Abstract
Primates, the mammalian order including our own species, comprise 480 species in 78 genera. Thus, they represent the third largest of the 18 orders of eutherian mammals. Although recent phylogenetic studies on primates are increasingly built on molecular datasets, most of these studies have focused on taxonomic subgroups within the order. Complete mitochondrial (mt) genomes have proven to be extremely useful in deciphering within-order relationships even up to deep nodes. Using 454 sequencing, we sequenced 32 new complete mt genomes adding 20 previously not represented genera to the phylogenetic reconstruction of the primate tree. With 13 new sequences, the number of complete mt genomes within the parvorder Platyrrhini was widely extended, resulting in a largely resolved branching pattern among New World monkey families. We added 10 new Strepsirrhini mt genomes to the 15 previously available ones, thus almost doubling the number of mt genomes within this clade. Our data allow precise date estimates of all nodes and offer new insights into primate evolution. One major result is a relatively young date for the most recent common ancestor of all living primates which was estimated to 66-69 million years ago, suggesting that the divergence of extant primates started close to the K/T-boundary. Although some relationships remain unclear, the large number of mt genomes used allowed us to reconstruct a robust primate phylogeny which is largely in agreement with previous publications. Finally, we show that mt genomes are a useful tool for resolving primate phylogenetic relationships on various taxonomic levels.
Introduction
An accurate and reliable phylogeny provides information about evolutionary relationships among species and higher taxa, and can be used to determine the timescale of their evolution. Thus, phylogenetic reconstructions serve as a basis for comparative analyses of adaptive processes and for the discrimination between ancestral and derived states (e.g. [1–4]). The use of sequence data and other genetic markers has strongly improved phylogenetic reconstructions. Depending on the mode of inheritance of the respective marker used (autosomal, Y chromosomal or mitochondrial), different questions concerning a phylogeny can be resolved. In animals, the mitochondrial (mt) genome is typically maternally inherited, non-recombining, and has a relatively high substitution rate and a smaller effective population size than the nuclear genome [5–9]. These properties can increase the probability of congruence between the mitochondrial gene tree and the species tree, helping to resolve relationships between recently divergent taxa [10], in particular if complete mt genome information is used instead of single gene information [11–14].
Although various studies on primate phylogeny combining mtDNA and nuclear DNA fragments in supermatrix approaches [15–17] or relying solely on nuclear DNA [18] were recently published, the molecular phylogeny of primates is still incompletely resolved and particularly a comprehensive phylogeny based on mt genomes alone is not yet available. Previous phylogenetic studies using only mitochondrial markers have mainly used fragments of the mt genome or, if sequence information of the complete mt genome was used, these studies either included only a few species (e.g., [19–21]) or focused on certain taxonomic groups within the primate order (e.g., strepsirrhines [22],; platyrrhines [23],; colobines [24–27],; gibbons [28,29],; chimpanzees [1],; and humans [30],). A generic mitochondrial phylogeny based on complete mt genome information is still lacking for primates. To overcome this limitation, we generated 32 complete primate mt genomes using next-generation-sequencing and combined them with 51 additional mt genome sequences from GenBank to reconstruct a robust family-level phylogeny of primates and to estimate the respective divergence times using solely primate fossil calibration points.
Results and Discussion
We produced complete mt genome sequences from 32 primate individuals. From each individual, we obtained an average of 1508 tagged reads with an average length of 235 bp, yielding approximately 356 kb of sequence data corresponding to 21-fold coverage. All newly sequenced mt genomes had lengths typical for primates (16,280–16,936 bp; Table S1), but the GC-content varied largely among taxa (37.78–46.32%, Table S2, Figure S1). All newly generated mt genomes consisted of 22 tRNA genes, 2 rRNA genes, 13 protein-coding genes and the control region in the order typical for mammals. By combining the 32 newly generated data with 51 additional primate mt genomes, the dataset represents all 16 primate families, 57 of the 78 recognized genera and 78 of the 480 currently recognized species [31].
