Abstract
Favorable associations between magnesium intake and glycemic traits, such as fasting glucose and insulin, are observed in observational and clinical studies, but whether genetic variation affects these associations is largely unknown. We hypothesized that single nucleotide polymorphisms (SNPs) associated with either glycemic traits or magnesium metabolism affect the association between magnesium intake and fasting glucose and insulin. Fifteen studies from the CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology) Consortium provided data from up to 52,684 participants of European descent without known diabetes. In fixed-effects meta-analyses, we quantified 1) cross-sectional associations of dietary magnesium intake with fasting glucose (mmol/L) and insulin (ln-pmol/L) and 2) interactions between magnesium intake and SNPs related to fasting glucose (16 SNPs), insulin (2 SNPs), or magnesium (8 SNPs) on fasting glucose and insulin. After adjustment for age, sex, energy intake, BMI, and behavioral risk factors, magnesium (per 50-mg/d increment) was inversely associated with fasting glucose [β = −0.009 mmol/L (95% CI: −0.013, −0.005), P < 0.0001] and insulin [−0.020 ln-pmol/L (95% CI: −0.024, −0.017), P < 0.0001]. No magnesium-related SNP or interaction between any SNP and magnesium reached significance after correction for multiple testing. However, rs2274924 in magnesium transporter-encoding TRPM6 showed a nominal association (uncorrected P = 0.03) with glucose, and rs11558471 in SLC30A8 and rs3740393 near CNNM2 showed a nominal interaction (uncorrected, both P = 0.02) with magnesium on glucose. Consistent with other studies, a higher magnesium intake was associated with lower fasting glucose and insulin. Nominal evidence of TRPM6 influence and magnesium interaction with select loci suggests that further investigation is warranted.
Introduction
Magnesium is an essential mineral found in many foods; rich sources include whole grains, green leafy vegetables, coffee, and legumes. Magnesium is a critical cofactor in >300 enzymatic reactions, including those related to energy metabolism (1). Evidence from cross-sectional and longitudinal observational studies suggests that diets higher in magnesium are associated with reduced risk of insulin resistance (2–8) and type 2 diabetes (9, 10), whereas in intervention studies, supplemental magnesium improves measures of glucose and insulin metabolism in generally healthy adults (11, 12), as well as in those with insulin resistance (13, 14) and type 2 diabetes (15, 16). However, little is known about potential interaction between magnesium intake and genetic variability on glycemic traits, in which genetic variants related to either magnesium transport and homeostasis or glucose and insulin metabolism may modify the pathways through which magnesium exerts its effects.
Single nucleotide polymorphisms (SNPs)48 associated with modest elevation in fasting glucose (FG) and fasting insulin (FI) concentrations have been identified through meta-analysis of genome-wide association studies (GWAS) (17). In addition, a GWAS meta-analysis of serum magnesium, a biomarker of magnesium status, identified 6 SNPs in genes linked to magnesium transport and homeostasis (18). Among these 6 SNPs, the C allele of rs4072037 in MUC1, which was associated with lower serum magnesium, was also associated with lower FG concentrations (18). Three studies have also investigated associations between magnesium-related loci in transient receptor potential cation channel, subfamily M, members 6 (TRPM6) or 7 (TRPM7) and diabetes or glycemic traits. One of these studies observed an association between carriers of the TRPM6 rs2274924 variant and elevated total glycosylated hemoglobin and odds of gestational diabetes in 997 women after delivery (19). The loci studied in these 2 genes in the other 2 studies did not modify either disease (20, 21) or glycemic traits (21); however, these were small studies and one included women only (20). The latter study (20) also examined interactions between magnesium intake and TRPM6 and TRPM7 loci on risk of type 2 diabetes, reporting increased odds of disease in women with a risk haplotype at rs3750425 and rs2274924 in TRPM6 only when magnesium intake was <250 mg/d.
Despite plausible biological mechanisms underlying associations between magnesium and glycemic traits, such as magnesium’s role as a cofactor for tyrosine kinase in the β subunit of the insulin receptor (22, 23), the interaction between dietary magnesium and glycemia-related genetic variants on glucose and insulin has yet to be examined. Furthermore, genetic factors related to magnesium transport and homeostasis may modify associations between magnesium intake and glycemic traits. Examining interactions between dietary magnesium and these variants may enhance our understanding of type 2 diabetes etiology and pathogenesis. Therefore, we examined cross-sectional associations of dietary magnesium intake with FG and FI, associations of magnesium-related SNPs with FG and FI, and interactions between dietary magnesium intake and both magnesium-related and glycemia-related SNPs on FG and FI in meta-analyses of 15 cohort studies.
Methods
Participating cohorts.
The sample for the cross-sectional meta-analyses included up to 52,684 participants of European descent from 15 cohort studies (Table 1) participating in the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium Nutrition Working Group (24): the Atherosclerosis Risk In Communities (ARIC) Study; the Family Heart Study (FamHS); the Framingham Heart Study Offspring and Generation 3 (FHS); the Cardiovascular Health Study (CHS); the Gene-Diet Attica Investigation on Childhood Obesity (GENDAI); the Greek Health Randomized Aging Study (GHRAS); the Gene-Lifestyle interactions And Complex traits Involved in Elevated disease Risk (GLACIER); the Health, Aging, and Body Composition (Health ABC) Study; Invecchiare in Chianti (InCHIANTI); the Malmö Diet and Cancer Study cardiovascular cohort (Malmö); the Multi-Ethnic Study of Atherosclerosis (MESA); the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS); the Rotterdam Study (Rotterdam); the Uppsala Longitudinal Study of Adult Men (ULSAM); and the Cardiovascular Risk in Young Finns Study (YFS). Participants provided written informed consent, and the protocol was approved by local institutional review boards. Participants within each cohort were excluded from analyses if they had diabetes, defined as diagnosed or self-reported diabetes, and/or fasting glucose ≥7 mmol/L, and/or use of diabetes medications.
TABLE 1.
