Abstract
Background
Immunization against influenza is considered an essential public health intervention to control both seasonal epidemics and pandemic influenza. According to the World Health Organization (WHO), there are five key policy and three key programmatic issues that decision-makers should consider before introducing a vaccine. These are (a) public health priority, (b) disease burden, (c) efficacy, quality and safety of the vaccine, (d) other inventions, (e) economic and financial issues, (f) vaccine presentation, (g) supply availability and (h) programmatic strength. We analyzed the body of evidence currently available on these eight issues in the WHO Western Pacific Region.
Methodology/Principal Findings
Studies indexed in PubMed and published in English between 1 January 2000 and 31 December 2010 from the 37 countries and areas of the Western Pacific Region were screened for keywords pertaining to the five policy and three programmatic issues. Studies were grouped according to country income level and vaccine target group. There were 133 articles that met the selection criteria, with most (90%) coming from high-income countries. Disease burden (n = 34), vaccine efficacy, quality and safety (n = 27) and public health priority (n = 27) were most frequently addressed by studies conducted in the Region. Many studies assessed influenza vaccine policy and programmatic issues in the general population (42%), in the elderly (24%) and in children (17%). Few studies (2%) addressed the eight issues relating to pregnant women.
Conclusions/Significance
The evidence for vaccine introduction in countries and areas in this Region remains limited, particularly in low- and middle-income countries that do not currently have influenza vaccination programmes. Surveillance activities and specialized studies can be used to assess the eight issues including disease burden among vaccine target groups and the cost-effectiveness of influenza vaccine. Multi-country studies should be considered to maximize resource utilization for cross-cutting issues such as vaccine presentation and other inventions.
Introduction
The Western Pacific Region of the World Health Organization (WHO) comprises 37 countries and areas, spanning from China in the north and west, to New Zealand in the south, and to French Polynesia in the east [1]. One of the most diverse regions of WHO, the Western Pacific Region is home to approximately 1.6 billion people and includes highly developed countries as well as countries with rapidly emerging economies.
Awareness of the public health importance of influenza has increased in this Region in recent years, motivated by the emergence of highly pathogenic avian influenza A(H5N1) and subsequently by the occurrence of the A(H1N1)pdm09 pandemic. The Region currently has 21 National Influenza Centres (NICs) in 15 countries that monitor the impact and evolution of influenza viruses and inform global vaccine strain selection [2]. Influenza vaccination policies are established in 18 countries and areas, with another seven providing vaccine recommendations (unpublished data).
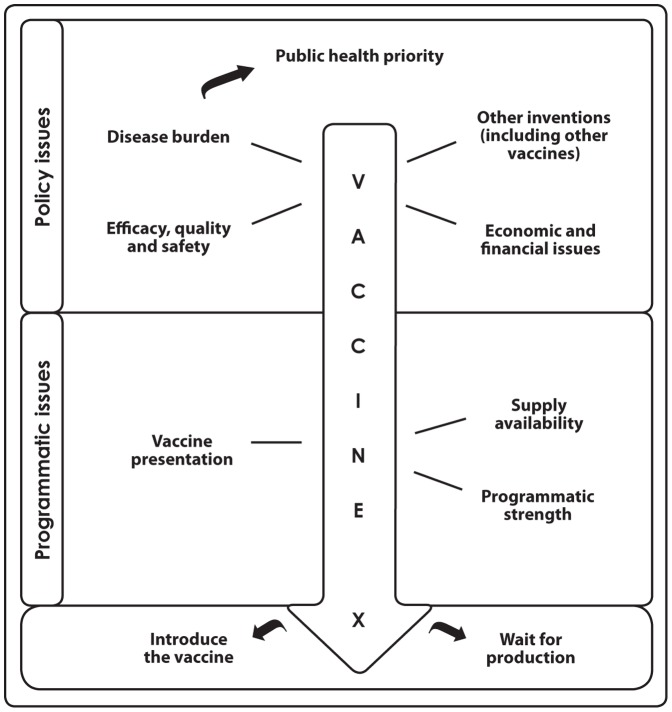
To assist countries with the development of vaccine policy, WHO published the Vaccine Introduction Guidelines in 2005 [3]. These guidelines highlight five key policy issues and three key programmatic issues that decision-makers should consider before introducing a vaccine (Figure 1). In 2012, WHO published new recommendations for the use of influenza vaccines [4]. WHO recommends that pregnant women should have the highest priority for influenza vaccination. Additional risk groups to be considered for vaccination, in no particular order of priority, are children aged 6–59 months, the elderly, individuals with specific chronic medical conditions, and health care workers.
Figure 1. Flowchart of key issues to be considered before vaccine introduction [Source: WHO, 3].
To better inform policy on influenza vaccine introduction in the Region, a literature review was conducted to summarize the body of evidence currently available on the eight policy and programmatic issues outlined in the WHO guidelines and in the context of the new recommendations for use of influenza vaccines. Although vaccine policy is developed and established by each country, reference to evidence from across the Region can support decision-making and identify future research needs that may be addressed collectively.
Materials and Methods
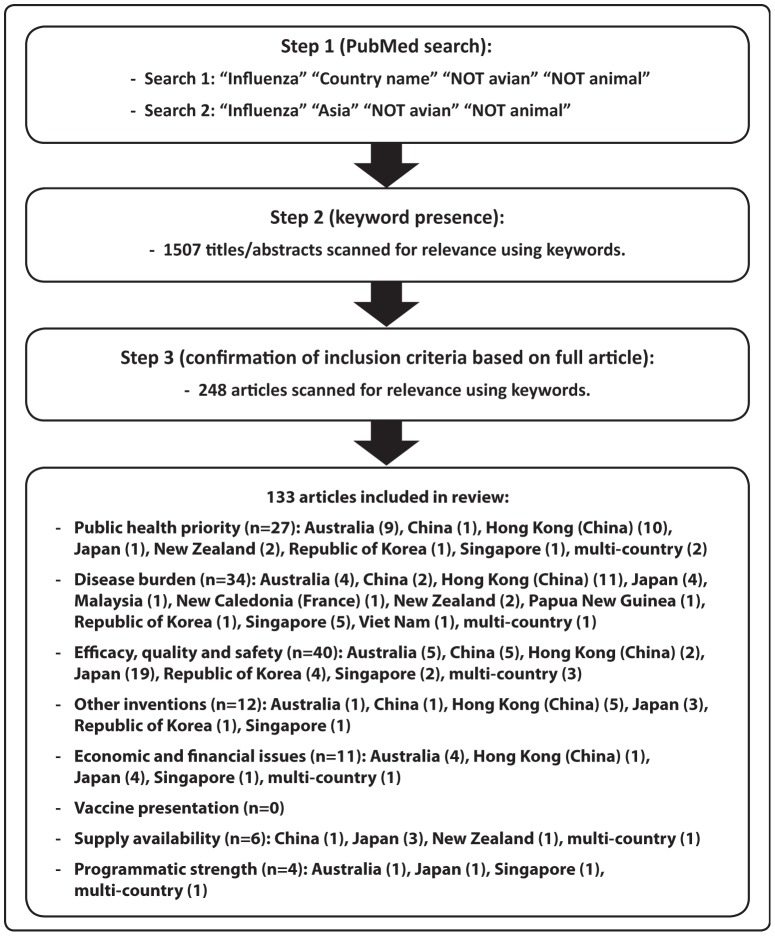
Data for this review were identified through a PubMed search and references from relevant articles. PubMed was used as it includes more than 19 million citations to biomedical literature from MEDLINE and life science journals, and it enabled the use of medical subject headings (MeSH) terms that are useful to explore publications based on key designated terms. Studies published in English between 1 January 2000 and 31 December 2010 from any of the 37 countries or areas of the Western Pacific Region [1] were included. The titles and abstracts of articles that included the search terms were screened for keywords pertaining to the five policy issues and three programmatic issues as per the WHO Vaccine Introduction Guidelines (Table 1), and the full articles that contained the keywords were collected and reviewed to confirm that the inclusion criteria were met (Figure 2).
