Abstract
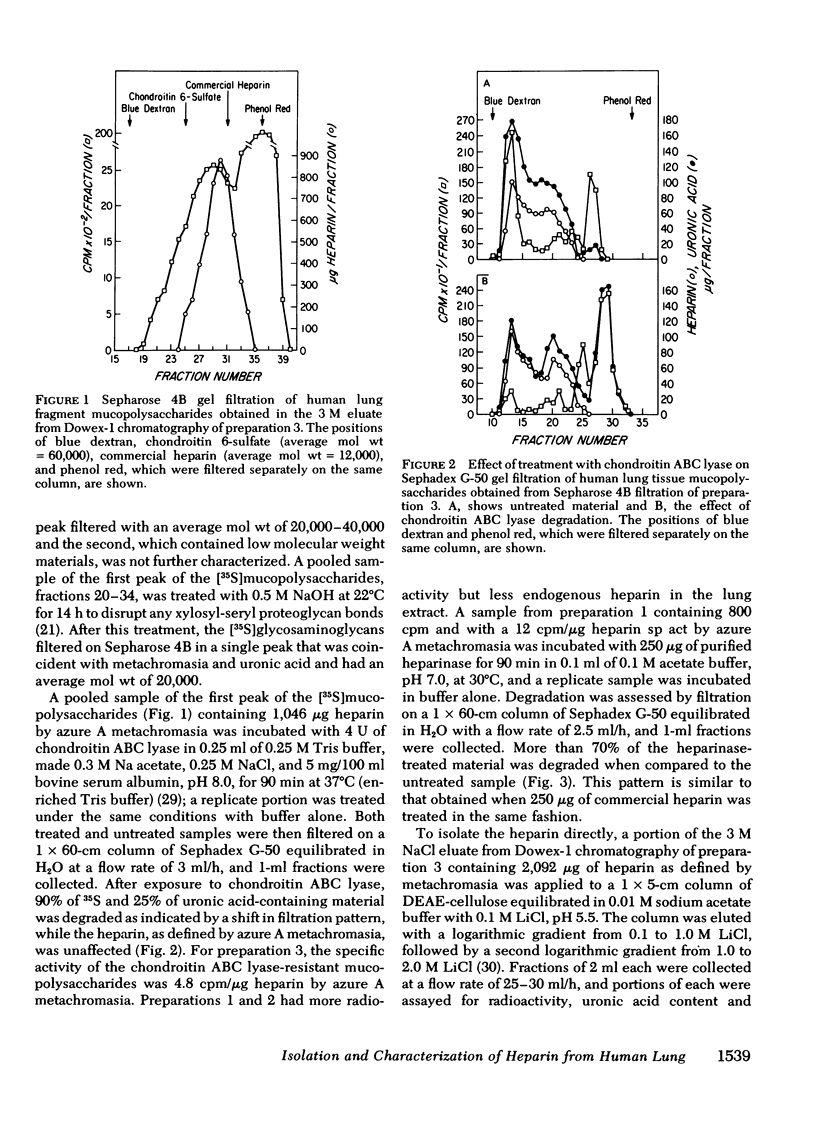
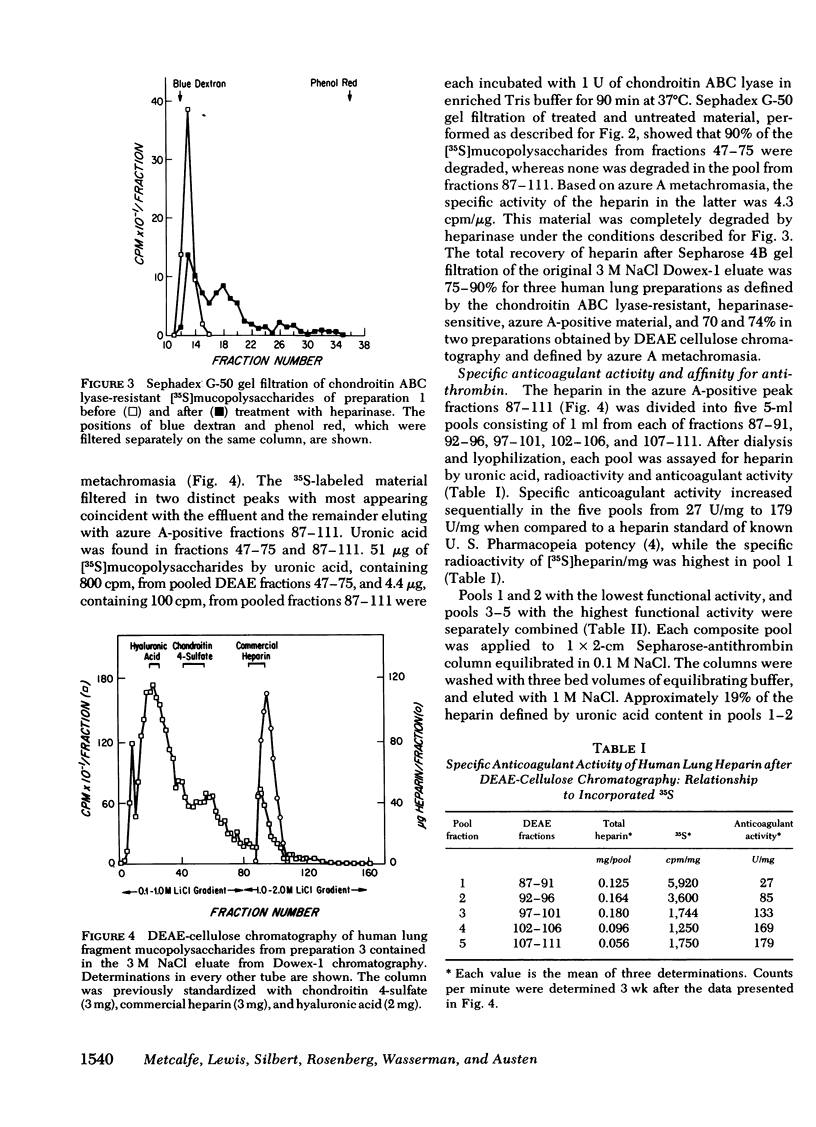
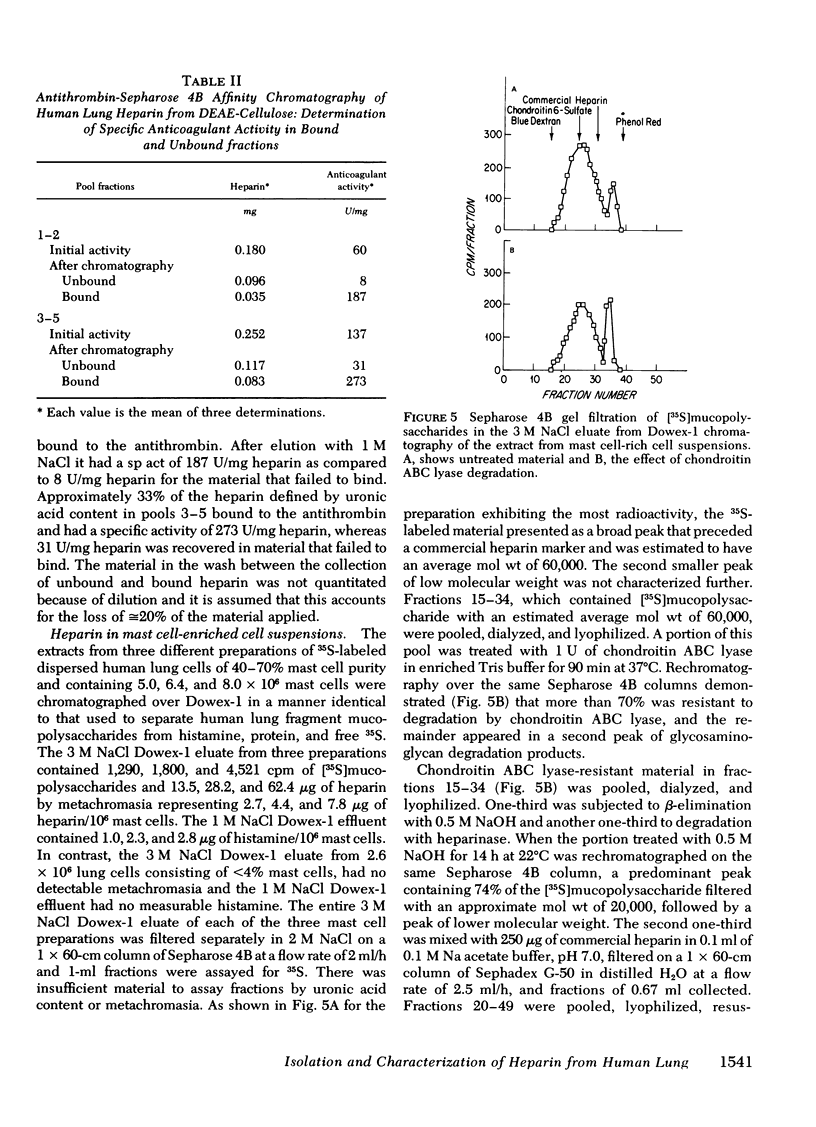
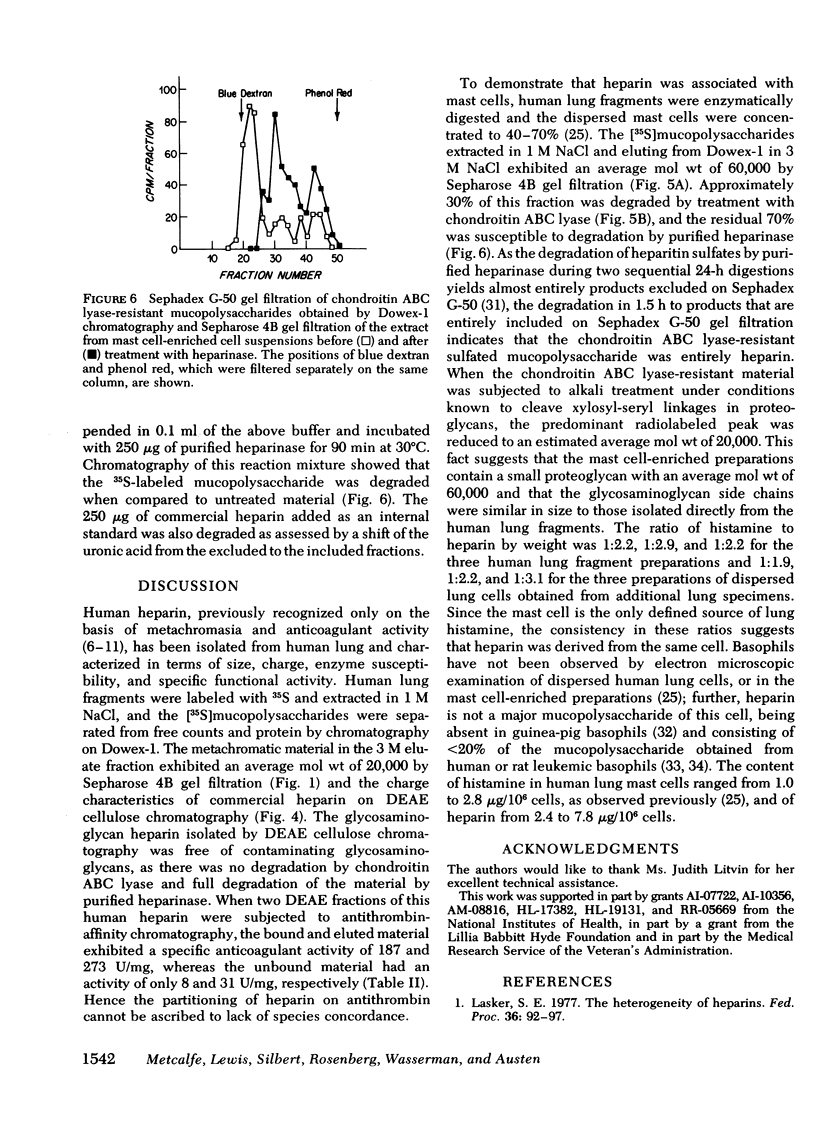
Heparin as measured by azure A metachromasia and anticoagulant activity has been extracted with 1 M NaCl from 35S-labeled human lung fragments or dispersed human lung cells enriched for mast cells. The 35S-labeled metachromatic material in the 3 M NaCl eluate from Dowex-1 chromatography of the extract from lung fragments exhibited an average mol wt of 20,000 by Sepharose 4B gel filtration. The 35S-labeled metachromatic material with the charge characteristics of commercial porcine heparin on DEAE cellulose chromatography was entirely heparin by the criteria of resistance to degradation by chondroitin ABC lyase and complete degradation by purified heparinase. Antithrombin affinity chromatography of purified heparin with an anticoagulant activity of 137 U/mg, revealed that the one-third that was bound and eluted had a 273 U/mg sp act, whereas the unbound activity was 31 U/mg. Thus, the previously observed heterogeneity of commercial porcine heparin for binding to human antithrombin was also observed with human heparin. The mast cell-enriched human lung cell preparations yielded [35S]mucopolysaccharides with an average mol wt of 60,000 by Sepharose 4B gel filtration. Approximately 30% of this fraction was degraded by chondroitin ABC lyase, and the residual 70% was degraded by purified