Participant characteristics of 15 US and European cohort studies1
| Cohort study (study acronym) (country) | n2 | Age | Sex | BMI | Fasting glucose | Fasting insulin3 | Dietary magnesium intake | Energy intake | Alcohol intake | Current smoker | Completed high school |
| y | % women | kg/m2 | mmol/L | pmol/L | mg/d | kcal/d | g/d | % | % | ||
| Atherosclerosis Risk in Communities (ARIC) Study (USA) | 8951 | 54 ± 5.7 | 53.7 | 27 ± 4.6 (n = 8586) | 5.5 ± 0.50 | 58.6 ± 1.93 (16.1, 213) | 260 ± 94.5 | 1640 ± 600 | 6.7 ± 1.0 (n = 8579) | 24.4 (n = 8587) | 84.1 (n = 8585) |
| Cardiovascular Health Study (CHS) (USA) | 2745 | 72 ± 5.4 | 62.3 | 26 ± 4.3 (n = 2737) | 5.5 ± 0.52 | 84.8 ± 1.54 (36.5, 197) | 413 ± 145 | 2020 ± 650 | 5.8 ± 13 (n = 2740) | 11.5 (n = 2744) | 76.2 (n = 2739) |
| Family Heart Study (FamHS) (US) | 3187 (3181) | 51 ± 14 | 53.6 | 27 ± 5.3 | 5.2 ± 0.50 | 60.3 ± 1.82 (18.6, 196) | 261 ± 95.8 | 1750 ± 620 | 6.4 ± 13 | 14.6 | 64.8 |
| Framingham Heart Study (FHS) (USA) | 5743 (5435) | 49 ± 14 | 54.9 | 27 ± 5.2 | 5.3 ± 0.50 | 79.8 ± 1.47 (14.9, 544) | 314 ± 115 | 1970 ± 660 | 11 ± 15 | 29.6 | 97.5 |
| Gene-Diet Attica Investigation on Childhood Obesity (GENDAI) (Greece) | 1087 (1064) | 11 ± 0.70 | 53.2 | 20 ± 3.4 | 4.8 ± 0.48 | 40.0 ± 1.72 (13.8, 116) | 225 ± 75.6 | 1890 ± 600 | NA | NA | NA |
| Greek Health Randomized Aging Study (GHRAS) (Greece) | 856 (670) | 72 ± 7.5 | 71.2 | 30 ± 4.8 | 5.8 ± 1.6 | 43.1 ± 1.75 (14.4, 129) | 237 ± 63.1 | 2160 ± 690 | 45 ± 90 | 14.5 | 64.0 |
| Gene-Lifestyle interactions And Complex traits In Elevated disease Risk (GLACIER) (Sweden) | 14,940 (892) | 52 ± 8.8 | 60.7 | 26 ± 4.0 | 5.4 ± 0.62 | 41.3 ± 1.90 (11.8, 145) | 291 ± 92.4 | 1720 ± 600 | 3.5 ± 4.5 | 21.6 | 79.1 |
| Health, Aging, and Body Composition Study (Health ABC) (USA) | 1281 (1256) | 74 ± 2.8 | 50.2 | 26 ± 4.0 | 5.2 ± 0.55 | 45.1 ± 1.70 (15.9, 128) | 294 ± 101 | 1810 ± 600 | 6.9 ± 14 | 6.10 | 89.1 |
| Invecchiare in Chianti (Aging in the Chianti Area; InCHIANTI) (Italy) | 1071 (1044) | 68 ± 16 | 56.3 | 27 ± 4.1 | 4.8 ± 0.61 | 65.4 ± 1.70 (23.1, 185) | 256 ± 74.6 | 2010 ± 600 | 15 ± 21 | 24.3 | 80.4 |
| Malmö Diet and Cancer Study (Malmö) (Sweden) | 4867 (4864) | 58 ± 5.9 | 60.0 | 25 ± 3.8 | 5.5 ± 0.52 | 37.3 ± 1.70 (13.2, 106) | 353 ± 91.3 | 2320 ± 670 | 10 ± 12 | 26.9 | 80.4 |
| Multi-Ethnic Study of Atherosclerosis (MESA) (USA) | 2145 | 63 ± 10 | 52.4 | 28 ± 5.0 | 4.9 ± 0.56 | 32.6 ± 1.84 (9.84, 108) | 276 ± 113 | 1700 ± 720 | 8.8 ± 16 | 11.0 | 79.3 |
| Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) (Sweden) | 727 | 70 ± 0.20 | 51.2 | 27 ± 4.1 | 4.9 ± 0.51 | 50.4 ± 1.68 (18.2, 140) | 317 ± 75.5 | 1890 ± 500 | 8.1 ± 7.9 | 10.6 | 44.7 |
| Rotterdam Study (The Netherlands) | 2340 (2273) | 72 ± 6.6 | 58.0 | 27 ± 3.8 | 5.5 ± 0.53 | 62.8 ± 1.68 (22.7, 174) | 314 ± 75.5 | 1990 ± 510 | 11 ± 14 | 15.8 | 90.3 |
| Uppsala Longitudinal Study of Adult Men (ULSAM) (Sweden) | 927 (919) | 71 ± 0.60 | 0 | 26 ± 3.2 | 5.4 ± 0.56 | 73.7 ± 1.72 (25.6, 212) | 286 ± 70.0 | 1750 ± 460 | 8.6 ± 13 | 20.2 | 41.0 |
| Cardiovascular Risk in Young Finns Study (YFS) (Finland) | 1788 (1783) | 38 ± 5.0 | 54.1 | 26 ± 4.7 | 5.3 ± 0.75 | 40.5 ± 2.16 (8.94, 183) | 480 ± 156 | 2410 ± 860 | 13 ± 20 | 18.3 | 84.6 |
Values are mean ± SD or percentages (%). Alcohol intake originally quantified as drinks/wk in CHS and FamHS and as drinks/d in YFS. Conversion to grams per day was based on 1 drink containing 14 g alcohol. NA, not applicable.
Maximum available observations, , for interactions between magnesium intake and single nucleotide polymorphisms in glucose outcome analyses (n for insulin interaction analyses in parentheses). Sample sizes vary in some cohorts depending on availability of genotype information.
Insulin was analyzed on the natural log scale and back-transformed to the geometric scale for presentation. Values are geometric mean ± SD (95% CI).
Dietary assessment.
Dietary data were collected via FFQ in 11 cohorts and via dietary recall (1 cohort), food record (2 cohorts), or a combination of food diary and FFQ (1 cohort). Daily intakes of dietary magnesium (from food and beverage sources), kilocalories, fiber, caffeine, and alcohol were estimated for each participant (Supplemental Table 1). By using data from 2 of our cohorts (ARIC and FHS), we assessed the rank ordering of participants using magnesium values derived from food/beverage sources only versus food/beverage and supplemental sources. Unadjusted Spearman correlations between food/beverage magnesium and total (food/beverage and supplemental) magnesium intake were 0.93 and 0.92 in ARIC and FHS, respectively, and 0.84 and 0.83 in ARIC and FHS, respectively, after adjustment for energy intake. Ranks did not vary appreciably and supplemental sources contributed, on average, just 8–16 mg/d of magnesium intake above that derived from food/beverage sources. Only 6 of the 15 studies had supplement use information on <8000 participants. Given these considerations, and to maximize sample size, we considered magnesium intake from food/beverage sources only.
Genotyping, imputation, and SNP selection.
SNPs were previously directly genotyped or imputed by participating cohorts before inclusion in this analysis (Supplemental Table 1). Of the 25 SNPs included in this meta-analysis, a previous GWAS meta-analysis in the Meta-Analyses of Glucose and Insulin-Related Traits Consortium (MAGIC)—which included data from CHS, FHS, InCHIANTI, and Rotterdam—identified 15 SNPs associated with FG, 1 with FI, and 1 with both FG and FI (17). Five SNPs were previously identified in another meta-analysis of GWAS of serum magnesium in MAGIC members, including ARIC, CHS, FHS, and Rotterdam (18). The remaining 3 SNPs associated with magnesium transport or homeostasis selected for this meta-analysis were based on the report by Song et al. (20). Not all SNPs were available in every cohort; total sample sizes for analyses vary accordingly (Supplemental Table 2).
FG and FI measurement.
FG (mmol/L) and FI (pmol/L) were quantified in each cohort by using similar procedures (Supplemental Table 1). FI was natural log-transformed to reduce skewness before data analysis.
Covariate measurement.
Cohort-specific assessment methods and definitions for BMI, educational level, smoking status, and physical activity are provided in Supplemental Table 1.
Cohort-specific analyses.