Table 1. Keywords used to include studies in the literature review and to allocate studies to the relevant issue according to the WHO Vaccination Introduction Guidelines.
| Key issues as per the WHO Vaccine Introduction Guidelines | Keywords used to classify studies to these issues |
| Public health priority | Priority, perception, Millennium Development Goals |
| Disease burden | Burden, incidence, prevalence, hospitalization, impact, mortality, cost, deaths, epidemiology, characteristic, etiology |
| Efficacy, quality and safety | Vaccine, efficacy, quality, safety, effectiveness, adverse event, standards, clinical trial |
| Other inventions | Antiviral, non-pharmaceutical |
| Economic and financial issues | Economic, budget, finance, funding, sustainability, cost-effectiveness, affordability, fiscal impact |
| Vaccine presentation | Presentation, formulation, dose |
| Supply availability | Supply, availability, manufacture, procurement, introduction strategy |
| Programmatic strength | Delivery, National Immunization Programme |
| Key issues as per the WHO Vaccine Introduction Guidelines | Keywords used to classify studies to these issues |
| Public health priority | Priority, perception, Millennium Development Goals |
| Disease burden | Burden, incidence, prevalence, hospitalization, impact, mortality, cost, deaths, epidemiology, characteristic, etiology |
| Efficacy, quality and safety | Vaccine, efficacy, quality, safety, effectiveness, adverse event, standards, clinical trial |
| Other inventions | Antiviral, non-pharmaceutical |
| Economic and financial issues | Economic, budget, finance, funding, sustainability, cost-effectiveness, affordability, fiscal impact |
| Vaccine presentation | Presentation, formulation, dose |
| Supply availability | Supply, availability, manufacture, procurement, introduction strategy |
| Programmatic strength | Delivery, National Immunization Programme |
Figure 2. Methods to identify studies included in the literature review.
Studies were excluded if they (a) stated virus name but focused on other diseases, (b) stated virus name that incorporated the countries of interest but did not involve research in any Western Pacific Region country or area, or (c) were a publication of non-original research data such as outbreak news reports, editorials and reviews. Studies included in the final analysis were categorized, summarized and appraised according to the relevant key policy or programmatic issues [3] and were reported by the income level of the country or area (high versus low and middle income as based on the World Bank classifications [5]) and by the five target groups recommended by the WHO position paper for influenza vaccination as well as studies that focus on the general population [4].
Results
The PubMed search using designated terms returned 1507 articles, of which 133 met the selection criteria and were categorized according to the WHO Vaccine Introduction Guidelines key issues (Figure 2). These studies were from 11 countries or areas (Australia, China, Hong Kong [China], Japan, Malaysia, New Caledonia [France], New Zealand, Papua New Guinea, the Republic of Korea, Singapore and Viet Nam).
Public health priority
Public health priority comprises issues associated with prioritizing a particular vaccine over other competing public health issues. Twenty-seven studies focused on the prioritization of influenza vaccination within various target groups, of which 96% (n = 26) were from high-income countries (Table 2). Eight studies focused on health care workers; three assessed perceptions and vaccine uptake among health care workers [6]–[8], four on the need for further education for health care workers to increase their vaccine coverage rates [9]–[12] and one on their role in impacting the likelihood of influenza vaccination for the elderly [13]. Nine other studies assessed the factors that impact the likelihood of vaccination among the elderly [13]–[17], individuals with chronic conditions [18], [19] and pregnant women [20], [21].
Table 2. Number of studies conducted for the eight key issues, by country income classification and vaccination target groups, N = 133*.
| Key Issue (Number of studies) | Number of studies by country income | Number of studies by target group | ||||||
| High income | Low and middle income | Children under 5 years | Health care workers | Pregnant women | People with chronic conditions/who are immunocompromised | Elderly | General population | |
| Public health priority (N = 27) | 26 | 1 | 0 | 9 | 2 | 3 | 10 | 4 |
| Disease burden (N = 34) | ||||||||
| (a) Incidence or prevalence (n = 12) | 10 | 2 | 3 | 0 | 0 | 0 | 2 | 6 |
| (b) Hospitalization (n = 15) | 13 | 2 | 5 | 0 | 0 | 1 | 1 | 6 |
| (c) Mortality (n = 9) | 7 | 2 | 1 | 0 | 0 | 0 | 1 | 7 |
| Efficacy, quality and safety (N = 39) | ||||||||
| (a) Efficacy (n = 27) | 22 | 5 | 9 | 3 | 0 | 1 | 4 | 11 |
| (b) Effectiveness (n = 10) | 10 | 0 | 2 | 0 | 0 | 2 | 4 | 0 |
| (c) Safety (n = 9) | 7 | 2 | 2 | 2 | 0 | 0 | 2 | 4 |
| Other inventions (N = 12) | 11 | 1 | 0 | 0 | 0 | 0 | 0 | 12 |
| Economic and financial issues (N = 11) | 11 | 0 | 0 | 0 | 0 | 0 | 7 | 4 |
| Vaccine presentation (N = 0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Supply availability (N = 6) | 5 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Programmatic strength (N = 4) | 4 | 0 | 1 | 2 | 0 | 0 | 1 | 2 |
*Some studies report on more than one key issue or target group.
Research also assessed the normative beliefs that favour vaccination, the underlying health belief models and strategies for appealing to these beliefs, and the increasing accessibility to immunization in the community – among the general public [22], [23] and the elderly [24]–[26]. Five other studies focused on concerns held by the public [27] and risk groups such as the elderly [28], [29], health care workers [30] and cancer patients over vaccine safety, efficacy and development [31]. Lastly, one multi-country study assessed how influenza is perceived by the general population and which populations are perceived as appropriate vaccination targets [32].
Disease burden
Thirty-four studies described disease burden, which was expressed in terms of (a) incidence or prevalence, (b) hospitalization and (c) mortality. Only 15% (n = 5) of studies were from low- and middle-income countries (Table 2). Since disease burden forms the basis for vaccine-introduction policy, each of the studies identified through the literature review has been summarized and tabulated (see Tables S1, S2, S3).
(a) Incidence or prevalence
Incidence or prevalence of influenza was assessed in 12 studies (83% [n = 10] from high-income countries or areas), with a focus on the general population [33]–[38] and risk groups such as indigenous people [39], children [37], [40], [41], the elderly [36], [38] and military populations [42]. The relationship between the incidence of influenza and climate parameters such as rainfall and humidity was also explored for a number of cities in the Region [43]. One study focused on methods for enumerating disease incidence [44].
(b) Hospitalization
Fifteen studies assessed influenza-associated hospitalization (87% [n = 13] from high-income countries or areas), with most studies focusing on describing rates in the general population [45]–[50] and risk groups such as the elderly [48], individuals with chronic conditions [51], children [52]–[56] and indigenous populations [54], [57]. Two studies from Hong Kong (China) compared influenza hospitalization rates to those in temperate regions [50], [52]. One study assessed admissions to intensive care units [58], and another assessed the direct costs of hospitalization for influenza patients during the 2009 pandemic [59].