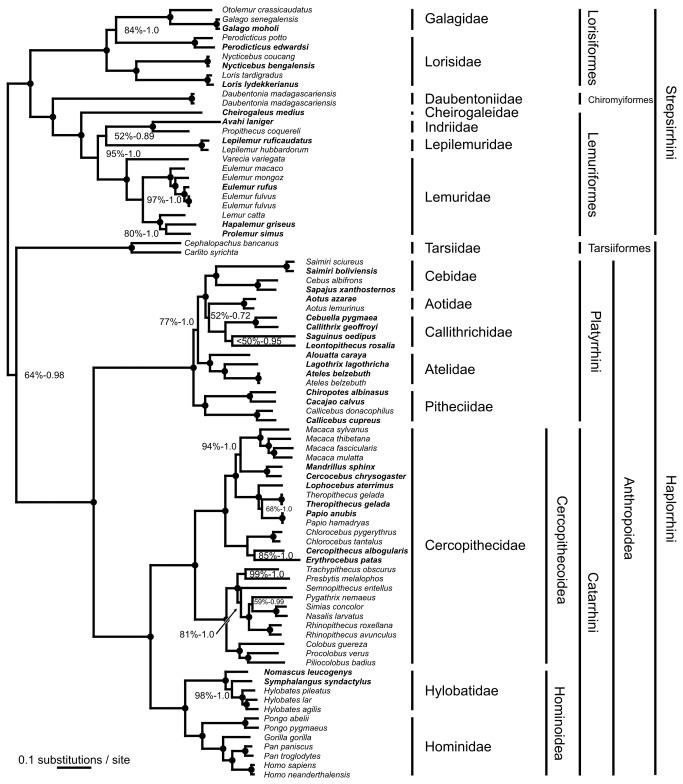
The phylogenetic relationships as revealed by both applied algorithms (maximum-likelihood [ML] and Bayesian inference) and for the different datasets are identical and predominantly strongly supported (Figure 1, S2, S3, S4). Only a few nodes obtained less statistical support and for the AGY datasets (mtDNA2, mtDNA4) support values were generally lower than for the AGTC datasets (mtDNA1, mtDNA3). Moreover, the tree topology is highly congruent with the ones obtained from nuclear sequence data [18], the presence/absence pattern of retroposon elements [26,32–42], supermatrix approaches [15–17] and mt genome data [19–29], although a few cases of incongruence remain (see below).
Figure 1. Phylogram showing the phylogenetic relationships among the investigated primate mt genomes as obtained from dataset mtDNA1.
Newly generated sequences are indicated in bold. Black dots on nodes indicate ML support and Bayesian posterior probabilities of 100% and 1.0, respectively. Lower values are shown at the respective branches.
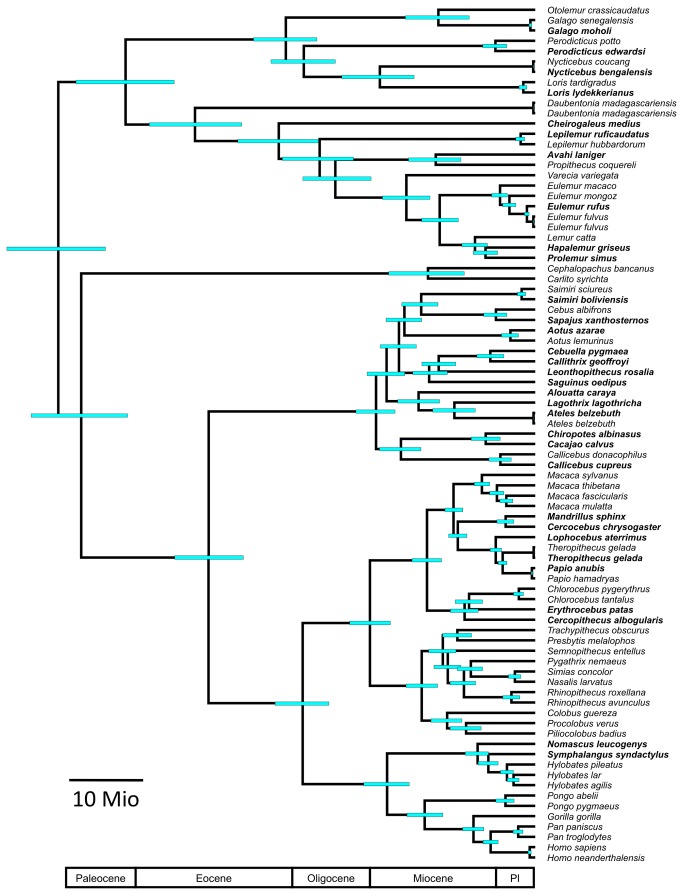
The estimated divergence ages from both AGCT datasets (mtDNA1, mtDNA3) are highly similar (Figure 2; Table S3). All estimates suggest that the most recent common ancestor (MRCA) of the primate order is more recent than suggested by various other genetic studies (e.g., [16,18,19,22,43–45], but see 15,46) and statistical modelling [47]. However, our estimate is in line with both fossil data [48,49], estimates based on expected life-history correlates of primates [50] and recent estimates based on a supermatrix approach [17].
Figure 2. Estimated divergence ages as obtained from dataset mtDNA1 along with their 95% credibility intervals (blue bars).
Newly generated sequences are indicated in bold. A geological time scale is given below the tree. For detailed information on estimated divergence ages see Table S3.
Early Primate Divergence
A longstanding problem in primate phylogeny and classification was the position of Tarsiiformes relative to Anthropoidea and Strepsirrhini [51]. Although only weakly supported in all our reconstructions, Tarsiiformes do always cluster together with Anthropoidea to the exclusion of Strepsirrhini. Accordingly, our findings are in agreement with retroposon integrations and nuclear sequence data [18,40] and support the initial primate divergence into Strepsirrhini versus Haplorrhini (Anthropoidea + Tarsiiformes). This initial split occurred 66.22-69.05 Ma (range of means from both estimates; for 95% credibility intervals see Table S3), suggesting a primate origin around the Cretaceous-Tertiary boundary [15,46]. Shortly afterwards, Tarsiiformes separated from Anthropoidea (63.07-64.81 Ma). Within Tarsiiformes, both analyzed genera, Carlito and Cephalopachus, diverged in the Middle Miocene, concordant with previous results [52].