Each cohort followed a uniform analysis plan to conduct the following analyses. First, main associations between magnesium intake and FG and FI were quantified with adjustment for age, sex, and energy intake (model 1); with additional adjustment for BMI (model 2); with additional adjustment for smoking, education, physical activity, and alcohol intake (model 3); and with additional adjustment for fiber and caffeine intake (model 4). Fiber and caffeine were considered as covariates to distinguish associations of magnesium with FG and FI from those due to nutrients contained in shared food sources displaying similar associations with glycemic traits (e.g., whole grains and coffee). Second, to verify earlier reported associations with FG (16 SNPs) and FI (2 SNPs), and to investigate associations between magnesium-related SNPs (8 SNPs) and FG and FI, cohorts regressed FG and FI on SNPs of interest by using an additive genetic model (per additional outcome-raising allele) adjusted for age and sex and, where relevant, study field center and/or family or population substructure. Third, magnesium-SNP interactions were investigated by including a first-order interaction term (magnesium intake × SNP) in a model including magnesium intake, SNP, age, sex, and energy intake. Before meta-analysis, the β coefficients and SEs involving magnesium intake (per 1 mg/d) reported by each cohort were multiplied by 50 to estimate the association of a 50-mg/d increment in magnesium intake, because 1-mg/d estimates were exceedingly small. Fifty milligrams of magnesium reflects intake from ∼2 ounces of espresso or 2 slices of whole-wheat bread. Assuming a hypothesis-consistent inverse association of magnesium with FG and FI, positive-interaction β coefficients indicate that the magnitude of the inverse association between magnesium intake and FG or FI is less in the presence of an FG- or FI-raising allele. That is, for individuals who carry 1 copy of an FG- or FI-raising allele, the lower FG or FI concentration observed in association with a 50-mg/d increment in magnesium intake would be diminished. Correspondingly, negative-interaction β coefficients indicate that the magnitude of the inverse association between magnesium intake and FG or FI is greater in the presence of an FG- or FI-raising allele. That is, for individuals who carry 1 copy of an FG- or FI-raising allele, the lower FG or FI concentration observed in association with a 50 mg/d higher magnesium intake would be stronger.
Meta-analyses.
We conducted inverse variance–weighted, fixed-effects meta-analyses for 1) main associations of magnesium intake on FG and FI by using STATA (version 12, Stata Corporation), 2) main associations of SNPs with respective outcomes by using METAL (University of Michigan, Center for Statistical Genetics; www.sph.umich.edu/csg/abecasis/metal/), and 3) interactions between SNPs and magnesium intake on respective outcomes by using METAL. The sample sizes for magnesium associations with FG ranged from 52,684 (model 1) to 48,588 (model 4), and with FI they ranged from 37,640 (model 1) to 34,137 (model 4). The sample sizes for interaction analyses on FG ranged from 29,280 (rs2274924) to 52,470 (rs4607517), and on FI they ranged from 28,851 (rs2274924) to 37,804 (rs780094). Heterogeneity across studies was tested by using Cochran’s Q statistic and quantified by using the I2 statistic (25). Approximately defined ranges for interpreting I2 for low, moderate, substantial, and considerable heterogeneity are 0–40%, 30–60%, 50–90%, and 75–100%, respectively (26). To assess potential sources of heterogeneity, we conducted meta-regression of the main magnesium association and of the interaction analyses. [For the main associations of the 8 magnesium-related SNPs on FG or FI (Supplemental Table 2), because no (0%) to low (28%) heterogeneity was observed, meta-regressions were not conducted for these associations.] Meta-regression covariates included region (northern Europe vs. Mediterranean vs. United States), mean age of cohort (<60 vs. ≥60 y), mean magnesium intake of cohort (<300 vs. ≥300 mg/d), mean BMI of cohort (<27 vs. ≥27 kg/m2), percentage of the cohort that was female, and sample size. We also conducted sensitivity analyses to assess the influence on the meta-analyzed estimate of any single cohort study by repeating analyses removing 1 cohort study at a time in the associations for magnesium, magnesium-related SNPs, and magnesium-SNP interactions. Random-effects meta-analyses were conducted secondarily; results were similar to those from the fixed-effects meta-analyses. Thus, we only present the results of the fixed-effects meta-analyses.
Power calculations for various magnitudes of association and sample sizes have been published elsewhere (24, 27). Statistical significance was defined at an α level of 0.0015, based on Bonferroni correction for 34 total interaction tests.
Results
The demographic, dietary, and outcome characteristics of participants in the 15 cohort studies are provided in Table 1. Mediterranean cohorts tended to have the lowest overall mean dietary magnesium intake and northern European cohorts the highest; the mean daily intake of dietary magnesium ranged from 224.7 mg/d in GENDAI (Greece) to 479.7 mg/d in YFS (Finland). Plots of mean intake across cohorts did not suggest that intake differed by either dietary assessment method or mean age (Supplemental Figs. 1 and 2).
Associations of magnesium intake with FG and FI.
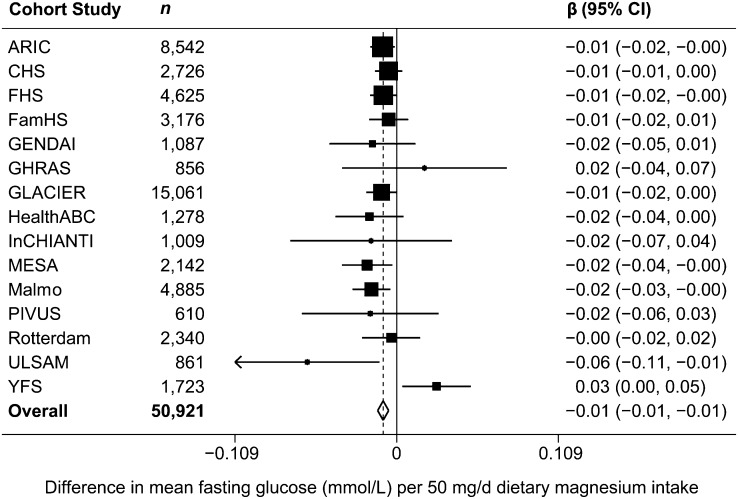
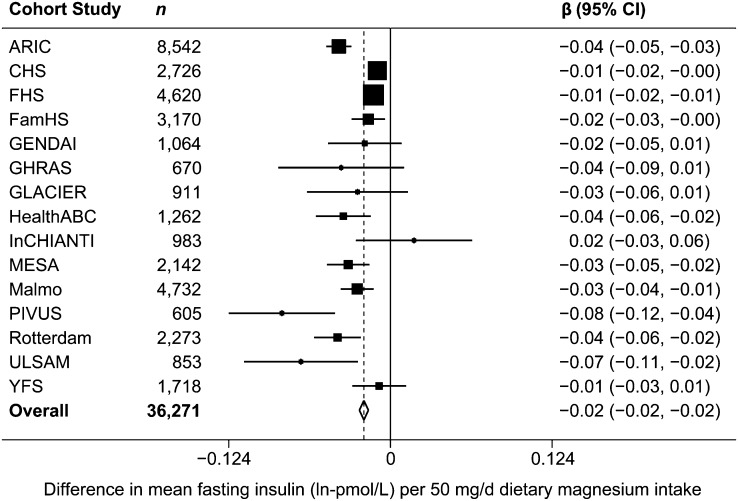
After adjustment for age, sex, alcohol, energy intake, BMI, smoking, education, and physical activity (model 3), magnesium intake was inversely associated with FG and FI concentrations. Per daily 50-mg increment of dietary magnesium, FG was 0.009 mmol/L lower [β = −0.009 mmol/L (95% CI: −0.013, −0.005), P < 0.0001] (Table 2 and Fig. 1) and FI was −0.020 ln-pmol/L lower [β = −0.020 ln-pmol/L (95% CI: −0.024, −0.017), P < 0.0001] (Table 2 and Fig. 2). After additional adjustment for caffeine and fiber intake (model 4), the association of magnesium with FG was attenuated [β = −0.001 mmol/L (95% CI: −0.006, 0.004), P = 0.78] (Table 2). However, magnesium intake remained significantly inversely associated with FI, although the magnitude of the association was mitigated [β = −0.012 ln-pmol/L (95% CI: −0.017, −0.007), P < 0.0001] (Table 2). Results of sensitivity analyses and meta-regressions did not substantively affect our conclusions, or reveal any clear sources of heterogeneity (results not shown).