(c) Mortality
Nine studies assessed influenza-associated mortality (seven from high-income countries or areas) by measuring excess mortality due to seasonal influenza [50], [60]–[64] and novel pandemic influenza in the general population [46], comparing mortality rates to findings in temperate countries [50], [61], [63], or assessing the impact of influenza vaccination on child mortality [65] or elderly mortality [66].
Efficacy, quality and safety
Efficacy, quality and safety were addressed by 39 studies in three areas: (a) vaccine efficacy to ensure the vaccine prevents the disease in the immunized population; (b) vaccine effectiveness to ensure that protection is achieved under programmatic implementation in the target population; and (c) vaccine safety to ensure that the safety profile is well-understood and that the vaccine meets international standards of quality and safety.
(a) Efficacy
Twenty-seven studies assessed efficacy or immunogenicity of influenza vaccines, of which 19% (n = 5) were from low- and middle-income countries and 11% (n = 3) were multi-country studies (Table 2). For seasonal influenza, three studies assessed vaccine efficacy in the general population [67]–[69], eight studies in children [68], [70]–[76], four studies in elderly populations [68], [77]–[79], two in health care workers [80], [81] and one in immunocompromised haemodialysis patients [82].
Nine additional studies assessed the immunogenicity of A(H1N1)pdm09 vaccines in the general population [83]–[89], in children [90] and in health care workers [91]. These studies were conducted in Australia, China, Hong Kong (China), Japan, the Republic of Korea and Singapore. One study conducted in Hong Kong (China) assessed the immunogenicity of pre-pandemic H5N1 vaccines in the general population [92]. Lastly, a qualitative study reported on the dangers of intentional misinterpretation of vaccine efficacy data by anti-vaccination campaigners, including the impact on vaccination rates and doctors' willingness to recommend vaccination [93].
(b) Effectiveness
Ten studies, all conducted in high-income countries and 80% (n = 8) in Japan, assessed influenza vaccine effectiveness (Table 2). Studies evaluated vaccine effectiveness in children [94], [95], the elderly [96]–[99] and the immunocompromised [95], [100]. Three studies reported on methods for estimating vaccine effectiveness including using routine surveillance data [101], school-based rapid diagnostic testing [102] and online surveys [103].
(c) Safety
Nine studies assessed influenza vaccine safety, of which only 22% (n = 2) were from low- and middle-income countries (Table 2). Four studies evaluated adverse events associated with seasonal influenza vaccines; one was conducted in children [76], one in health care workers [104], one in the general population [68] and two in the elderly [68], [77]. Pandemic A(H1N1)pdm09 vaccines were assessed for their safety in the general population [83], [105], in health care workers [106] and in children [90]. The pre-pandemic AS03-adjuvanted H5N1 vaccine was assessed for its safety profile in one study in the general population [92].
Other inventions
Twelve studies on other inventions were conducted in the Region, of which only 8% (n = 1) was from a low- and middle-income country (Table 2). All the studies were conducted in the general population. Research was conducted on hand hygiene [107], [108], infection control [109], facemask use [108], [110], school closure [111], [112], physical exercise [113], alternative therapies including tea catechin [114], passive immunotherapy for management of severe cases of influenza infection [115] and use of traditional medicine [116]. One modelling study assessed methods to improve the effectiveness of different antiviral strategies [117], and another evaluated combinations of methods including enhanced surveillance with isolation, segregation and personal protective equipment to limit influenza transmission in closed environments [118].
Economic and financial issues
Eleven studies, all from high-income countries, assessed the cost-effectiveness or economic efficiency for seasonal and pre-pandemic influenza vaccine (Table 2). Seven studies focused on the elderly, of which four evaluated the cost-effectiveness of seasonal influenza vaccination for the elderly [119]–[122], two assessed the cost-effectiveness of combining influenza and pneumococcal vaccination compared to influenza vaccination alone [120], [123], and two assessed the impact of subsidizing the cost of vaccination [124], [125]. One study from Hong Kong (China) assessed the cost-effectiveness of vaccine including subsidy in the general population [126]. One multi-country study assessed vaccine coverage in relation to gross national income per capita as well as the impact of increasing income and education on coverage rates [32]. Two studies assessed the factors that impact cost-effectiveness of pre-pandemic and pandemic vaccine including vaccine strain match, availability and cost [127], [128].
Vaccine presentation
No studies met the criteria for vaccine presentation, formulation or dosage.
Supply availability
Six studies examined influenza vaccine supply and production issues (Table 2). The five studies conducted by individual countries reviewed national vaccine needs and production capacities [129], [130] including for pregnant women [131] and people with chronic conditions [131] (Table 2), discussed challenges of having limited suppliers for seasonal influenza vaccine and the potential implications of supply disruption [132], and presented plans for safety tests for pre-pandemic candidate vaccines to inform vaccine introduction policy [133]. A multi-country study assessed global vaccine usage and an increase in uptake between 1994 and 2003 [134].
Programmatic strength
Four studies, all from high-income countries, assessed programmatic strengths to deliver influenza vaccine to the target populations (Table 2). Two studies assessed methods to improve the uptake of influenza vaccination by hospital-based health care workers, including conducting onsite vaccination clinics [135] and providing senior management support for vaccination [136]. One study described lessons learnt for increasing vaccination rates among high-risk groups, including likely hindrances such as the vocal anti-vaccination campaign and the reporting of improperly conducted vaccine efficacy studies [137]. The fourth study was a multi-country study that assessed influenza vaccination coverage rates among adults, the elderly and children [32].
Discussion
Immunization against influenza is considered an essential public health intervention to control both seasonal epidemics and pandemic influenza [138]. In the Western Pacific Region, published literature on influenza vaccine policy and programmatic issues is mostly limited to countries with existing influenza vaccination programmes. Of the 11 countries and areas with studies included in this report, eight reported that seasonal influenza vaccine was available through both government funding and private market purchase, two reported vaccine was available through private market purchase only (Singapore and Viet Nam) and one reported the lack of any vaccination programme (Papua New Guinea, unpublished data).
Most research conducted in the Region focused on the general population and the elderly. Future work should consider other target groups recommended for influenza vaccine by WHO, especially pregnant women since this group is deemed a priority [4].
The perception of the public and the medical community on influenza disease and vaccines is a significant factor in determining if vaccine introduction is a priority [3]. No research was found that linked influenza to national health priorities, Millennium Development Goals or national decision-making groups that may be relevant to countries considering vaccine-introduction policy.
Defining disease burden is key to providing the rationale for vaccine introduction. However, although 34 disease burden studies were identified, the majority were conducted in countries and areas that have already established influenza vaccination policy (Australia, Hong Kong [China], Japan, New Zealand and Singapore). As many countries in the Region have not yet published data on influenza disease burden, future work is needed to understand the burden in different populations and different risk groups. Most studies analysed data arising from influenza surveillance systems that linked case counts to laboratory findings and hospitalization and mortality registries. Many countries in the Region have sentinel surveillance systems for influenza, which can be used to inform disease burden. Surveillance system data not only inform the decision to introduce vaccine, but also enable evaluation and continued measurement of vaccine impact. Options for determining disease burden include (a) utilizing data from countries of similar social and demographic characteristics, (b) deriving estimates from mathematical modelling and (c) conducting active surveillance studies. Specialized and targeted studies would be most useful in countries with limited laboratory access [3].