heparinase. When the chondroitin ABC lyase-resistant fraction was subjected to alkali degradation the average mol wt was reduced to 20,000. The calculated human lung mast cell heparin content of 2.4-7.8 μg/106 cells gave a ratio to histamine on a weight basis similar to that of intact lung fragments, thereby implying that heparin in the lung fragments was largely restricted to the mast cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- BOLLET A. J. The measurement of tissue acid mucopolysaccharides. J Clin Invest. 1958 Jun;37(6):858–863. doi: 10.1172/JCI103676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCKLEHURST W. E. The release of histamine and formation of a slow-reacting substance (SRS-A) during anaphylactic shock. J Physiol. 1960 Jun;151:416–435. doi: 10.1113/jphysiol.1960.sp006449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- ENGELBERG H. Plasma heparin levels in normal man. Circulation. 1961 Apr;23:578–581. doi: 10.1161/01.cir.23.4.578. [DOI] [PubMed] [Google Scholar]
- Gould K. G., Jr, Clements J. A., Jones A. L., Felts J. M. Dispersal of rabbit lung into individual viable cells: a new model for the study of lung metabolism. Science. 1972 Dec 15;178(4066):1209–1210. doi: 10.1126/science.178.4066.1209. [DOI] [PubMed] [Google Scholar]
- Horner A. A. The concept of macromolecular heparin and its physiological significance. Fed Proc. 1977 Jan;36(1):35–39. [PubMed] [Google Scholar]