TABLE 2.
Meta-analyzed associations between magnesium intake and fasting glucose and fasting insulin in 15 cohort studies
| Association2 of fasting glucose (mmol/L) per 50-mg/d increment in magnesium intake |
Association2 of fasting insulin (ln-pmol/L) per 50-mg/d increment in magnesium intake |
|||||||
| Model1 | n3 | β (95% CI) | P | I 2(95% CI) | n3 | β (95% CI) | P | I2 (95% CI) |
| 1 | 52,684 | −0.016(−0.019, −0.012) | <0.0001 | 50 (9, 72) | 37,640 | −0.028(−0.032, −0.024) | <0.0001 | 80 (68, 88) |
| 2 | 52,568 | −0.013(−0.017, −0.010) | <0.0001 | 20 (0, 56) | 37,527 | −0.023(−0.026, −0.020) | <0.0001 | 81 (69, 88) |
| 3 | 50,921 | −0.009(−0.013, −0.005) | <0.0001 | 30 (0, 62) | 36,271 | −0.020(−0.024, −0.017) | <0.0001 | 75 (59, 85) |
| 4 | 48,588 | −0.001(−0.006, 0.005) | 0.78 | 30 (0, 65) | 34,137 | −0.012(−0.017, −0.007) | <0.0001 | 46 (0, 73) |
Model 1 adjusted for age, sex, energy intake, study center (in Atherosclerosis Risk in Communities Study; Cardiovascular Health Study; Family Heart Study; Health, Aging, and Body Composition Study; Invecchiare in Chianti; Multi-Ethnic Study of Atherosclerosis), and family or population substructure (in Cardiovascular Health Study, Family Heart Study, Framingham Heart Study, Multi-Ethnic Study of Atherosclerosis, Cardiovascular Risk in Young Finns Study). Model 2 adjusted for model 1 with additional adjustment for BMI. Model 3 adjusted for model 2 with additional adjustment for smoking, education, physical activity, and alcohol intake. The Gene-Diet Attica Investigation on Childhood Obesity (child/adolescent cohort) did not adjust for smoking, education, or alcohol intake, because these variables are not applicable in this cohort. The Rotterdam Study did not adjust for physical activity, because this variable was not available in this cohort. Model 4 adjusted for model 3 with additional adjustment for fiber and caffeine. The Greek Health Randomized Aging Study, Prospective Investigation of the Vasculature in Uppsala Seniors, and Uppsala Longitudinal Study of Adult Men were excluded from model 4 analysis, because these cohorts did not have information on either fiber or caffeine intake.
β coefficient and 95% CI shown as β (95% CI).
The number of independent observations in each analysis.
FIGURE 1.
Forest plot of associations between dietary magnesium (50 mg/d) and fasting glucose (mmol/L) in 15 US and European cohort studies. The estimate from each cohort study, indicated by a filled square, was adjusted for age, sex, BMI, smoking, education, physical activity, alcohol intake, energy intake, study center (in ARIC, CHS, FamHS, HealthABC, InCHIANTI, MESA), and/or family or population substructure (in CHS, FamHS, FHS, MESA, YFS). GENDAI (child/adolescent cohort) did not adjust for smoking, education, or alcohol intake, because these variables were not applicable in this study. Rotterdam did not adjust for physical activity, because this variable was not available in the study. The size of the square is proportional to the weight of the cohort study in the overall fixed-effects estimate, and the horizontal line represents the 95% CI. The overall summary estimate and its 95% CI are indicated by the open diamond. ARIC, Atherosclerosis Risk in Communities Study; CHS, Cardiovascular Health Study; FamHS, Family Heart Study; FHS, Framingham Heart Study; GENDAI, Gene-Diet Attica Investigation on Childhood Obesity; GHRAS, Greek Health Randomized Aging Study, GLACIER, Gene-Lifestyle interactions And Complex traits Involved in Elevated disease Risk; HealthABC, Health, Aging, and Body Composition Study; InCHIANTI, Invecchiare in Chianti; MESA, Multi-Ethnic Study of Atherosclerosis; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors; ULSAM, Uppsala Longitudinal Study of Adult Men; YFS, Cardiovascular Risk in Young Finns Study.
FIGURE 2.
Forest plot of associations between dietary magnesium (50 mg/d) and fasting insulin (ln-pmol/L) in 15 US and European cohort studies. The estimate from each cohort study, indicated by a filled square, was adjusted for age, sex, BMI, smoking, education, physical activity, alcohol intake, energy intake, study center (in ARIC, CHS, FamHS, HealthABC, InCHIANTI, MESA), and/or family or population substructure (in CHS, FamHS, FHS, MESA, YFS). GENDAI (child/adolescent cohort) did not adjust for smoking, education, or alcohol intake, because these variables were not applicable in this study. Rotterdam did not adjust for physical activity, because this variable was not available in the study. The size of the square is proportional to the weight of the cohort study in the overall fixed-effects estimate, and the horizontal line represents the 95% CI. The overall summary estimate and its 95% CI are indicated by the open diamond. ARIC, Atherosclerosis Risk in Communities Study; CHS, Cardiovascular Health Study; FamHS, Family Heart Study; FHS, Framingham Heart Study; GENDAI, Gene-Diet Attica Investigation on Childhood Obesity; GHRAS, Greek Health Randomized Aging Study, GLACIER, Gene-Lifestyle interactions And Complex traits Involved in Elevated disease Risk; HealthABC, Health, Aging, and Body Composition Study; InCHIANTI, Invecchiare in Chianti; MESA, Multi-Ethnic Study of Atherosclerosis; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors; ULSAM, Uppsala Longitudinal Study of Adult Men; YFS, Cardiovascular Risk in Young Finns Study.
Associations of SNPs with FG and FI.
Meta-analyzed estimates of SNP associations with FG and FI are presented in Supplemental Table 2. The direction and magnitude of the associations of the 16 glucose- and 2 insulin-related SNPs on FG or FI, respectively, were consistent with those previously reported (17). The 8 SNPs related to magnesium homeostasis and transport showed no significant association with either FG or FI (Supplemental Table 2). A nominally significant inverse association with FG was observed per additional G allele at rs2274924 in TRPM6 [β = −0.013 (95% CI: −0.024, −0.001) mmol/L, P = 0.03]. Results of sensitivity analyses and meta-regressions did not substantively affect our conclusions, or reveal any clear sources of heterogeneity (results not shown).
Magnesium-SNP interactions on FG and FI.