Research on vaccine efficacy, effectiveness and safety in the Region adds evidence that influenza vaccines are safe and provide adequate protection when matched to circulating strains. The body of evidence provided in these areas, which included both seasonal and pandemic influenza vaccination, was mainly from high-income countries, with Japan conducting the majority of studies on vaccine effectiveness. Studies on adverse events from influenza vaccination have been conducted in three of the target groups for vaccination, namely children, health care workers and the elderly. Despite the evidence presented, studies from more countries in the Region are needed to determine the efficacy, effectiveness and safety of influenza vaccines in different populations and settings and to assess their suitability in different countries across the Region. Since research in this area is resource-intensive, enhanced cross-country collaboration and public–private partnerships, as well as ongoing participation in the Global Influenza Surveillance and Response System (GISRS), are recommended [139].
Some studies focused on other public health measures as alternatives to vaccination. The effectiveness of these other mechanisms should be determined and shared to support the exploration of such public health measures being considered in other regions.
The body of published literature on the economic and financial aspects of influenza vaccine policy is very limited. None of the countries in the Region currently without influenza vaccination policy conducted research on the potential costs and benefits of adding influenza vaccine to their schedule or the potential impact it would have on limited national health budgets. Assessing the economic and financial implications of new vaccines should be considered carefully so that decision-makers can assess (a) the cost-effectiveness relative to other uses of scare resources, (b) the long-term resource requirements, (c) the funding gaps and whether additional domestic or external funding could be mobilized, and (d) the potential financial sustainability of the new vaccine [3].
A Global Action Plan was developed to increase seasonal vaccine use, increase production capacity and enable research and development [140]. There is evidence that vaccine production capacity is increasing globally [138]. In 2009, countries of the Western Pacific Region were projected to produce 23% (133 million doses) of the 573 million doses of seasonal trivalent vaccine produced globally [138]. Yet, as influenza vaccine production increases globally and more countries are considering introducing influenza vaccine policy [134], [138], [140], gaps in knowledge remain about country-level preferences for vaccine formulations and dosing, vaccination delivery systems, the additional resources required and the marketing component required to promote vaccine uptake.
This literature review had some limitations. First, only studies published in English were considered, which may have excluded a large volume of research in national languages, especially research that is nationally relevant but of limited international interest, such as programmatic issues. Second, the literature review searched only for studies indexed by PubMed, which may have underestimated the volume of research and excluded research on socio-behavioural or operational research, which are more likely to be indexed by other databases or in “grey” literature [141]. Nevertheless, PubMed is of value as it reflects the knowledge that is widely available for shared learning nationally and internationally.
In conclusion, the evidence for vaccine introduction in countries and areas in the Western Pacific Region remains limited, particularly in low- and middle-income countries that do not currently have influenza vaccination programmes. Few countries have conducted analyses of disease burden that provide the basis for vaccine introduction policy. To move forward, countries with influenza surveillance systems, especially those with National Influenza Centres carrying out virological surveillance, may consider utilizing surveillance activities and specialized studies to assess key issues, including identification of risk groups, seasonal trends and costs of influenza, and the cost-effectiveness of influenza vaccine. Importantly, a number of multi-country studies were conducted in the Region over the past 10 years, and opportunities for further collaboration should be explored to maximize resource utilization.
Supporting Information
Studies reporting on influenza incidence and prevalence.
(DOCX)
Studies reporting on influenza-associated hospitalization.
(DOCX)
Studies reporting on influenza-associated mortality.
(DOCX)
Acknowledgments
The authors would like to thank Nikki Angcao for assistance with the literature search, and Marc-Alain Widdowson and Anne Moen for their review of the manuscript.
Funding Statement
The authors have no funding or support to report.
References
- 1.World Health Organization (2005) Countries and areas. Available: www.wpro.who.int/countries/list.htm. Accessed 2011 November 23.
- 2.World Health Organization (2010) National Influenza Centres. Available: www.who.int/csr/disease/influenza/centres/en/index.html. Accessed 2011 March 15.
- 3.World Health Organization (2005) Vaccine Introduction Guidelines. Adding a vaccine to a national immunization programme: decision and implementation. Available: http://www.who.int/vaccines-documents/DoxGen/H3DoxList.htm. Accessed 2011 November 1.
- 4. World Health Organization (2012) Vaccines against influenza WHO position paper - November 2012. Releve epidemiologique hebdomadaire/Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record/Health Section of the Secretariat of the League of Nations 87: 461–476. [PubMed] [Google Scholar]
- 5.World Bank (2011) Country classifications Available: http://data.worldbank.org/about/country-classifications/country-and-lending-groups. Accessed 2011 March 15.
- 6. Seale H, Leask J, MacIntyre CR (2011) Attitudes amongst Australian hospital healthcare workers towards seasonal influenza and vaccination. Influenza Other Respi Viruses 4: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murray SB, Skull SA (2002) Poor health care worker vaccination coverage and knowledge of vaccination recommendations in a tertiary Australia hospital. Aust N Z J Public Health 26: 65–68. [DOI] [PubMed] [Google Scholar]
- 8. Seale H, Wang Q, Yang P, Dwyer DE, Wang X, et al. Influenza vaccination amongst hospital health care workers in Beijing. Occup Med (Lond) 60: 335–339. [DOI] [PubMed] [Google Scholar]
- 9. Ridda I, Lindley IR, Gao Z, McIntyre P, Macintyre CR (2008) Differences in attitudes, beliefs and knowledge of hospital health care workers and community doctors to vaccination of older people. Vaccine 26: 5633–5640. [DOI] [PubMed] [Google Scholar]
- 10. Brunton C, Weir R, Jennings L (2005) Knowledge and attitudes about influenza vaccination amongst general practitioners, practice nurses, and people aged 65 and over. N Z Med J 118: U1434. [PubMed] [Google Scholar]