Strepsirrhini
Among strepsirrhines we found a division into the Malagasy lemurs (Chiromyiformes and Lemuriformes) and Lorisiformes, with both lineages separating in the Late Paleocene or Early Eocene (56.89-58.57 Ma). Within Lorisiformes, Loris idae appeared as a paraphyletic group with the African Perodicticus either forming a sister lineage to Galagidae or, to a clade containing Galagidae plus Nycticebus and Loris. However, support values for either branching pattern are low and a monophyletic Loris idae clade is statistically not rejected (P > 0.05, Table S4). Therefore, divergence age estimates are based on an a-priori constrained monophyly of Loris idae as suggested by retroposon integrations and nuclear sequence data [18,38]. According to this approach, both Lorisiformes families diverged around the Eocene-Oligocene boundary ca. 35 Ma, followed by a subsequent separation of African and Asian lorisids about 32 Ma. The genera within Galagidae and the Asian Loris idae emerged about 13 Ma and 21 Ma, respectively.
Malagasy lemurs appeared as a monophyletic clade with the basal split dating to about 47 Ma separating the Chiromyiformes ( Daubentonia madagascariensis ) from the Lemuriformes. As in earlier studies [15,16,18,38,53], the relationships among the four Lemuriformes families are not well resolved and various alternative relationships are not rejected. However, one retroposon integration supports an Indri idae + Lemur idae clade [38] and hence, both families were constrained to be monophyletic for divergence age estimations. Based on our estimates, lemuriform families emerged 26.52-35.47 Ma. Within Indri idae , the nocturnal Avahi and the diurnal Propithecus separated about 13 Ma. Within the family Lemur idae , Varecia diverged first (~ 17 Ma), while the remaining genera split into Eulemur and a clade consisting of Lemur , Hapalemur and Prolemur about 13 Ma. In the latter clade, Lemur appears as sister lineage to Hapalemur + Prolemur, suggesting a common origin of the bamboo lemurs ( Hapalemur and Prolemur). The branching of those three genera gained only weak statistical support and the divergence time estimates suggest a rapid divergence within a short time period during the Late Miocene.
New World Monkeys (Platyrrhini)
We found Platyrrhini to have separated from Catarrhini about 46 Ma, which is in line with earlier studies [18,45,46,54,55]. Although only weakly supported, the branching pattern among platyrrhines with Pitheciidae diverging first and Atelidae forming a sister family to the remaining families is in agreement with various recent studies [16,18,23,36,37,56,57]. While Cebidae, Aotidae and Callithrichidae are strongly suggested as a monophyletic clade, the phylogenetic relationships among them remain unresolved and various alternative relationships are not rejected. According to our divergence age estimates, Pitheciidae split from other platyrrhine families about 22 Ma and further diverged into Callicebinae ( Callicebus ) and Pitheciinae ( Cacajao and Chiropotes ) about 18 Ma. Atelidae split from Cebidae, Aotidae and Callithrichidae about 20 Ma, while the latter three families originated during a short time period (18.03-18.97 Ma) in the Early Miocene. Further differentiation of families into subfamilies (Atelidae: Atelinae [Ateles, Lagothrix ] - Alouattinae [Alouatta]; Cebidae: Cebinae [Cebus, Sapajus] - Samirinae [Saimiri]) and initial splits within Callithrichidae occurred slightly later in the Middle Miocene. In Callithrichidae, the branching pattern among Saguinus , Leontopithecus and the Callithrix + Cebuella clade remains unresolved, but nuclear sequence data and retroposon integrations strongly suggest that the basal split separated Saguinus from all other lineages within the family; therefore, this branching pattern was fixed for divergence time estimations [18,36]. The genera within the different subfamilies originated during the Late Miocene ( Chiropotes - Cacajao , Ateles - Lagothrix , Callithrix - Cebuella) or Early Pliocene (Cebus - Sapajus).