Meta-analyzed estimates of interactions between magnesium intake and SNPs on FG and FI are presented in Table 3 (with additional information in Supplemental Figs. 3 and 4). There was no significant interaction on either FG or FI, after correction for multiple testing (i.e., at P < 0.0015). Two nominally significant interactions were observed between SNPs and magnesium on FG. The first at rs11558471 in SLC30A8 (previously associated with FG levels) suggested a stronger glucose-lowering association with higher magnesium intake in those with the A (risk) allele at this locus [interaction β = −0.0045 (95% CI: −0.0082, −0.0008) mmol/L per A allele and 50-mg/d increment in magnesium, P = 0.02]. The second at rs3740393 near CNNM2 (previously associated with magnesium transport/homeostasis) suggested a weaker inverse association between magnesium intake and FG in those with the G (risk) allele at this locus [interaction β = 0.0064 (95% CI: 0.0009, 0.0119) mmol/L per G allele with 50 mg/d higher magnesium, P = 0.02]. Results of sensitivity analyses and meta-regressions did not substantively affect our conclusions, or reveal any clear sources of heterogeneity (results not shown).
TABLE 3.
Meta-analyzed interactions between magnesium intake and SNPs on fasting glucose and fasting insulin in 15 cohort studies1
| SNP | Nearest gene | Coded allele | Other allele | Coded allele frequency | Number of cohorts | Interaction2 between 50-mg/d increment in magnesium intake × SNP on outcome |
|||
| n3 | β (95% CI) | P | I 2(95% CI) | ||||||
| Glucose-related SNPs | Outcome is fasting glucose (mmol/L) |
||||||||
| rs10830963 | MTNR1B | G | C | 0.28 | 15 | 51,257 | 0.0033 (−0.0006, 0.0072) | 0.10 | 47 (4, 71) |
| rs10885122 | ADRA2A | G | T | 0.89 | 15 | 52,068 | −0.0013 (−0.0064, 0.0038) | 0.64 | 15 (0, 53) |
| rs11071657 | C2CD4B | A | G | 0.62 | 15 | 52,098 | 0.0023 (−0.0012, 0.0058) | 0.21 | 0 (0, 35) |
| rs11558471 | SLC30A8 | A | G | 0.69 | 13 | 50,329 | −0.0045 (−0.0082, −0.0008) | 0.02 | 5 (0, 37) |
| rs11605924 | CRY2 | A | C | 0.48 | 15 | 52,264 | 0.0017 (−0.0016, 0.0050) | 0.30 | 41 (0, 68) |
| rs11708067 | ADCY5 | A | G | 0.78 | 14 | 50,829 | −0.0006 (−0.0047, 0.0035) | 0.78 | 12 (0, 51) |
| rs11920090 | SLC2A2 | T | A | 0.86 | 14 | 51,441 | −0.0019 (−0.0066, 0.0028) | 0.43 | 35 (0, 65) |
| rs174550 | FADS1 | T | C | 0.67 | 15 | 52,305 | −0.0022 (−0.0055, 0.0011) | 0.20 | 27 (0, 61) |
| rs2191349 | DGKB-TMEM195 | T | G | 0.53 | 15 | 52,241 | −0.0024 (−0.0057, 0.0009) | 0.14 | 31 (0, 63) |
| rs340874 | PROX1 | C | T | 0.53 | 14 | 51,449 | −0.0004 (−0.0037, 0.0029) | 0.81 | 12 (0, 50) |
| rs4506565 | TCF7L2 | T | A | 0.29 | 13 | 49,253 | −0.0001 (−0.0038, 0.0036) | 0.97 | 54 (14, 75) |
| rs4607517 | GCK | A | G | 0.17 | 15 | 52,470 | 0.0020 (−0.0023, 0.0063) | 0.35 | 0 (0, 22) |
| rs560887 | G6PC2 | C | T | 0.71 | 15 | 51,479 | 0.0026 (−0.0009, 0.0061) | 0.15 | 0 (0, 28) |
| rs7034200 | GLIS3 | A | C | 0.48 | 15 | 52,016 | 0.0026 (−0.0007, 0.0059) | 0.11 | 5 (0, 56) |
| rs780094 | GCKR | C | T | 0.39 | 15 | 52,442 | 0.0005 (−0.0028, 0.0038) | 0.74 | 41 (0, 68) |
| rs7944584 | MADD | A | T | 0.73 | 15 | 51,263 | 0.0017 (−0.0020, 0.0054) | 0.36 | 46 (2, 71) |
| Magnesium-related SNPs | |||||||||
| rs11144134 | TRPM6 | T | C | 0.92 | 10 | 29,978 | −0.0015 (−0.0091, 0.0061) | 0.70 | 41 (0, 72) |
| rs2274924 | TRPM6 | A | G | 0.84 | 10 | 29,280 | 0.0042 (−0.0013, 0.0097) | 0.12 | 0 (0, 46) |
| rs3740393 | CNNM2 | G | C | 0.85 | 11 | 30,904 | 0.0064 (0.0009, 0.0119) | 0.02 | 38 (0, 70) |
| rs3750425 | TRPM6 | G | A | 0.91 | 10 | 29,978 | 0.0052 (−0.0017, 0.0121) | 0.14 | 0 (0, 2) |
| rs4072037 | MUC1 | C | T | 0.45 | 11 | 30,905 | −0.0028 (−0.0069, 0.0013) | 0.17 | 31 (0, 66) |
| rs6746896 | CNNM4 | A | G | 0.67 | 11 | 30,210 | −0.0001 (−0.0042, 0.0040) | 0.95 | 16 (0, 56) |
| rs8042919 | TRPM7 | G | A | 0.90 | 11 | 30,905 | −0.0049 (−0.0116, 0.0018) | 0.15 | 4 (0, 62) |
| rs994430 | CNNM3 | A | T | 0.60 | 11 | 30,905 | −0.0004 (−0.0043, 0.0035) | 0.84 | 0 (0, 54) |
| Insulin-related SNPs | Outcome is fasting insulin (ln-pmol/L) |
||||||||
| rs35767 | IGF1 | G | A | 0.84 | 15 | 37,485 | 0.0031 (−0.0020, 0.0082) | 0.22 | 21 (0, 57) |
| rs780094 | GCKR | C | T | 0.58 | 15 | 37,804 | −0.0028 (−0.0063, 0.0007) | 0.12 | 36 (0, 66) |
| Magnesium-related SNPs | |||||||||
| rs11144134 | TRPM6 | T | C | 0.92 | 10 | 29,549 | −0.0004 (−0.0080, 0.0072) | 0.94 | 6 (0, 65) |
| rs2274924 | TRPM6 | A | G | 0.83 | 10 | 28,851 | 0.0041 (−0.0012, 0.0094) | 0.13 | 0 (0, 60) |
| rs3740393 | CNNM2 | G | C | 0.85 | 11 | 30,467 | 0.0013 (−0.0042, 0.0068) | 0.64 | 0 (0, 49) |
| rs3750425 | TRPM6 | G | A | 0.91 | 10 | 29,549 | 0.0046 (−0.0021, 0.0113) | 0.17 | 0 (0, 58) |
| rs4072037 | MUC1 | C | T | 0.45 | 11 | 30,468 | −0.0003 (−0.0044, 0.0038) | 0.88 | 27 (0, 64) |
| rs6746896 | CNNM4 | A | G | 0.68 | 11 | 29,773 | 0.0009 (−0.0030, 0.0048) | 0.65 | 6 (0, 63) |
| rs8042919 | TRPM7 | G | A | 0.90 | 11 | 30,468 | −0.0004 (−0.0071, 0.0063) | 0.91 | 0 (0, 58) |
| rs994430 | CNNM3 | A | T | 0.61 | 11 | 30,468 | 0.0004 (−0.0035, 0.0043) | 0.84 | 7 (0, 63) |
Additive allele model, adjusted for age, sex, total energy intake, study center (in Atherosclerosis Risk in Communities Study; Cardiovascular Health Study; Family Heart Study; Health, Aging, and Body Composition Study; Invecchiare in Chianti; Multi-Ethnic Study of Atherosclerosis), and family or population substructure (in Cardiovascular Health Study, Family Heart Study, Framingham Heart Study, Multi-Ethnic Study of Atherosclerosis, Cardiovascular Risk in Young Finns Study). SNP, single nucleotide polymorphism.