- 11. Ward K, Seale H, Zwar N, Leask J, Macintyre CR (2011) Annual influenza vaccination: coverage and attitudes of primary care staff in Australia. Influenza Other Respi Viruses 5: 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong SY, Wong EL, Chor J, Kung K, Chan PK, et al. (2010) Willingness to accept H1N1 pandemic influenza vaccine: a cross-sectional study of Hong Kong community nurses. BMC infectious diseases 10: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lau JT, Yang X, Tsui HY, Kim JH (2006) Prevalence of influenza vaccination and associated factors among community-dwelling Hong Kong residents of age 65 or above. Vaccine 24: 5526–5534. [DOI] [PubMed] [Google Scholar]
- 14. Mok E, Yeung SH, Chan MF (2006) Prevalence of influenza vaccination and correlates of intention to be vaccinated among Hong Kong Chinese. Public Health Nurs 23: 506–515. [DOI] [PubMed] [Google Scholar]
- 15. Kee SY, Lee JS, Cheong HJ, Chun BC, Song JY, et al. (2007) Influenza vaccine coverage rates and perceptions on vaccination in South Korea. J Infect 55: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curry E, Kerr N, Yang J, Briggs S (2006) Influenza immunisation rate for 2005 and factors associated with receiving this vaccine in patients aged 65 years and over admitted to a general medical ward at Auckland City Hospital. N Z Med J 119: U2254. [PubMed] [Google Scholar]
- 17. Kwong EW, Lam IO, Chan TM (2009) What factors affect influenza vaccine uptake among community-dwelling older Chinese people in Hong Kong general outpatient clinics? J Clin Nurs 18: 960–971. [DOI] [PubMed] [Google Scholar]
- 18. Tan EK, Lim LH, Teoh YL, Ong G, Bock HL Influenza and seasonal influenza vaccination among diabetics in Singapore: knowledge, attitudes and practices. Singapore Med J 51: 623–630. [PubMed] [Google Scholar]
- 19. Takahashi O, Noguchi Y, Rahman M, Shimbo T, Goto M, et al. (2003) Influence of family on acceptance of influenza vaccination among Japanese patients. Fam Pract 20: 162–166. [DOI] [PubMed] [Google Scholar]
- 20. Lau JT, Cai Y, Tsui HY, Choi KC (2010) Prevalence of influenza vaccination and associated factors among pregnant women in Hong Kong. Vaccine 28: 5389–5397. [DOI] [PubMed] [Google Scholar]
- 21. White SW, Petersen RW, Quinlivan JA (2010) Pandemic (H1N1) 2009 influenza vaccine uptake in pregnant women entering the 2010 influenza season in Western Australia. Med J Aust 193: 405–407. [DOI] [PubMed] [Google Scholar]
- 22. Siu W Fear appeals and public service advertising: applications to influenza in Hong Kong. Health Commun 25: 580. [DOI] [PubMed] [Google Scholar]
- 23. Seale H, Heywood AE, McLaws ML, Ward KF, Lowbridge CP, et al. Why do I need it? I am not at risk! Public perceptions towards the pandemic (H1N1) 2009 vaccine. BMC Infect Dis 10: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kwong EW, Pang SM, Choi PP, Wong TK (2010) Influenza vaccine preference and uptake among older people in nine countries. J Adv Nurs 66: 2297–2308. [DOI] [PubMed] [Google Scholar]
- 25. Gill TK, Taylor AW, Watson M (2007) Trends in influenza immunisation amongst an elderly Australian community. Vaccine 25: 5428–5432. [DOI] [PubMed] [Google Scholar]
- 26. Kwong EW, Lam IO (2008) Chinese older people in Hong Kong: health beliefs about influenza vaccination. Nurs Older People 20: 29–33. [DOI] [PubMed] [Google Scholar]
- 27. Lau JT, Yeung NC, Choi KC, Cheng MY, Tsui HY, et al. (2009) Acceptability of A/H1N1 vaccination during pandemic phase of influenza A/H1N1 in Hong Kong: population based cross sectional survey. Bmj 339: b4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lau JT, Kim JH, Yang X, Tsui HY (2008) Cross-sectional and longitudinal factors predicting influenza vaccination in Hong Kong Chinese elderly aged 65 and above. J Infect 56: 460–468. [DOI] [PubMed] [Google Scholar]
- 29. Gill T, Taylor AW, Kempe A, Pickering S, Watson M (2005) Prevalence of influenza vaccination in South Australian aged care homes. Aust N Z J Public Health 29: 38–43. [DOI] [PubMed] [Google Scholar]
- 30. Chor JS, Ngai KL, Goggins WB, Wong MC, Wong SY, et al. (2009) Willingness of Hong Kong healthcare workers to accept pre-pandemic influenza vaccination at different WHO alert levels: two questionnaire surveys. Bmj 339: b3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crawford NW, Heath JA, Ashley D, Downie P, Buttery JP (2010) Survivors of childhood cancer: an Australian audit of vaccination status after treatment. Pediatr Blood Cancer 54: 128–133. [DOI] [PubMed] [Google Scholar]
- 32. de Lataillade C, Auvergne S, Delannoy I (2009) 2005 and 2006 seasonal influenza vaccination coverage rates in 10 countries in Africa, Asia Pacific, Europe, Latin America and the Middle East. J Public Health Policy 30: 83–101. [DOI] [PubMed] [Google Scholar]
- 33. Puzelli S, Boros S, Affinito C, Calzoletti L, Facchini M, et al. (2006) Prevalence of antibodies against A and B influenza viruses in South-Western Papua New Guinea. J Med Virol 78: 820–824. [DOI] [PubMed] [Google Scholar]
- 34. Leo YS, Lye DC, Barkham T, Krishnan P, Seow E, et al. (2010) Pandemic (H1N1) 2009 surveillance and prevalence of seasonal influenza, Singapore. Emerg Infect Dis 16: 103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Phuong HL, Nga TT, van Doornum GJ, Groen J, Binh TQ, et al. (2010) Viral respiratory tract infections among patients with acute undifferentiated fever in Vietnam. Southeast Asian J Trop Med Public Health 41: 1116–1126. [PubMed] [Google Scholar]
- 36. Ng TP, Pwee KH, Niti M, Goh LG (2002) Influenza in Singapore: assessing the burden of illness in the community. Ann Acad Med Singapore 31: 182–188. [PubMed] [Google Scholar]
- 37. Wada T, Morishima T, Okumura A, Tashiro M, Hosoya M, et al. (2009) Differences in clinical manifestations of influenza-associated encephalopathy by age. Microbiol Immunol 53: 83–88. [DOI] [PubMed] [Google Scholar]
- 38. Huang QS, Lopez LD, McCallum L, Adlam B (2008) Influenza surveillance and immunisation in New Zealand, 1997–2006. Influenza Other Respi Viruses 2: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Flint SM, Davis JS, Su JY, Oliver-Landry EP, Rogers BA, et al. (2010) Disproportionate impact of pandemic (H1N1) 2009 influenza on Indigenous people in the Top End of Australia's Northern Territory. Med J Aust 192: 617–622. [DOI] [PubMed] [Google Scholar]
- 40. Nelson EA, Tam JS, Yu LM, Li AM, Chan PK, et al. (2007) Assessing disease burden of respiratory disorders in Hong Kong children with hospital discharge data and linked laboratory data. Hong Kong Med J 13: 114–121. [PubMed] [Google Scholar]
- 41. Tang JW, Lai FY, Wong F, Hon KL (2010) Incidence of common respiratory viral infections related to climate factors in hospitalized children in Hong Kong. Epidemiol Infect 138: 226–235. [DOI] [PubMed] [Google Scholar]
- 42. Seah SG, Lim EA, Kok-Yong S, Liaw JC, Lee V, et al. (2010) Viral agents responsible for febrile respiratory illnesses among military recruits training in tropical Singapore. J Clin Virol 47: 289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tang JW, Lai FY, Nymadawa P, Deng YM, Ratnamohan M, et al. (2010) Comparison of the incidence of influenza in relation to climate factors during 2000–2007 in five countries. J Med Virol 82: 1958–1965. [DOI] [PubMed] [Google Scholar]
