Åman P, Sandberg A-S. Digestion of barley malt porridges in a gastrointestinal model: iron dialysability, iron uptake by Caco-2 cells and degradation of β-glucan. J Cereal Sci. 2005;42:243–54 [Google Scholar]
- 41.Trotter PJ, Storch J. Fatty acid uptake and metabolism in a human intestinal cell line (Caco-2): comparison of apical and basolateral incubation. J Lipid Res. 1991;32:293–304 [PubMed] [Google Scholar]
- 42.Draper HH, Polensek L, Hadley M, McGirr L. Urinary malondialdehyde as an indicator of lipid peroxidation in the diet and in the tissues. Lipids. 1984;19:836–43 [DOI] [PubMed] [Google Scholar]
- 43.Jenkinson A, Franklin MF, Wahle K, Duthie GG. Dietary intakes of polyunsaturated fatty acids and indices of oxidative stress in human volunteers. Eur J Clin Nutr. 1999;53:523–8 [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Yeo HC. Overvik-Douki E, Hagen T, Doniger SJ, Chu DW, Brooks GA, Ames BN. Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol. 2000;89:21–8 [DOI] [PubMed] [Google Scholar]
- 45.Tholstrup T, Hellgren LI, Petersen M, Basu S, Straarup EM, Schnohr P, Sandstram B. A solid dietary fat containing fish oil redistributes lipoprotein subclasses without increasing oxidative stress in men. J Nutr. 2004;134:1051–7 [DOI] [PubMed] [Google Scholar]
- 46.Kikugawa K, Yasuhara Y, Ando K, Koyama K, Hiramoto K, Suzuki M. Protective effect of supplementation of fish oil with high n-3 polyunsaturated fatty acids against oxidative stress-induced DNA damage of rat liver in vivo. J Agric Food Chem. 2003;51:6073–9 [DOI] [PubMed] [Google Scholar]
- 47.Pazos M, Alonso A, Fernández-Bolaños J, Torres JL, Medina I. Physicochemical properties of natural phenolics from grapes and olive oil by-products and their antioxidant activity in frozen horse mackerel fillets. J Agric Food Chem. 2006;54:366–73 [DOI] [PubMed] [Google Scholar]
- 48.Maestre R, Pazos M, Iglesias J, Medina I. Capacity of reductants and chelators to prevent lipid oxidation catalyzed by fish hemoglobin. J Agric Food Chem. 2009;57:9190–6 [DOI] [PubMed] [Google Scholar]
- 49.Kanazawa K, Ashida H. Dietary hydroperoxides of linoleic acid decompose to aldehydes in stomach before being absorbed into the body. Biochim Biophys Acta. 1998;1393:349–61 [DOI] [PubMed] [Google Scholar]
- 50.Kanner J. Oxidative processes in meat and meat products quality implications. Meat Sci. 1994;36:169–89 [DOI] [PubMed] [Google Scholar]
- 51.Frankel EN. Lipid oxidation. Dundee (Scotland): The Oily Press; 1998.
- 52.Kanazawa K, Ashida H. Catabolic fate of dietary trilinoleoylglycerol hydroperoxides in rat gastrointestines. Biochim Biophys Acta. 1998;1393:336–48 [DOI] [PubMed] [Google Scholar]
- 53.Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46:4113–7 [Google Scholar]
- 54.González MJ, Torres JLs, Medina I. Impact of thermal processing on the activity of gallotannins and condensed tannins from hamamelis virginiana used as functional ingredients in seafood. J Agric Food Chem. 2010;58:4274–83 [DOI] [PubMed] [Google Scholar]
- 55.Pinto M, Robine-Leon S, Appay M. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–30 [Google Scholar]
- 56.Grasset E, Pinto M, Dussaulx E, Zweibaum A, Desjeux JF. Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am J Physiol. 1984;247:C260–7 [DOI] [PubMed] [Google Scholar]
- 57.Levin MS, Talkad VD, Gordon JI, Stenson WF. Trafficking of exogenous fatty acids within Caco-2 cells. J Lipid Res. 1992;33:9–19 [PubMed] [Google Scholar]
- 58.Aw TY. Cellular redox: a modulator of intestinal epithelial cell proliferation. News Physiology Sci. 2003;18:201–4 [DOI] [PubMed] [Google Scholar]
- 59.Wijeratne SSK, Cuppett SL. Lipid hydroperoxide induced oxidative stress damage and antioxidant enzyme response in Caco-2 human colon cells. J Agric Food Chem. 2006;54:4476–81 [DOI] [PubMed] [Google Scholar]
- 60.Aw TY. Determinants of intestinal detoxication of lipid hydroperoxides. Free Radic Res. 1998;28:637–46 [DOI] [PubMed] [Google Scholar]
- 61.Lindley KJ, Goss-Sampson MA, Muller DP, Milla PJ. Lipid peroxidation and electrogenic ion transport in the jejunum of the vitamin E deficient rat. Gut. 1994;35:34–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chamulitrat W, Jordan SJ, Mason RP. Fatty acid radical formation in rats administered oxidized fatty acids: In vivo spin trapping investigation. Arch Biochem Biophys. 1992;299:361–7 [DOI] [PubMed] [Google Scholar]
- 63.Caturla N, Vera-Samper E, Villalaín J, Mateo CR, Micol V. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic Biol Med. 2003;34:648–62 [DOI] [PubMed] [Google Scholar]
- 64.Davies KJ, Lin SW, Pacifici RE. Protein damage and degradation by oxygen radicals. IV. Degradation of denatured protein. J Biol Chem. 1987;262:9914–20 [PubMed] [Google Scholar]
- 65.Löest HB, Noh SK, Koo SI. Green tea extract inhibits the lymphatic absorption of cholesterol and alpha-tocopherol in ovariectomized rats. J Nutr. 2002;132:1282–8 [DOI] [PubMed] [Google Scholar]
- 66.Borradaile NM, de Dreu LE, Barrett PHR, Behrsin CD, Huff MW. Hepatocyte apoB-containing lipoprotein secretion is decreased by the grapefruit flavonoid, naringenin, via inhibition of MTP-mediated microsomal triglyceride accumulation. Biochemistry. 2003;42:1283–91 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.