Interaction coefficient and 95% shown as β (95% CI).
The number of independent observations in each interaction analysis.
Discussion
In this cross-sectional meta-analysis involving >50,000 participants free of diabetes in 15 cohort studies from the CHARGE Consortium, we observed inverse associations between magnesium intake and FG and FI concentrations, even after adjustment for BMI and other demographic and lifestyle factors known to influence diabetes risk. After further adjustment for fiber and caffeine intake, the inverse association of magnesium with FI remained significant, but not the association with FG. However, including these dietary components in the model may be an overadjustment, reflecting common food sources, thus leading to the observed mitigated associations. Our study is among the largest, to our knowledge, to investigate these tightly controlled measures of glucose homeostasis in generally healthy populations. Our findings support those of recent meta-analyses of studies on magnesium and incident type 2 diabetes, which estimated ∼14% reduced risk of disease per daily 100-mg increment in magnesium intake (9, 10). Previous prospective cohort studies investigating whole-grain (28) and coffee (29–33) consumption on type 2 diabetes risk have observed beneficial associations with higher consumption. From a reductionist viewpoint, it remains of interest whether the whole foods themselves or their key components (e.g., magnesium or fiber in whole grains, or magnesium, caffeine, or other polyphenols in coffee) exert health benefits. Our observations suggest that the association between magnesium intake and FI is at least partly independent of other dietary constituents found in magnesium-containing foods, such as whole grains and coffee, a phenomenon previously observed in at least 2 smaller observational studies (2, 6). In prospective studies of magnesium intake and type 2 diabetes, associations of magnesium intake do not appear to be substantially affected after accounting for fiber intake (9). However, results of previous cross-sectional studies in adults free of diabetes are inconsistent with respect to magnesium’s associations with FG, irrespective of adjustment for fiber intake (6, 34, 35). Because blood glucose is generally under tight homeostatic control in diabetes-free populations, such as those included here, our observations lend support to the hypothesis of magnesium’s actions in insulin sensitivity and resistance with downstream, mitigating effects on diabetes pathogenesis (23).
Our findings on the associations of 16 glucose- and 2 insulin-related SNPs with FG and FI are in line with those previously reported (17). We also investigated 8 magnesium-related loci in relation to FG and FI, hypothesizing that if magnesium is causally related to these traits, genes that influence magnesium transport and homeostasis might be expected to affect FG and FI. However, we found no significant evidence that variation at these loci influences FG or FI. Our strongest, nominally significant association with FG was at rs2274924 (P = 0.03) in TRPM6, a gene encoding a magnesium-permeable epithelial channel with a critical role in magnesium reabsorption in the kidney. The missense mutation (A → G) at rs2274924 causes a Lys1584Glu amino acid change in exon 27 of the resulting channel protein. Nair et al. (19) recently reported that, in the presence of this polymorphism, the insulin signaling cascade is unable to activate the phosphorylation of the amino acid adjacent to the substituted amino acid resulting from the polymorphism, thereby rendering the variant TRPM6 channel insensitive to the activating effects of insulin. Furthermore, increased glycosylated hemoglobin and greater risk of gestational diabetes were observed in GG homozygotes compared to AA homozygotes in a cohort of 997 women (19). In contrast, rs2274924 was not associated with type 2 diabetes in 1 small case-control study in women (20). Consistent with other reports, we found no association of other previously studied TRPM6 or TRPM7 loci with either FG or FI (18, 21). Taken together, it is unlikely that the most of the loci we studied implicated in magnesium transport and homeostasis are meaningfully affecting fasting measures of glucose or insulin, with the possible suggestive exception of TRPM6 rs2274924 on glucose. Given recent evidence (19) and our cross-sectional approach, it is possible that this locus has deleterious downstream effects on intracellular magnesium and glucose handling secondary to the variant product’s reduced sensitivity to insulin. Regardless of whether these loci are themselves playing a direct role does not preclude the involvement of magnesium-dependent pathways in the regulation of glucose and insulin homeostasis.
We observed no significant interactions between magnesium intake and loci on FG or FI, suggesting that magnesium’s favorable associations with these traits are independent of genetic variation at the loci studied. Our strongest, albeit not significant, magnesium × SNP interaction on FG was at rs11558471 in SLC30A8, which we previously reported showed some evidence of interaction with total zinc intake on FG, although not below the multiple testing-corrected significance threshold in that study (P < 0.0025) (27). The interaction we report here with magnesium was in the same direction as that reported for zinc, which may reflect shared chemical properties of zinc and magnesium cations, or reflect similar affinity for these cations by the transmembrane transporter encoded by SLC30A8. The second nominally significant interaction with magnesium intake on FG was at rs3740393 near CNNM2. The gene encodes a membrane protein required for renal magnesium handling; the G allele at this locus is associated with lower serum magnesium (18). If replicated in future studies, the interaction suggests that the magnitude of the inverse association between magnesium intake and FG is diminished in the presence of the serum magnesium–lowering G allele, versus the C allele. This interaction may plausibly indicate a higher dietary magnesium requirement in those with a propensity for lower serum magnesium to observe beneficial effects on fasting glucose.
To date, the only other study examining interactions between magnesium intake and loci in TRPM6 and TRPM7 was a small case-control study of type 2 diabetes in predominantly white, older women followed for 10 y. The authors reported that women who were carriers of 2 rare alleles from nonsynonymous SNPs in TRPM6 (rs3750425 and rs2274924) had nearly 5 times the odds of type 2 diabetes when their magnesium intake was <250 mg/d (20). Despite our null interaction findings in relation to fasting glucose and insulin, we cannot rule out the possibility that in the presence of chronically low magnesium intake, these loci affect long-term risk of diabetes, which may not be reflected in the cross-sectional homeostatic measures analyzed in our study in individuals without known diabetes.
To our knowledge, this is one of the largest observational studies to investigate magnesium intake’s associations with FG and FI, and it is the largest meta-analysis investigating interactions between magnesium intake and risk loci on FG and FI. In addition to following a uniform, a priori analysis plan in each cohort, we used cross-cohort exposure, covariate, and outcome definitions in a consortium-based meta-analytic context that minimizes the typical recall and publication bias associated with literature-based meta-analyses (36). The favorable inverse associations we report between magnesium intake and FG and FI are consistent with other studies investigating similar relationships.