- 44. Lau EH, Cowling BJ, Ho LM, Leung GM (2008) Optimizing use of multistream influenza sentinel surveillance data. Emerg Infect Dis 14: 1154–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mermond S, Berlioz-Arthaud A, Estivals M, Baumann F, Levenes H, et al. (2010) Aetiology of community-acquired pneumonia in hospitalized adult patients in New Caledonia. Trop Med Int Health 15: 1517–1524. [DOI] [PubMed] [Google Scholar]
- 46. Yu H, Feng L, Peng Z, Feng Z, Shay DK, et al. (2009) Estimates of the impact of a future influenza pandemic in China. Influenza Other Respi Viruses 3: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beard F, McIntyre P, Gidding H, Watson M (2006) Influenza related hospitalisations in Sydney, New South Wales, Australia. Arch Dis Child 91: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yap FH, Ho PL, Lam KF, Chan PK, Cheng YH, et al. (2004) Excess hospital admissions for pneumonia, chronic obstructive pulmonary disease, and heart failure during influenza seasons in Hong Kong. J Med Virol 73: 617–623. [DOI] [PubMed] [Google Scholar]
- 49. Wong CM, Yang L, Chan KP, Leung GM, Chan KH, et al. (2006) Influenza-associated hospitalization in a subtropical city. PLoS Med 3: e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li CK, Choi BC, Wong TW (2006) Influenza-related deaths and hospitalizations in Hong Kong: a subtropical area. Public Health 120: 517–524. [DOI] [PubMed] [Google Scholar]
- 51. Tomizuka T, Takayama Y, Shobayashi T, Fukushima Y, Suzuki Y (2010) Underlying medical conditions and hospitalization for pandemic (H1N1) 2009, Japan. Emerging infectious diseases 16: 1646–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chiu SS, Lau YL, Chan KH, Wong WH, Peiris JS (2002) Influenza-related hospitalizations among children in Hong Kong. N Engl J Med 347: 2097–2103. [DOI] [PubMed] [Google Scholar]
- 53. Chiu SS, Chan KH, Chen H, Young BW, Lim W, et al. (2009) Virologically confirmed population-based burden of hospitalization caused by influenza A and B among children in Hong Kong. Clin Infect Dis 49: 1016–1021. [DOI] [PubMed] [Google Scholar]
- 54. D'Onise K, Raupach JC (2008) The burden of influenza in healthy children in South Australia. Med J Aust 188: 510–513. [DOI] [PubMed] [Google Scholar]
- 55. Kwong KL, Lung D, Wong SN, Que TL, Kwong NS (2009) Influenza-related hospitalisations in children. J Paediatr Child Health 45: 660–664. [DOI] [PubMed] [Google Scholar]
- 56. Shin SY, Kim JH, Kim HS, Kang YA, Lee HG, et al. (2010) Clinical characteristics of Korean pediatric patients critically ill with influenza A (H1N1) virus. Pediatr Pulmonol 45: 1014–1020. [DOI] [PubMed] [Google Scholar]
- 57. Verrall A, Norton K, Rooker S, Dee S, Olsen L, et al. (2010) Hospitalizations for pandemic (H1N1) 2009 among Maori and Pacific Islanders, New Zealand. Emerg Infect Dis 16: 100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Drennan K, Hicks P, Hart G (2010) Impact of pandemic (H1N1) 2009 on Australasian critical care units. Crit Care Resusc 12: 223–229. [PubMed] [Google Scholar]
- 59. Ong MP, Sam IC, Azwa H, Mohd Zakaria IE, Kamarulzaman A, et al. (2010) High direct healthcare costs of patients hospitalised with pandemic (H1N1) 2009 influenza in Malaysia. The Journal of infection 61: 440–442. [DOI] [PubMed] [Google Scholar]
- 60. Lee VJ, Yap J, Ong JB, Chan KP, Lin RT, et al. (2009) Influenza excess mortality from 1950–2000 in tropical Singapore. PLoS One 4: e8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chow A, Ma S, Ling AE, Chew SK (2006) Influenza-associated deaths in tropical Singapore. Emerg Infect Dis 12: 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. He J, Gu D, Wu X, Reynolds K, Duan X, et al. (2005) Major causes of death among men and women in China. N Engl J Med 353: 1124–1134. [DOI] [PubMed] [Google Scholar]
- 63. Wong CM, Chan KP, Hedley AJ, Peiris JS (2004) Influenza-associated mortality in Hong Kong. Clin Infect Dis 39: 1611–1617. [DOI] [PubMed] [Google Scholar]
- 64. Yang L, Wong CM, Chan KP, Chau PY, Ou CQ, et al. (2009) Seasonal effects of influenza on mortality in a subtropical city. BMC Infect Dis 9: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sugaya N, Takeuchi Y (2005) Mass vaccination of schoolchildren against influenza and its impact on the influenza-associated mortality rate among children in Japan. Clin Infect Dis 41: 939–947. [DOI] [PubMed] [Google Scholar]
- 66. Reichert TA, Sugaya N, Fedson DS, Glezen P, Simonsen L, et al. (2001) The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med 344: 889–896. [DOI] [PubMed] [Google Scholar]
- 67. Takahashi H, Tanaka Y, Ohyama T, Sunagawa T, Nakashima K, et al. (2001) Evaluation of a mass influenza vaccination campaign. Jpn J Infect Dis 54: 184–188. [PubMed] [Google Scholar]
- 68. Zhu FC, Zhou W, Pan H, Lu L, Gerez L, et al. (2008) Safety and immunogenicity of two subunit influenza vaccines in healthy children, adults and the elderly: a randomized controlled trial in China. Vaccine 26: 4579–4584. [DOI] [PubMed] [Google Scholar]
- 69. Kawai N, Ikematsu H, Iwaki N, Satoh I, Kawashima T, et al. (2003) A prospective, Internet-based study of the effectiveness and safety of influenza vaccination in the 2001-2002 influenza season. Vaccine 21: 4507–4513. [DOI] [PubMed] [Google Scholar]
- 70. Ono S, Kudo M, Aoki K, Ezaki F, Misumi J (2003) Effect of mass immunization against influenza encephalopathy on mortality rates in children. Pediatr Int 45: 680–687. [DOI] [PubMed] [Google Scholar]
- 71. Sasaki Y, Kusuhara K, Saito M, Hikino S, Murayama Y, et al. (2006) Serum immunoglobulin levels do not affect antibody responses to influenza HA vaccine in preterm infants. Vaccine 24: 2208–2212. [DOI] [PubMed] [Google Scholar]
- 72. Kamada M, Nagai T, Kumagai T, Igarashi M, Ihara T, et al. (2006) Efficacy of inactivated trivalent influenza vaccine in alleviating the febrile illness of culture-confirmed influenza in children in the 2000–2001 influenza season. Vaccine 24: 3618–3623. [DOI] [PubMed] [Google Scholar]
- 73. Block SL, Toback SL, Yi T, Ambrose CS (2009) Efficacy of a single dose of live attenuated influenza vaccine in previously unvaccinated children: a post hoc analysis of three studies of children aged 2 to 6 years. Clin Ther 31: 2140–2147. [DOI] [PubMed] [Google Scholar]
- 74. Sugimura T, Ito Y, Tananari Y, Ozaki Y, Maeno Y, et al. (2008) Improved antibody responses in infants less than 1 year old using intradermal influenza vaccination. Vaccine 26: 2700–2705. [DOI] [PubMed] [Google Scholar]
- 75. Tam JS, Capeding MR, Lum LC, Chotpitayasunondh T, Jiang Z, et al. (2007) Efficacy and safety of a live attenuated, cold-adapted influenza vaccine, trivalent against culture-confirmed influenza in young children in Asia. Pediatr Infect Dis J 26: 619–628. [DOI] [PubMed] [Google Scholar]
- 76. Nolan T, Richmond PC, McVernon J, Skeljo MV, Hartel GF, et al. (2009) Safety and immunogenicity of an inactivated thimerosal-free influenza vaccine in infants and children. Influenza Other Respi Viruses 3: 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Deguchi Y, Nishimura K (2001) Efficacy of Influenza Vaccine in Elderly Persons in Welfare Nursing Homes: Reduction in Risks of Mortality and Morbidity During an Influenza A (H3N2) Epidemic. J Gerontol A Biol Sci Med Sci 56: M391–394. [DOI] [PubMed] [Google Scholar]