Old World Monkeys (Cercopithecoidea)
Old World monkeys separated from hominoids about 32 Ma, which is in broad agreement with earlier studies [18,22,45,54,55]. They further diverged into the subfamilies Cercopithecinae and Colobinae in the Early Miocene. During the Middle Miocene, Cercopithecinae further separated into Cercopithecini ( Chlorocebus , Erythrocebus , Cercopithecus ) and Papionini (Macaca, Mandrillus , Cercocebus , Papio, Theropithecus , Lophocebus ), while Colobinae diverged into the African Colobini (Colobus, Procolobus , Piliocolobus) and Asian Presbytini ( Presbytis , Trachypithecus , Semnopithecus , Rhinopithecus, Pygathrix , Nasalis, Simias). Interestingly, in both subfamilies we found several discordances between mt genome and nuclear data. In our mt genome data, Erythrocebus clusters with Cercopithecus and not with Chlorocebus as suggested by all available nuclear sequence and retroposon data [18,42,58,59], but monophyly of Erythrocebus and Chlorocebus is not rejected by alternative tree topology tests (P > 0.05). Thus, Erythrocebus and Chlorocebus were constrained in a monophyletic clade for calculating divergence ages, which resulted in estimates for the differentiation of Cercopithecini lineages between 8.88 and 9.59 Ma. In the Papionini, we found the Mandrillus + Cercocebus clade to be closer related to Macaca than to the other African genera (Papio, Theropithecus , Lophocebus ), which is in disagreement with nuclear sequence and retroposon data [18,41]. However, a sister position of Macaca to all other Papionini is not rejected and hence, for divergence age estimations, Macaca was constrained as sister group to all other members of Papionini. Accordingly, Macaca separated first (ca. 11.25 Ma), followed shortly afterwards by the separation of the Mandrillus + Cercocebus clade from the Papio + Theropithecus + Lophocebus clade about 10.5 Ma. The relationships within the latter clade are unresolved and suggest a rapid diversification about 5.2 Ma. In both the African and Asian colobines, the branching pattern among genera and respective divergence ages are similar to those found in other mt genome studies [26,27]. Similar to previous studies on colobines, our study provides evidence for a monophyletic odd-nosed monkey clade (Rhinopithecus, Pygathrix, Nasalis, Simias), which originated in the Late Miocene, but support for a monophyletic langur clade ( Presbytis , Trachypithecus , Semnopithecus ) [18,24,26,27,60,61] is missing. While nuclear sequence and retroposon data suggest a Semnopithecus + Trachypithecus clade [18,26,35,61], mt genome data indicate Trachypithecus to be related with Presbytis [26,27], thus supporting the hybridization scenario proposed by Roos et al. [26].
Apes and Humans (Hominoidea)
In agreement with earlier studies [15–18,25,28,29,46,62–64], hominoids diverged into small apes or gibbons (Hylobatidae) and great apes and humans (Hominidae) in the Early Miocene. Within Hylobatidae, Nomascus separated first (ca. 7.8 Ma), followed by the divergence of Symphalangus and Hylobates ca. 6.2 Ma, which is concordant with other mtDNA data sets [28,29,64,65]. In concordance with other studies [15–18,22,25,45,66], in Hominidae, orang-utans ( Pongo ) diverged ca. 15.2 Ma from the African great apes and humans, while Gorilla separated from the Homo + Pan clade ca. 8.4 Ma. Finally, chimpanzees and humans separated in the Latest Miocene, about 5.9 Ma.
Conclusions
Our study based on complete mt genomes of a large number of primates revealed a robust primate phylogeny with well-resolved phylogenetic relationships and predominantly strong node support. Moreover, the obtained phylogeny is largely in agreement with nuclear sequence and retroposon data, suggesting that the reconstructed relationships are indeed correct. However, there are some discordances between nuclear and mt genome phylogenies, some of which can be explained by hybridization and secondary gene flow, while for others, branching patterns as suggested by nuclear data cannot be excluded for the mt genome data. We also found that the observed shifts in G/C content among taxa have no major influence on the overall phylogeny. Interestingly, our estimate for the MRCA of all living primates dates to the Cretaceous-Tertiary boundary and thus much more recent than some other genetic studies have suggested. However, since we used only primate internal calibration points and since our estimates are in agreement with fossil data and expected life-history correlates of primates, we believe that this estimate is reliable. Overall, our study shows that complete mt genomes provide a better resolution of phylogenetic relationships on various taxonomic levels than short mt genome fragments or nuclear sequence data. Since hybridization among primate taxa is common [67], data from sex-specific inherited markers, i.e. mtDNA or Y-chromosomal loci is essential to trace such events and thus, our study will serve as basis for future studies on primate evolution and possible hybridization events.
Ethical Statement
Samples were not specifically acquired for this study and all samples were provided by zoos in Amsterdam, Berlin, Cologne, Duisburg, Dresden, Gettorf, Mannheim, Munich, Romagne and Wuppertal, or by Prof Yves Rumpler. Most samples derived from zoo specimens. Respective samples were taken during routine veterinary care under general anaesthesia with a 2mg/kg injection of ketamine solution. Skin biopsies from Avahi laniger and Lepilemur ruficaudatus were obtained from wild animals, which were already used in earlier molecular studies ( [68,69]). Permission for field work and biopsy collection was provided by the Direction des Eaux et Forêts of Antananarivo and the Association Nationale pour la Gestion des Aires Protégées of Antananarivo to Prof Yves Rumpler. Sample collection was approved by the Animal Welfare Body of the German Primate Center and adhered to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Non-Human Primates (see www.asp.org/society/policy.cfm). No animals were sacrificed for this study.