The loci for this analysis were selected a priori from those identified and replicated in previous GWAS meta-analyses. Inherent to the GWAS method is that emergent loci have a homogenous effect both within and across populations; that is, these are areas of no environmental interaction, despite potentially widely varying environmental exposures, such as diet (37). Gene × environment approaches such as ours that rely on prior GWAS therefore must overcome the limitations inherent to both the homogeneity and the relatively small effect sizes conveyed by these loci to determine whether the variants modify the effects of an environmental exposure (37). Although the clinical importance of interactions even smaller than those detectable [due to power (24)] in analyses such as ours may be limited, they nevertheless remain of considerable mechanistic interest. Despite our hypothesis, the glycemia- and magnesium-related loci we investigated may yet be implicated in pathways through which magnesium acts to ultimately affect diabetes risk. Short-term magnesium supplementation (500 mg/d) in healthy adults has been shown to up- and downregulate >50 genes involved in inflammatory and metabolic pathways, and magnesium regulation, as well as genomic regions with unknown function (38). Furthermore, magnesium’s interactions with loci to regulate insulin and glucose metabolism may be more evident in postchallenge measures of related traits, rather than the fasting traits used in our study (13, 39, 40). Therefore, our observations regarding the specific loci in the present study do not rule out the possibility that other genetic variants or genomic regions associated with glycemic traits interact with dietary magnesium.
In conclusion, our results indicate that higher dietary magnesium intake is inversely associated with FG and FI in individuals free of diabetes, generally irrespective of genetic variation at glycemia- and magnesium-related loci investigated. Nominal evidence for the influence of a TRPM6 locus on FG and for magnesium interaction with loci in SLC30A8 and CNNM2 on FG indicate that future research, including GWAS, is necessary and may reveal genomic regions that more strongly influence associations between magnesium intake and traits related to glucose homeostasis (37).
Supplementary Material
Acknowledgments
J.A.N. and N.M.M. conceived the present study. Cohort-specific contributions were as follows: L.D., K.M., D.S.S. (CHS), J.I.R. (MESA), I.B.B. (Family HS), L.A.C. (FHS), G.V.D. (GENDAI/GHRAS), C.P., M.Y. (GENDAI), P.W.F. (GLACIER), S.B.K., Y.L. (HealthABC), S.B., L.F. (InCHIANTI), M.O.-M. (Malmö), L.L., E.I. (PIVUS/ULSAM), A.G.U., A.H., O.H.F. (Rotterdam), T.L., O.T.R., J.V., V.M., and M.K. (YFS), cohort study design; J.A.N. (ARIC/MESA), D.S.S. (CHS), M.I.M. (GENDAI/GHRAS), J.I.R. (MESA), T.L., O.T.R., J.V., and M.K. (YFS), study-specific CHARGE-related participation; K.E.N., J.S.P., W.H.L.K. (ARIC), D.M. (CHS), I.B.B., M.K.W. (FamHS), L.A.C., P.F.J., J.B.M. (FHS), G.V.D., C.J.G., I.P., N.H., M.I.M. (GENDAI/GHRAS), I.N. (GENDAI), S.K., E.G. (GHRAS), F.B.H., G.H., I.J. (GLACIER), S.B. (InCHIANTI), M.O.-M. (Malmö), J.I.R. (MESA), U.R., A.-C.S. (PIVUS/ULSAM), A.G.U., J.C.M.W. (Rotterdam), T.L., O.T.R., J.V., V.M., and M.K. (YFS), study-specific dietary, phenotypic, and/or genotypic data collection and management; J.A.N. (ARIC/MESA), R.N.L. (CHS), M.K.W. (Family HS), L.A.C., J.S.N. (FHS), I.N. (GENDAI), S.K. (GHRAS), F.B.H., G.H., I.J., F.R. (GLACIER), D.K.H., K.K.L. (HealthABC), T.T. (InCHIANTI), E.S. (Malmö), A.M. (MESA), E.I., A.G. (PIVUS/ULSAM), F.J.A.v.R. (Rotterdam), and T.L. (YFS), study-specific analysis and/or analysis critical review. A.H. and J.S.N. conducted the primary and confirmatory meta-analyses, respectively; A.H. drafted the manuscript and generated tables and figures; and A.H., P.W.F., N.M.M., J.B.M., J.A.N., F.R., and M.K.W. revised and edited the manuscript (Writing Group). All authors reviewed and approved the final manuscript.
Footnotes
Abbreviations used: ARIC, Atherosclerosis Risk in Communities Study; CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology; CHS, Cardiovascular Health Study; FamHS, Family Heart Study; FG, fasting glucose; FHS, Framingham Heart Study; FI, fasting insulin; GENDAI, Gene-Diet Attica Investigation on Childhood Obesity; GHRAS, Greek Health Randomized Aging Study; GLACIER, Gene-Lifestyle interactions And Complex traits Involved in Elevated disease Risk; GWAS, genome-wide association study; HealthABC, Health, Aging, and Body Composition Study; InCHIANTI, Invecchiare in Chianti; MAGIC, Meta-Analyses of Glucose and Insulin-Related Traits Consortium; MESA, Multi-Ethnic Study of Atherosclerosis; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors; SNP, single nucleotide polymorphism; ULSAM, Uppsala Longitudinal Study of Adult Men; YFS, Cardiovascular Risk in Young Finns Study.
Literature Cited
- 1.Shils ME, Shike M. Modern nutrition in health and disease. Philadelphia: Lippincott, Williams, & Wilkins; 2006 [Google Scholar]
- 2.Fung TT, Manson JE, Solomon CG, Liu S, Willett WC, Hu FB. The association between magnesium intake and fasting insulin concentration in healthy middle-aged women. J Am Coll Nutr. 2003;22:533–8 [DOI] [PubMed] [Google Scholar]
- 3.Kim DJ, Xun P, Liu K, Loria C, Yokota K, Jacobs DR, He K. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care. 2010;33:2604–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphries S, Kushner H, Falkner B. Low dietary magnesium is associated with insulin resistance in a sample of young, nondiabetic Black Americans. Am J Hypertens. 1999;12:747–56 [DOI] [PubMed] [Google Scholar]
- 5.Ma B, Lawson AB, Liese AD, Bell RA, Mayer-Davis EJ. Dairy, magnesium, and calcium intake in relation to insulin sensitivity: approaches to modeling a dose-dependent association. Am J Epidemiol. 2006;164:449–58 [DOI] [PubMed] [Google Scholar]
- 6.Rumawas ME, McKeown NM, Rogers G, Meigs JB, Wilson PWF, Jacques PF. Magnesium intake is related to improved insulin homeostasis in the Framingham Offspring cohort. J Am Coll Nutr. 2006;25:486–92 [DOI] [PubMed] [Google Scholar]
- 7.Song Y, Manson JE, Buring JE, Liu S. Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes Care. 2004;27:59–65 [DOI] [PubMed] [Google Scholar]
- 8.He K, Liu K, Daviglus ML, Morris SJ, Loria CM, Van Horn L, Jacobs DR, Savage PJ. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation. 2006;113:1675–82 [DOI] [PubMed] [Google Scholar]
- 9.Dong J-Y, Xun P, He K, Qin L-Q. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care. 2011;34:2116–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson SC, Wolk A. Magnesium intake and risk of type 2 diabetes: a meta-analysis. J Intern Med. 2007;262:208–14 [DOI] [PubMed] [Google Scholar]