- 78. Hung IF, Leung AY, Chu DW, Leung D, Cheung T, et al. (2010) Prevention of acute myocardial infarction and stroke among elderly persons by dual pneumococcal and influenza vaccination: a prospective cohort study. Clin Infect Dis 51: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 79. Miyagawa K, Hayashi Y, Kurihara S, Maeda A (2008) Co-administration of l-cystine and l-theanine enhances efficacy of influenza vaccination in elderly persons: nutritional status-dependent immunogenicity. Geriatr Gerontol Int 8: 243–250. [DOI] [PubMed] [Google Scholar]
- 80. Di B, Xu X, Li T, Lu E, Wu J, et al. (2010) Immunogenicity associated with the routine use of an influenza A H1N1 vaccine in health care personnel in Guangzhou, China. Clin Vaccine Immunol 17: 1478–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kheok SW, Chong CY, McCarthy G, Lim WY, Goh KT, et al. (2008) The efficacy of influenza vaccination in healthcare workers in a tropical setting: a prospective investigator blinded observational study. Ann Acad Med Singapore 37: 465–469. [PubMed] [Google Scholar]
- 82. Song JY, Cheong HJ, Ha SH, Kee SY, Jeong HW, et al. (2006) Active influenza immunization in hemodialysis patients: comparison between single-dose and booster vaccination. Am J Nephrol 26: 206–211. [DOI] [PubMed] [Google Scholar]
- 83. Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 375: 56–66. [DOI] [PubMed] [Google Scholar]
- 84. Wu J, Zhong X, Li CK, Zhou JF, Lu M, et al. (2011) Optimal vaccination strategies for 2009 pandemic H1N1 and seasonal influenza vaccines in humans. Vaccine 29: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 85. Cheong HJ, Song JY, Heo JY, Noh JY, Choi WS, et al. (2011) Immunogenicity and safety of influenza A (H1N1) 2009 monovalent inactivated split vaccine in Korea. Vaccine 29: 523–527. [DOI] [PubMed] [Google Scholar]
- 86. Cowling BJ, Ng S, Ma ES, Cheng CK, Wai W, et al. (2010) Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus infection during 2009 in Hong Kong. Clin Infect Dis 51: 1370–1379. [DOI] [PubMed] [Google Scholar]
- 87. Liu W, de Vlas SJ, Tang F, Ma MJ, Wei MT, et al. (2010) Clinical and immunological characteristics of patients with 2009 pandemic influenza A (H1N1) virus infection after vaccination. Clin Infect Dis 51: 1028–1032. [DOI] [PubMed] [Google Scholar]
- 88. Ikematsu H, Nagai H, Kawashima M, Kawakami Y, Tenjinbaru K, et al. (2010) Immunogenicity and safety of a novel AS03(A)-adjuvanted H1N1 2009 pandemic influenza vaccine in adults in Japan. Hum Vaccin 6: 888–893. [DOI] [PubMed] [Google Scholar]
- 89. Lee VJ, Tay JK, Chen MI, Phoon MC, Xie ML, et al. (2010) Inactivated trivalent seasonal influenza vaccine induces limited cross-reactive neutralizing antibody responses against 2009 pandemic and 1934 PR8 H1N1 strains. Vaccine 28: 6852–6857. [DOI] [PubMed] [Google Scholar]
- 90. Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, et al. (2009) Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. Jama 303: 37–46. [DOI] [PubMed] [Google Scholar]
- 91. Igari H, Segawa S, Watanabe A, Suzuki A, Watanabe M, et al. (2010) Immunogenicity of a monovalent pandemic influenza A H1N1 vaccine in health-care workers of a university hospital in Japan. Microbiol Immunol 54: 618–624. [DOI] [PubMed] [Google Scholar]
- 92. Chu DW, Hwang SJ, Lim FS, Oh HM, Thongcharoen P, et al. (2009) Immunogenicity and tolerability of an AS03(A)-adjuvanted prepandemic influenza vaccine: a phase III study in a large population of Asian adults. Vaccine 27: 7428–7435. [DOI] [PubMed] [Google Scholar]
- 93. Hirota Y (2008) Ecological fallacy and scepticism about influenza vaccine efficacy in Japan: the Maebashi study. Vaccine 26: 6473–6476. [DOI] [PubMed] [Google Scholar]
- 94. Ochiai H, Fujieda M, Ohfuji S, Fukushima W, Kondo K, et al. (2009) Inactivated influenza vaccine effectiveness against influenza-like illness among young children in Japan–with special reference to minimizing outcome misclassification. Vaccine 27: 7031–7035. [DOI] [PubMed] [Google Scholar]
- 95. Gotoh K, Ito Y, Suzuki E, Kaneko K, Kiuchi T, et al. (2011) Effectiveness and safety of inactivated influenza vaccination in pediatric liver transplant recipients over three influenza seasons. Pediatr Transplant 15: 112–116. [DOI] [PubMed] [Google Scholar]
- 96. Hara M, Sakamoto T, Tanaka K (2006) Effectiveness of influenza vaccination in preventing influenza-like illness among community-dwelling elderly: population-based cohort study in Japan. Vaccine 24: 5546–5551. [DOI] [PubMed] [Google Scholar]
- 97. Hara M, Tanaka K, Kase T, Maeda A, Hirota Y (2010) Evaluation of seasonal influenza vaccination effectiveness based on antibody efficacy among the institutionalized elderly in Japan. Vaccine 28: 5664–5668. [DOI] [PubMed] [Google Scholar]
- 98. Deguchi Y, Takasugi Y, Nishimura K (2000) Vaccine effectiveness for influenza in the elderly in welfare nursing homes during an influenza A (H3N2) epidemic. Epidemiol Infect 125: 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ozasa K, Kawahito Y, Doi T, Watanabe Y, Washio M, et al. (2006) Retrospective assessment of influenza vaccine effectiveness among the non-institutionalized elderly population in Japan. Vaccine 24: 2537–2543. [DOI] [PubMed] [Google Scholar]
- 100. Otsuka T, Fujinaka H, Katsuyama K, Iizawa M, Kinoshita S, et al. (2007) Influenza vaccination for severely multiply handicapped persons/children in the 2005–2006 season. Vaccine 25: 4521–4524. [DOI] [PubMed] [Google Scholar]
- 101. Kelly H, Carville K, Grant K, Jacoby P, Tran T, et al. (2009) Estimation of influenza vaccine effectiveness from routine surveillance data. PLoS One 4: e5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yamaguchi S, Ohfuji S, Hirota Y (2010) Influenza vaccine effectiveness in primary school children in Japan: a prospective cohort study using rapid diagnostic test results. J Infect Chemother 16: 407–413. [DOI] [PubMed] [Google Scholar]
- 103. Carlson SJ, Durrheim DN, Dalton CB (2010) Flutracking provides a measure of field influenza vaccine effectiveness, Australia, 2007–2009. Vaccine 28: 6809–6810. [DOI] [PubMed] [Google Scholar]
- 104. Lee CS, Lee KH, Jung MH, Lee HB (2008) Rate of influenza vaccination and its adverse reactions seen in health care personnel in a single tertiary hospital in Korea. Jpn J Infect Dis 61: 457–460. [PubMed] [Google Scholar]
- 105. Mahajan D, Roomiani I, Gold MS, Lawrence GL, McIntyre PB, et al. (2010) Annual report: surveillance of adverse events following immunisation in Australia, 2009. Commun Dis Intell 34: 259–276. [PubMed] [Google Scholar]
- 106. Park SW, Lee JH, Kim ES, Kwak YG, Moon CS, et al. (2011) Adverse events associated with the 2009 H1N1 influenza vaccination and the vaccination coverage rate in health care workers. Am J Infect Control 39: 69–71. [DOI] [PubMed] [Google Scholar]
- 107. Park JH, Cheong HK, Son DY, Kim SU, Ha CM (2010) Perceptions and behaviors related to hand hygiene for the prevention of H1N1 influenza transmission among Korean university students during the peak pandemic period. BMC Infect Dis 10: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cowling BJ, Chan KH, Fang VJ, Cheng CK, Fung RO, et al. (2009) Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med 151: 437–446. [DOI] [PubMed] [Google Scholar]