Materials and Methods
Primate DNA samples were obtained from the long-term collections of the authors or from colleagues (see Table S1 for a full list of samples). Two overlapping PCR fragments with sizes of 8 kb (primers 5’-GGCTTTCTCAACTTTTAAAGGATA-3’; 5’-TGTCCTGATCCAACATCGAG-3’) and 10 kb (primers 5’-CCGTGCAAAGGTAGCATAATC-3’; 5’-TTACTTTTATTTGGAGTTGCACCA-3’), respectively, that cover the entire mt genome were amplified using the Expand Long Range dNTPack (Roche). Initial denaturation was at 92° C for 2’, followed by (92° C for 10″, 60° C for 15″, 68° C for 8’) for 10 cycles, (92° C for 10″, 60° C for 15″, 68° C for 8’+20”/cycle) for 25 cycles, and a final extension at 68° C for 7’. After SPRI bead purification (AMPure, Beckman Coulter), PCR products were quantified on a Nanodrop and PCR products from identical samples were pooled in equimolar ratios. PCR products were then converted into bar-coded 454 sequencing libraries according to the Parallel-Tagged-Sequencing protocol [70]. Final library quantification was done via qPCR [71]. Pooled DNA libraries were sequenced on the 454 Flx Sequencing platform (Roche). Sequencing reads were sorted according to their molecular bar code using the program Untag [70] and assembled via the Newbler assembly program of the Roche 454 software tools. The consensus sequence of each mt genome was built on a >50%-majority rule. Gaps in genomic sequences and regions below 3-fold coverage were re-sequenced from shorter PCR fragments using Sanger sequencing. Mitochondrial genomes were annotated automatically using the DOGMA annotation software [72]. If the reading frame of protein coding genes was disrupted due to homopolymer length misidentification by the 454 post-processing software, the original read assembly was revised and corrected manually. All 454 sequences are available at the European Nucleotide Archive under study accession number ERP002564. Accession numbers for sample specific reads are given in Table S1. All assembled and annotated primate mt genomes are available at GenBank, accession numbers are given in Table S1.
To expand the dataset, we added 51 additional primate mt genomes available from GenBank as well as four non-primate mt genomes used as outgroups (Table S1). Data from GenBank were selected to represent complete mt genomes with no more than 10 ambiguous sites. Accordingly, the final dataset consisted of 87 mt genomes. An alignment was generated using MAFFT v 6.708b [73] with default settings (Data S1). Four different datasets were generated for phylogenetic reconstructions. For Dataset 1 (mtDNA1), poorly aligned positions and indels were removed with Gblocks v 0.91b [74] using default settings, and also the D-loop region was excluded (total length: 13,281 bp). Due to extreme shifts in C/T content among taxa as calculated in PAUP v4.0b10 [75] (Table S2, Figure S1), positions with C and T were replaced with Y (AGY) in the second dataset (mtDNA2). Dataset 3 (mtDNA3) and 4 (mtDNA4) were generated in Mesquite v 2.75 [76] and consisted only of the 12 protein-coding genes on the heavy strand (total length: 10,773 bp). In mtDNA4, C and T were again replaced with Y. Phylogenetic trees were constructed with ML and Bayesian algorithms, using the programs GARLI 2.0 [77] and MrBayes 3.1.2 [78,79]. For all reconstructions, the optimal nucleotide substitution model for each locus was chosen using the Bayesian information criterion (BIC) as implemented in jModeltest 2.1 [80]. For phylogenetic analyses, the datasets were whenever appropriate partitioned treating each locus separately and each with its own substitution model. In GARLI, only the model specification settings were adjusted, while all other settings were left at their default value. Relative support of internal nodes was assessed by bootstrap analyses with 500 replications and ML majority-rule consensus trees were calculated in PAUP. For Bayesian analyses, we used four independent Markov Chain Monte Carlo (MCMC) runs with the default temperature of 0.2. Four repetitions were run for 10 million generations with tree and parameter sampling occurring every 100 generations. Acceptance rates were in the optimal range of 10-70%. The first 25% of samples were discarded as burn-in, leaving 75,001 trees per run. The adequacy of this burn-in and convergence of all parameters was assessed by examining the uncorrected potential scale reduction factor (PSRF) [81] as calculated by MrBayes, which should approach 1 as runs converge and by visual inspection of the trace of the parameters across generations using the software TRACER 1.5 [82]. AWTY [83] was used to check whether posterior clade probabilities were also converging. Posterior probabilities for each split and a phylogram with mean branch lengths were calculated from the posterior density of trees. For the mtDNA1 dataset, various alternative phylogenetic relationships were tested with the Kishino-Hasegawa [84] and Shimodaira-Hasegawa [85] tests with full optimization and 1,000 bootstrap replications in PAUP.
To estimate divergence ages from datasets mtDNA1 and mtDNA3 we applied a Bayesian MCMC method, which employs a relaxed molecular clock approach [86] as implemented in BEAST 1.6.1 [87]. Therefore, we assumed a relaxed uncorrelated lognormal model of lineage variation and a Birth-Death Process prior for branching rates. Dataset mtDNA3 was further partitioned into codon positions and the substitution model, rate heterogeneity and base frequencies were unlinked across codon positions (1–3). Because some depicted branching patterns were only weakly supported or contradicted the nuclear phylogeny [18], these relationships were constrained if respective alternative relationships were not rejected by alternative tree topology tests (Table S4). Four replicates were run for 25 million generations with tree and parameter sampling occurring every 100 generations. The adequacy of a 10% burn-in and convergence of all parameters were assessed by visual inspection of the trace of the parameters across generations using TRACER v 1.5 [82]. Subsequently, the sampling distributions were combined (25% burn-in) using the software LogCombiner v 1.6.1 and a consensus chronogram with node height distribution was generated and visualized with TreeAnnotator v 1.6.1 and FigTree v 1.3.1 [88].