- Hovingh P., Linker A. The enzymatic degradation of heparin and heparitin sulfate. 3. Purification of a heparitinase and a heparinase from flavobacteria. J Biol Chem. 1970 Nov 25;245(22):6170–6175. [PubMed] [Google Scholar]
- JAQUES L. B., MONKHOUSE F. C., STEWART M. A method for the determination of heparin in blood. J Physiol. 1949 Aug;109(1-2):41–48. doi: 10.1113/jphysiol.1949.sp004367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaques L. B., Wollin A. A modified method for the colorimetric determination of heparin. Can J Physiol Pharmacol. 1967 Sep;45(5):787–794. doi: 10.1139/y67-093. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Lasker S. E. The heterogeneity of heparins. Fed Proc. 1977 Jan;36(1):92–97. [PubMed] [Google Scholar]
- Lewis R. A., Wasserman S. I., Goetzi E. J., Austen K. F. Formation of slow-reacting substance of anaphylaxis in human lung tissue and cells before release. J Exp Med. 1974 Nov 1;140(5):1133–1146. doi: 10.1084/jem.140.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. G., Spencer A. F., Silbert J. E. Biosynthesis of glycosaminoglycans by cultured mastocytoma cells. Biochem J. 1973 Jun;134(2):455–463. doi: 10.1042/bj1340455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker A., Hovingh P. Structural studies of heparitin sulfates. Biochim Biophys Acta. 1975 Apr 7;385(2):324–333. doi: 10.1016/0304-4165(75)90360-8. [DOI] [PubMed] [Google Scholar]
- Olsson I., Berg B., Fransson L. A., Nordén A. The identity of the metachromatic substance of basophilic leucocytes. Scand J Haematol. 1970;7(6):440–444. doi: 10.1111/j.1600-0609.1970.tb01929.x. [DOI] [PubMed] [Google Scholar]