- 109. Dan YY, Tambyah PA, Sim J, Lim J, Hsu LY, et al. (2009) Cost-effectiveness analysis of hospital infection control response to an epidemic respiratory virus threat. Emerg Infect Dis 15: 1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cheng VC, Tai JW, Wong LM, Chan JF, Li IW, et al. (2009) Prevention of nosocomial transmission of swine-origin pandemic influenza virus A/H1N1 by infection control bundle. J Hosp Infect 74: 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sasaki A, Hoen AG, Ozonoff A, Suzuki H, Tanabe N, et al. (2009) Evidence-based tool for triggering school closures during influenza outbreaks, Japan. Emerg Infect Dis 15: 1841–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cowling BJ, Lau EH, Lam CL, Cheng CK, Kovar J, et al. (2008) Effects of school closures, 2008 winter influenza season, Hong Kong. Emerg Infect Dis 14: 1660–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wong CM, Lai HK, Ou CQ, Ho SY, Chan KP, et al. (2008) Is exercise protective against influenza-associated mortality? PLoS One 3: e2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yamada H, Takuma N, Daimon T, Hara Y (2006) Gargling with tea catechin extracts for the prevention of influenza infection in elderly nursing home residents: a prospective clinical study. J Altern Complement Med 12: 669–672. [DOI] [PubMed] [Google Scholar]
- 115. Wu JT, Lee CK, Cowling BJ, Yuen KY (2010) Logistical feasibility and potential benefits of a population-wide passive-immunotherapy program during an influenza pandemic. Proc Natl Acad Sci U S A 107: 3269–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lu CJ, Luo Y, Zhou H, Qin XL, Chen BJ, et al. (2010) A preliminary study on the medical expenditure of Chinese medicine and integrative medicine treatment for influenza A (H1N1) in the fever clinics. Chin J Integr Med 16: 493–497. [DOI] [PubMed] [Google Scholar]
- 117. Kelso JK, Halder N, Milne GJ (2010) The impact of case diagnosis coverage and diagnosis delays on the effectiveness of antiviral strategies in mitigating pandemic influenza A/H1N1 2009. PLoS One 5: e13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lee VJ, Yap J, Cook AR, Chen MI, Tay JK, et al. (2010) Effectiveness of public health measures in mitigating pandemic influenza spread: a prospective sero-epidemiological cohort study. J Infect Dis 202: 1319–1326. [DOI] [PubMed] [Google Scholar]
- 119. Newall AT, Scuffham PA, Kelly H, Harsley S, Macintyre CR (2008) The cost-effectiveness of a universal influenza vaccination program for adults aged 50–64 years in Australia. Vaccine 26: 2142–2153. [DOI] [PubMed] [Google Scholar]
- 120. Cai L, Uchiyama H, Yanagisawa S, Kamae I (2006) Cost-effectiveness analysis of influenza and pneumococcal vaccinations among elderly people in Japan. Kobe J Med Sci 52: 97–109. [PubMed] [Google Scholar]
- 121. Hoshi SL, Kondo M, Honda Y, Okubo I (2007) Cost-effectiveness analysis of influenza vaccination for people aged 65 and over in Japan. Vaccine 25: 6511–6521. [DOI] [PubMed] [Google Scholar]
- 122. Mogasale V, Barendregt J (2011) Cost-effectiveness of influenza vaccination of people aged 50–64 years in Australia: results are inconclusive. Aust N Z J Public Health 35: 180–186. [DOI] [PubMed] [Google Scholar]
- 123. Kelly H, Attia J, Andrews R, Heller RF (2004) The number needed to vaccinate (NNV) and population estimates of the NNV: comparison of influenza and pneumococcal vaccine programmes for people aged 65 years and over. Vaccine 22: 2192–2198. [DOI] [PubMed] [Google Scholar]
- 124. Ohkusa Y (2005) Policy evaluation for the subsidy for influenza vaccination in elderly. Vaccine 23: 2256–2260. [DOI] [PubMed] [Google Scholar]
- 125. Kondo M, Hoshi SL, Okubo I (2009) Does subsidy work? Price elasticity of demand for influenza vaccination among the elderly in Japan. Health Policy 91: 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Fitzner KA, Shortridge KF, McGhee SM, Hedley AJ (2001) Cost-effectiveness study on influenza prevention in Hong Kong. Health Policy 56: 215–234. [DOI] [PubMed] [Google Scholar]
- 127. Newall AT, Wood JG, Oudin N, MacIntyre CR (2010) Cost-effectiveness of pharmaceutical-based pandemic influenza mitigation strategies. Emerg Infect Dis 16: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lee VJ, Tok MY, Chow VT, Phua KH, Ooi EE, et al. (2009) Economic analysis of pandemic influenza vaccination strategies in Singapore. PLoS One 4: e7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Feng L, Mounts AW, Feng Y, Luo Y, Yang P, et al. (2010) Seasonal influenza vaccine supply and target vaccinated population in China, 2004–2009. Vaccine 28: 6778–6782. [DOI] [PubMed] [Google Scholar]
- 130. Gronvall GK, Borio LL (2006) Removing barriers to global pandemic influenza vaccination. Biosecur Bioterror 4: 168–175. [DOI] [PubMed] [Google Scholar]
- 131. Morimoto T, Ishikawa H (2010) Evaluation of vaccination strategies to suppress a novel influenza pandemic using an individual-based model. Nihon Eiseigaku Zasshi 65: 459–466. [DOI] [PubMed] [Google Scholar]
- 132.Blackmore T (2005) Sole supply of influenza vaccine: economic common sense or a disaster waiting to happen? N Z Med J 118: : U1596; discussion U1601. [PubMed] [Google Scholar]
- 133. Masuda M, Sugita S, Kuroda K, Nishimura H (2009) H5N1 influenza vaccination policy in Japan. Lancet Infect Dis 9: 266–267. [DOI] [PubMed] [Google Scholar]
- 134. The Macroepidemiology of Influenza Vaccination (MIV) Study Group (2005) The macro-epidemiology of influenza vaccination in 56 countries, 1997–2003. Vaccine 23: 5133–5143. [DOI] [PubMed] [Google Scholar]
- 135. Lee HY, Fong YT (2007) On-site influenza vaccination arrangements improved influenza vaccination rate of employees of a tertiary hospital in Singapore. Am J Infect Control 35: 481–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ballestas T, McEvoy SP, Doyle J (2009) Co-ordinated approach to healthcare worker influenza vaccination in an area health service. J Hosp Infect 73: 203–209. [DOI] [PubMed] [Google Scholar]
- 137. Hirota Y, Kaji M (2008) History of influenza vaccination programs in Japan. Vaccine 26: 6451–6454. [DOI] [PubMed] [Google Scholar]
- 138. Partridge J, Kieny MP (2010) Global production of seasonal and pandemic (H1N1) influenza vaccines in 2009–2010 and comparison with previous estimates and global action plan targets. Vaccine 28: 4709–4712. [DOI] [PubMed] [Google Scholar]
- 139.World Health Organization (2005) Global Influenza Surveillance and Response System (GISRS) Available: http://www.who.int/influenza/gisrs_laboratory/en/. Accessed 2011 October 20.
- 140.World Health Organization (2011) Global action plan for influenza vaccines. Available: www.who.int/influenza_vaccines_plan/objectives/en/. Accessed 2012 January 7.
- 141. Gonzalez-Block MA (2004) Health policy and systems research agendas in developing countries. Health Res Policy Syst 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Studies reporting on influenza incidence and prevalence.
(DOCX)
Studies reporting on influenza-associated hospitalization.
(DOCX)
Studies reporting on influenza-associated mortality.
(DOCX)