As calibration points, we used the same as in Perelman et al. [18] (Table S5): MRCA of Lorisiformes 40 Ma (SD = 3.0) [89], MRCA of Anthropoidea 43 Ma (SD = 4.5) [90,91], MRCA of Catarrhini 29.0 Ma (SD = 6.0) [91,92], MRCA of Platyrrhini 23.5 Ma (SD = 3.0) [23,93], MRCA of Papionini 7.0 Ma (SD = 1.0) [94], MRCA of Theropithecus and Papio 4.0 Ma (SD = 0.4) [58,60], MRCA of Hominidae 15.5 Ma (SD = 2.5) [22], MRCA of Homo and Pan 6.5 Ma (SD = 0.8) [95], and a primate MRCA of 90.0 Ma (SD = 6.0) [19,45,91]. All calibration points were applied as normal priors.
Supporting Information
Information on the studied species including mt genome length along with accession numbers for GenBank and the European Nucleotide Archive.
(XLS)
Base composition of individual mt genomes and average base composition for the studied genera (in bold).
(XLS)
Estimated divergence ages and 95% credibility intervals (in parentheses) for datasets mtDNA1 and mtDNA3 based on 9 internal calibration points and comparable estimates from earlier studies ( [15,17,18]).
(XLS)
Results from alternative tree topology (Kishino-Hasegawa and Shimodaira-Hasegawa) tests for questionable relationships based on 1000 bootstraps. Shown are likelihoods and differences to the most probable topology. Significant (P<0.05) results are labeled with an asterisk.
(DOC)
Calibration points used for divergence time estimates.
(XLS)
Diagram showing the G/C content of the mt genomes of the studied genera.
(TIF)
Phylogram as obtained from dataset mtDNA2. Newly generated sequences are indicated in bold. Black dots on nodes indicate ML support and Bayesian posterior probabilities of 100% and 1.0, respectively. Values below are shown at the respective branches.
(TIF)
Phylogram as obtained from dataset mtDNA3. Newly generated sequences are indicated in bold. Black dots on nodes indicate ML support and Bayesian posterior probabilities of 100% and 1.0, respectively. Values below are shown at the respective branches.
(TIF)
Phylogram as obtained from dataset mtDNA4. Newly generated sequences are indicated in bold. Black dots on nodes indicate ML support and Bayesian posterior probabilities of 100% and 1.0, respectively. Values below are shown at the respective branches.
(TIF)
Original alignment of the 83 studied primate individuals and four outgroup taxa.
(FA)
Acknowledgments
We thank the zoos in Amsterdam, Berlin, Cologne, Duisburg, Dresden, Gettorf, Mannheim, Munich, Romagne and Wuppertal as well as Karl Amman, Yves Rumpler and Helga Schulze for providing valuable primate samples. We thank Mark Springer and two anonymous reviewers for helpful comments on an earlier version of the manuscript.
Funding Statement
This work was funded by the Max Planck Society and the German Primate Center. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bjork A, Liu W, Wertheim JO, Hahn BH, Worobey M (2011) Evolutionary history of chimpanzees inferred from complete mitochondrial genomes. Mol Biol Evol 28: 615-623. doi:10.1093/molbev/msq227. PubMed: 20802239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rohland N, Malaspinas AS, Pollack JL, Slatkin M, Matheus P et al. (2007) Proboscidean mitogenomics: chronology and mode of elephant evolution using mastodon as outgroup. PLOS Biol 5: e207. doi:10.1371/journal.pbio.0050207. PubMed: 17676977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shultz S, Opie C, Atkinson QD (2011) Stepwise evolution of stable sociality in primates. Nature 479: 219-222. doi:10.1038/nature10601. PubMed: 22071768. [DOI] [PubMed] [Google Scholar]
- 4. Zinner D, Groeneveld LF, Keller C, Roos C (2009) Mitochondrial phylogeography of baboons (Papio spp.): indication for introgressive hybridization? BMC Evol Biol 9: 83. doi:10.1186/1471-2148-9-83. PubMed: 19389236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Avise J (1994) Molecular markers, natural history and evolution. New York: Springer Verlag. 511pp. [Google Scholar]
- 6. Avise JC (2000) Phylogeography: the history and formation of species. Cambridge: Harvard University Press. 447pp. [Google Scholar]
- 7. Brown WM, Prager EM, Wang A, Wilson AC (1982) Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol 18: 225-239. doi:10.1007/BF01734101. PubMed: 6284948. [DOI] [PubMed] [Google Scholar]
- 8. Melnick DJ, Hoelzer GA (2005) What is mtDNA good for in the study of primate evolution? Evol Anthropol 2: 2-10. doi:10.1002/evan.1360020103. [Google Scholar]