- Orenstein N. S., Galli S. J., Dvorak A. M., Silbert J. E., Dvorak H. F. Sulfated glycosaminoglycans of guinea pig basophilic leukocytes. J Immunol. 1978 Aug;121(2):586–592. [PubMed] [Google Scholar]
- Paterson N. A., Wasserman S. I., Said J. W., Austen K. F. Release of chemical mediators from partially purified human lung mast cells. J Immunol. 1976 Oct;117(4):1356–1362. [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Cassady I. M. Separation of mast cells in successive stages of differentiation using programmed gradient sedimentation. Am J Pathol. 1970 Dec;61(3):323–340. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. D., Armand G., Lam L. Structure-function relationships of heparin species. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3065–3069. doi: 10.1073/pnas.75.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. D. Biologic actions of heparin. Semin Hematol. 1977 Oct;14(4):427–440. [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Slorach S. A. Histamine and heparin release from isolated rat mast cells exposed to compound 48-80. Acta Physiol Scand. 1971 May;82(1):91–97. doi: 10.1111/j.1748-1716.1971.tb04945.x. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]
- Yurt R. W., Leid R. W., Jr, Austen K. F. Native heparin from rat peritoneal mast cells. J Biol Chem. 1977 Jan 25;252(2):518–521. [PubMed] [Google Scholar]



