Abstract
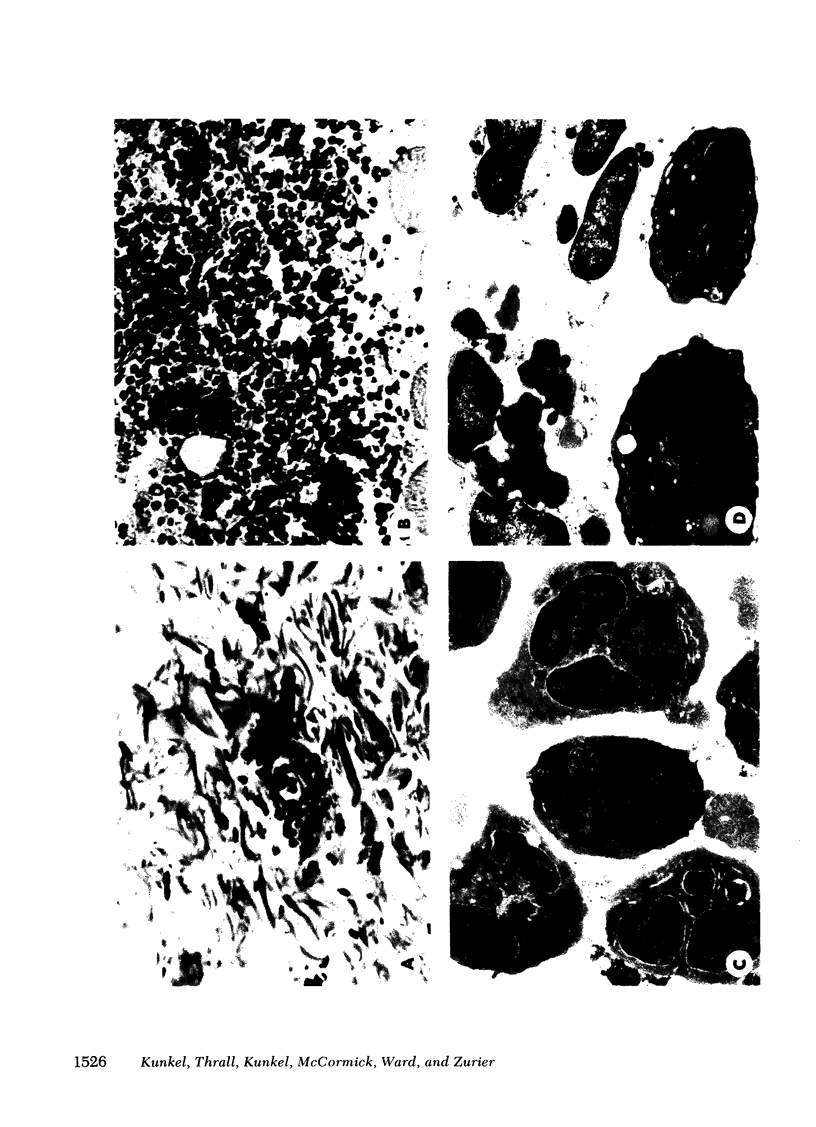
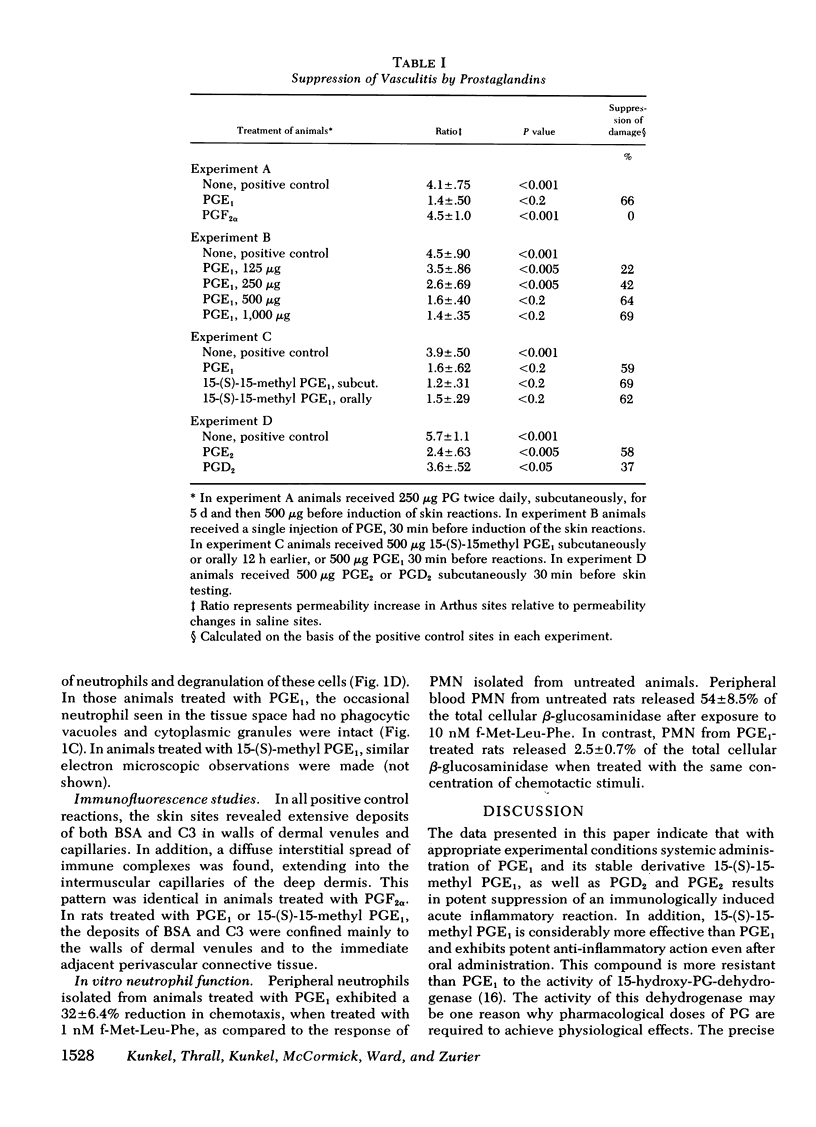
Immune complex-induced vascular damage can be markedly suppressed by treatment of rats with either prostaglandin (PG)E1 or its stable derivative, 15-(S)-15-methyl PGE1, but not with PGF2 alpha. In addition, PGD2 and PGE2 also show suppressive effects. The PGE1 derivative is considerably more effective than PGE1 and shows potent anti-inflammatory activity even after oral administration. Suppression of the vasculitis reaction is reflected by a greatly diminished increase in vasopermeability, indicating little or no vascular damage. In suppressed animals, the infiltration of neutrophils is greatly reduced, and those leukocytes that have appeared at tissue sites fail to show phagocytic uptake of immune complexes. In suppressed animals, the skin sites nevertheless show deposits of immune complexes and C3 fixation in vascular walls. Neutrophils harvested from the blood of rats treated with PGE1 show depressed responsiveness in chemotaxis and in enzyme secretion after incubation with chemotactic peptide. These studies indicate that certain PG have potent anti-inflammatory activity, which may be related to their effects on leukocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonta I. L., Parnham M. J., Van Vliet L. Combination of theophylline and prostaglandin E1 as inhibitors of the adjuvant-induced arthritis syndrome of rats. Ann Rheum Dis. 1978 Jun;37(3):212–217. doi: 10.1136/ard.37.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G. Immunologic tissue injury mediated by neutrophilic leukocytes. Adv Immunol. 1968;9:97–162. doi: 10.1016/s0065-2776(08)60442-3. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H. The mechanism of Arthus reactions. II. The role of polymorphonuclear leucocytes and platelets in reversed passive reactions in the guinea-pig. Br J Exp Pathol. 1955 Jun;36(3):283–289. [PMC free article] [PubMed] [Google Scholar]