- 9. Vilstrup JT, Ho SY, Foote AD, Morin PA, Kreb D et al. (2011) Mitogenomic phylogenetic analyses of the Delphinidae with an emphasis on the Globicephalinae. BMC Evol Biol 11: 65. doi:10.1186/1471-2148-11-65. PubMed: 21392378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moore WS (1995) Inferring phylogenies from mtDNA variation: mitochondrial-gene trees versus nuclear-gene trees. Evolution 49: 718-726. doi:10.2307/2410325. [DOI] [PubMed] [Google Scholar]
- 11. DeFilippis VR, Moore WS (2000) Resolution of phylogenetic relationships among recently evolved species as a function of amount of DNA sequence: an empirical study based on woodpeckers (Aves: Picidae). Mol Phylogenet Evol 16: 143-160. doi:10.1006/mpev.2000.0780. PubMed: 10877947. [DOI] [PubMed] [Google Scholar]
- 12. Duchêne S, Archer FI, Vilstrup J, Caballero S, Morin PA (2011) Mitogenome phylogenetics: the impact of using single regions and partitioning schemes on topology, substitution rate and divergence time estimation. PLOS ONE 6: e27138. doi:10.1371/journal.pone.0027138. PubMed: 22073275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rokas A, Carroll SB (2005) More genes or more taxa? The relative contribution of gene number and taxon number to phylogenetic accuracy. Mol Biol Evol 22: 1337-1344. doi:10.1093/molbev/msi121. PubMed: 15746014. [DOI] [PubMed] [Google Scholar]
- 14. Zinner D, Wertheimer J, Liedigk R, Groeneveld LF, Roos C (2013) Baboon phylogeny as inferred from complete mitochondrial genomes. Am J Phys Anthropol 150: 133-140. doi:10.1002/ajpa.22185. PubMed: 23180628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chatterjee HJ, Ho SY, Barnes I, Groves C (2009) Estimating the phylogeny and divergence times of primates using a supermatrix approach. BMC Evol Biol 9: 259. doi:10.1186/1471-2148-9-259. PubMed: 19860891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fabre PH, Rodrigues A, Douzery EJ (2009) Patterns of macroevolution among primates inferred from a supermatrix of mitochondrial and nuclear DNA. Mol Phylogenet Evol 53: 808-825. doi:10.1016/j.ympev.2009.08.004. PubMed: 19682589. [DOI] [PubMed] [Google Scholar]
- 17. Springer MS, Meredith RW, Gatesy J, Emerling CA, Park J et al. (2012) Macroevolutionary dynamics and historical biogeography of primate diversification inferred from a species supermatrix. PLOS ONE 7: e49521 PubMed: 23166696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perelman P, Johnson WE, Roos C, Seuanez HN, Horvath JE et al. (2011) A molecular phylogeny of living primates. PLOS Genet 7: e1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arnason U, Adegoke JA, Gullberg A, Harley EH, Janke A et al. (2008) Mitogenomic relationships of placental mammals and molecular estimates of their divergences. Gene 421: 37-51. doi:10.1016/j.gene.2008.05.024. PubMed: 18590805. [DOI] [PubMed] [Google Scholar]
- 20. Arnason U, Gullberg A, Burguete AS, Janke A (2000) Molecular estimates of primate divergences and new hypotheses for primate dispersal and the origin of modern humans. Hereditas 133: 217-228. PubMed: 11433966. [DOI] [PubMed] [Google Scholar]
- 21. Arnason U, Gullberg A, Janke A (1998) Molecular timing of primate divergences as estimated by two nonprimate calibration points. J Mol Evol 47: 718-727. doi:10.1007/PL00006431. PubMed: 9847414. [DOI] [PubMed] [Google Scholar]
- 22. Matsui A, Rakotondraparany F, Munechika I, Hasegawa M, Horai S (2009) Molecular phylogeny and evolution of prosimians based on complete sequences of mitochondrial DNAs. Gene 441: 53-66. doi:10.1016/j.gene.2008.08.024. PubMed: 18824224. [DOI] [PubMed] [Google Scholar]
- 23. Hodgson JA, Sterner KN, Matthews LJ, Burrell AS, Jani RA et al. (2009) Successive radiations, not stasis, in the South American primate fauna. Proc Natl Acad Sci U S A 106: 5534-5539. doi:10.1073/pnas.0810346106. PubMed: 19321426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liedigk R, Yang M, Jablonski NG, Momberg F, Geissmann T et al. (2012) Evolutionary history of the odd-nosed monkeys and the phylogenetic position of the newly described Myanmar snub-nosed monkey Rhinopithecus strykeri . PLOS ONE 7: e37418. doi:10.1371/journal.pone.0037418. PubMed: 22616004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raaum RL, Sterner KN, Noviello CM, Stewart CB, Disotell TR (2005) Catarrhine primate divergence dates estimated from complete mitochondrial genomes: concordance with fossil and nuclear DNA evidence. J Hum Evol 48: 237-257. doi:10.1016/j.jhevol.2004.11.007. PubMed: 15737392. [DOI] [PubMed] [Google Scholar]