- 11.Guerrero-Romero F, Rodríguez-Morán M. Magnesium improves the beta-cell function to compensate variation of insulin sensitivity: double-blind, randomized clinical trial. Eur J Clin Invest. 2011;41:405–10 [DOI] [PubMed] [Google Scholar]
- 12.Hadjistavri LS, Sarafidis PA, Georgianos PI, Tziolas IM, Aroditis CP, Hitoglou-Makedou A, Zebekakis PE, Pikilidou MI, Lasaridis AN. Beneficial effects of oral magnesium supplementation on insulin sensitivity and serum lipid profile. Med Sci Monit. 2010;16:CR307–12 [PubMed] [Google Scholar]
- 13.Mooren FC, Krüger K, Völker K, Golf SW, Wadepuhl M, Kraus A. Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects—a double-blind, placebo-controlled, randomized trial. Diabetes Obes Metab. 2011;13:281–4 [DOI] [PubMed] [Google Scholar]
- 14.Guerrero-Romero F, Tamez-Perez HE, González-González G, Salinas-Martínez AM, Montes-Villarreal J, Treviño-Ortiz JH, Rodríguez-Morán M. Oral magnesium supplementation improves insulin sensitivity in non-diabetic subjects with insulin resistance: a double-blind placebo-controlled randomized trial. Diabetes Metab. 2004;30:253–8 [DOI] [PubMed] [Google Scholar]
- 15.Yokota K, Kato M, Lister F, Ii H, Hayakawa T, Kikuta T, Kageyama S, Tajima N. Clinical efficacy of magnesium supplementation in patients with type 2 diabetes. J Am Coll Nutr. 2004;23:506S–9S [DOI] [PubMed] [Google Scholar]
- 16.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in Type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med. 2006;23:1050–6 [DOI] [PubMed] [Google Scholar]
- 17.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer TE, Verwoert GC, Hwang S-J, Glazer NL, Smith AV, Van Rooij FJA, Ehret GB, Boerwinkle E, Felix JF, et al. ; The Genetic Factors for Osteoporosis (GEFOS) Consortium Meta-Analysis of Glucose and Insulin Related Traits Consortium (MAGIC): genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six loci influencing serum magnesium levels. PLoS Genet. 2010;6:e1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair AV, Hocher B, Verkaart S, Van Zeeland F, Pfab T, Slowinski T, Chen Y-P, Schlingmann KP, Schaller A, et al. Loss of insulin-induced activation of TRPM6 magnesium channels results in impaired glucose tolerance during pregnancy. Proc Natl Acad Sci USA. 2012;109:11324–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Y, Hsu Y-H, Niu T, Manson JE, Buring JE, Liu S. Common genetic variants of the ion channel transient receptor potential membrane melastatin 6 and 7 (TRPM6 and TRPM7), magnesium intake, and risk of type 2 diabetes in women. BMC Med Genet. 2009;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero JR, Castonguay AJ, Barton NS, Germer S, Martin M, Zee RYL. Gene variation of the transient receptor potential cation channel, subfamily M, members 6 (TRPM6) and 7 (TRPM7), and type 2 diabetes mellitus: a case-control study. Transl Res. 2010;156:235–41 [DOI] [PubMed] [Google Scholar]
- 22.Suárez A, Pulido N, Casla A, Casanova B, Arrieta FJ, Rovira A. Impaired tyrosine-kinase activity of muscle insulin receptors from hypomagnesaemic rats. Diabetologia. 1995;38:1262–70 [DOI] [PubMed] [Google Scholar]
- 23.Günther T. The biochemical function of Mg2+ in insulin secretion, insulin signal transduction and insulin resistance. Magnes Res. 2010;23:5–18 [DOI] [PubMed] [Google Scholar]
- 24.Nettleton JA, McKeown NM, Kanoni S, Lemaitre RN, Hivert M-F, Ngwa J, Van Rooij FJA, Sonestedt E, Wojczynski MK, et al. Interactions of dietary whole-grain intake with fasting glucose- and insulin-related genetic loci in individuals of European descent. Diabetes Care. 2010;33:2684–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta-analyses. In: Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. Higgins JPT, Green S, editors. The Cochrane Collaboration; 2011 [cited 2012 Oct 22]. Available from: www.cochrane-handbook.org. [Google Scholar]
- 27.Kanoni S, Nettleton JA, Hivert M-F, Ye Z, Van Rooij FJA, Shungin D, Sonestedt E, Ngwa JS, Wojczynski MK, et al. Total zinc intake may modify the glucose-raising effect of a zinc transporter (SLC30A8) variant. Diabetes. 2011;60:2407–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr. 2012;142:1304–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salazar-Martinez E, Willett WC, Ascherio A, Manson JE, Leitzmann MF, Stampfer MJ, Hu FB. Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med. 2004;140:1–8 [DOI] [PubMed] [Google Scholar]
- 30.van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, caffeine, and risk of type 2 diabetes. Diabetes Care. 2006;29:398–403 [DOI] [PubMed] [Google Scholar]
- 31.van Dam RM, Feskens EJ. Coffee consumption and risk of type 2 diabetes mellitus. Lancet. 2002;360:1477–8 [DOI] [PubMed] [Google Scholar]
- 32.Tuomilehto J. Coffee consumption and risk of type 2 diabetes mellitus among middle-aged Finnish men and women. JAMA. 2004;291:1213–9 [DOI] [PubMed] [Google Scholar]
- 33.Rosengren A, Dotevall A, Wilhelmsen L, Thelle D, Johansson S. Coffee and incidence of diabetes in Swedish women: a prospective 18-year follow-up study. J Intern Med. 2004;255:89–95 [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Folsom AR, Melnick SL, Eckfeldt JH, Sharrett AR, Nabulsi AA, Hutchinson RG, Metcalf PA. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. Atherosclerosis Risk in Communities Study. J Clin Epidemiol. 1995;48:927–40 [DOI] [PubMed] [Google Scholar]
- 35.Mirmiran P, Shab-Bidar S, Hosseini-Esfahani F, Asghari G, Hosseinpour-Niazi S, Azizi F. Magnesium intake and prevalence of metabolic syndrome in adults: Tehran Lipid and Glucose Study. Public Health Nutr. 2012;15(4):693–701 [DOI] [PubMed] [Google Scholar]
- 36.Palla L, Higgins JPT, Wareham NJ, Sharp SJ. Challenges in the use of literature-based meta-analysis to examine gene-environment interactions. Am J Epidemiol. 2010;171:1225–32 [DOI] [PubMed] [Google Scholar]
- 37.Franks PW. Gene × environment interactions in type 2 diabetes. Curr Diab Rep. 2011;11:552–61 [DOI] [PubMed] [Google Scholar]
- 38.Chacko SA, Sul J, Song Y, Li X, LeBlanc J, You Y, Butch A, Liu S. Magnesium supplementation, metabolic and inflammatory markers, and global genomic and proteomic profiling: a randomized, double-blind, controlled, crossover trial in overweight individuals. Am J Clin Nutr. 2011;93:463–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gow IF, O’Donnell M, Flint L, Flapan AD. Infusion of Mg in humans acutely reduces serum insulin levels: a pilot study. Magnes Res. 2011;24:189–95 [DOI] [PubMed] [Google Scholar]
- 40.Paolisso G, Sgambato S, Gambardella A, Pizza G, Tesauro P, Varricchio M, D’Onofrio F. Daily magnesium supplements improve glucose handling in elderly subjects. Am J Clin Nutr. 1992;55:1161–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.