- Hansen H. S. 15-hydroxyprostaglandin dehydrogenase. A review. Prostaglandins. 1976 Oct;12(4):647–679. doi: 10.1016/0090-6980(76)90044-7. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Hornsta G. Degree and duration of prostaglandin E 1-induced inhibition of platelet aggregation in the rat. Eur J Pharmacol. 1971;15(3):343–349. doi: 10.1016/0014-2999(71)90101-4. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Acute immunologic pulmonary alveolitis. J Clin Invest. 1974 Aug;54(2):349–357. doi: 10.1172/JCI107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., DeBernardo R. The immediate allergic response: in vitro action of cyclic AMP-active and other drugs on the two stages of histamine release. J Immunol. 1971 Oct;107(4):1131–1136. [PubMed] [Google Scholar]
- Nishizawa E. E., Miller W. L., Gorman R. R., Bundy G. L., Svensson J., Hamberg M. Prostaglandin d2 as a potential antithrombotic agent. Prostaglandins. 1975 Jan;9(1):109–121. doi: 10.1016/s0090-6980(75)80122-5. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Chemotactic factor influences on the aggregation, swelling, and foreign surface adhesiveness of human leukocytes. Am J Pathol. 1978 Mar;90(3):537–550. [PMC free article] [PubMed] [Google Scholar]
- Orange R. P., Austen W. G., Austen K. F. Immunological release of histamine and slow-reacting substance of anaphylaxis from human lung. I. Modulation by agents influencing cellular levels of cyclic 3',5'-adenosine monophosphate. J Exp Med. 1971 Sep 1;134(3 Pt 2):136s–148s. [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD P. A., COCHRANE C. G., MUELLER-EBERHARD H. J. THE ROLE OF SERUM COMPLEMENT IN CHEMOTAXIS OF LEUKOCYTES IN VITRO. J Exp Med. 1965 Aug 1;122:327–346. doi: 10.1084/jem.122.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurier R. B., Quagliata F. Effect of prostaglandin E 1 on adjuvant arthritis. Nature. 1971 Dec 3;234(5327):304–305. doi: 10.1038/234304a0. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]