- 26. Roos C, Zinner D, Kubatko LS, Schwarz C, Yang M et al. (2011) Nuclear versus mitochondrial DNA: evidence for hybridization in colobine monkeys. BMC Evol Biol 11: 77. doi:10.1186/1471-2148-11-77. PubMed: 21435245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sterner KN, Raaum RL, Zhang YP, Stewart CB, Disotell TR (2006) Mitochondrial data support an odd-nosed colobine clade. Mol Phylogenet Evol 40: 1-7. doi:10.1016/j.ympev.2006.01.017. PubMed: 16500120. [DOI] [PubMed] [Google Scholar]
- 28. Chan YC, Roos C, Inoue-Murayama M, Inoue E, Shih CC et al. (2010) Mitochondrial genome sequences effectively reveal the phylogeny of Hylobates gibbons. PLOS ONE 5: e14419. doi:10.1371/journal.pone.0014419. PubMed: 21203450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsudaira K, Ishida T (2010) Phylogenetic relationships and divergence dates of the whole mitochondrial genome sequences among three gibbon genera. Mol Phylogenet Evol 55: 454-459. doi:10.1016/j.ympev.2010.01.032. PubMed: 20138221. [DOI] [PubMed] [Google Scholar]
- 30. Gonder MK, Mortensen HM, Reed FA, de Sousa A, Tishkoff SA (2007) Whole-mtDNA genome sequence analysis of ancient African lineages. Mol Biol Evol 24: 757-768. PubMed: 17194802. [DOI] [PubMed] [Google Scholar]
- 31. Mittermeier RA, Rylands AB, Wilson DE (2013) Handbook of the Mammals of the World, Volume 3, Primates. Barcelona: Lynx Edicions. 952pp. [Google Scholar]
- 32. Herke SW, Xing J, Ray DA, Zimmerman JW, Cordaux R et al. (2007) A SINE-based dichotomous key for primate identification. Gene 390: 39-51. doi:10.1016/j.gene.2006.08.015. PubMed: 17056208. [DOI] [PubMed] [Google Scholar]
- 33. Konkel MK, Walker JA, Batzer MA (2010) LINEs and SINEs of primate evolution. Evol Anthropol 19: 236-249. doi:10.1002/evan.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li J, Han K, Xing J, Kim HS, Rogers J et al. (2009) Phylogeny of the macaques (Cercopithecidae: Macaca) based on Alu elements. Gene 448: 242-249. doi:10.1016/j.gene.2009.05.013. PubMed: 19497354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Osterholz M, Walter L, Roos C (2008) Phylogenetic position of the langur genera Semnopithecus and Trachypithecus among Asian colobines, and genus affiliations of their species groups. BMC Evol Biol 8: 58. doi:10.1186/1471-2148-8-58. PubMed: 18298809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Osterholz M, Walter L, Roos C (2009) Retropositional events consolidate the branching order among New World monkey genera. Mol Phylogenet Evol 50: 507-513. doi:10.1016/j.ympev.2008.12.014. PubMed: 19135536. [DOI] [PubMed] [Google Scholar]
- 37. Ray DA, Xing J, Hedges DJ, Hall MA, Laborde ME et al. (2005) Alu insertion loci and platyrrhine primate phylogeny. Mol Phylogenet Evol 35: 117-126. doi:10.1016/j.ympev.2004.10.023. PubMed: 15737586. [DOI] [PubMed] [Google Scholar]
- 38. Roos C, Schmitz J, Zischler H (2004) Primate jumping genes elucidate strepsirrhine phylogeny. Proc Natl Acad Sci U S A 101: 10650-10654. doi:10.1073/pnas.0403852101. PubMed: 15249661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Salem AH, Ray DA, Xing J, Callinan PA, Myers JS et al. (2003) Alu elements and hominid phylogenetics. Proc Natl Acad Sci U S A 100: 12787-12791. doi:10.1073/pnas.2133766100. PubMed: 14561894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schmitz J, Roos C, Zischler H (2005) Primate phylogeny: molecular evidence from retroposons. Cytogenet Genome Res 108: 26-37. doi:10.1159/000080799. PubMed: 15545713. [DOI] [PubMed] [Google Scholar]
- 41. Xing J, Wang H, Han K, Ray DA, Huang CH et al. (2005) A mobile element based phylogeny of Old World monkeys. Mol Phylogenet Evol 37: 872-880. doi:10.1016/j.ympev.2005.04.015. PubMed: 15936216. [DOI] [PubMed] [Google Scholar]
- 42. Xing J, Wang H, Zhang Y, Ray DA, Tosi AJ et al. (2007) A mobile element-based evolutionary history of guenons (tribe Cercopithecini). BMC Biol 5: 5. doi:10.1186/1741-7007-5-5. PubMed: 17266768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. dos Reis M, Inoue J, Hasegawa M, Asher RJ, Donoghue PC et al. (2012) Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proc Biol Sci 279: 3491-3500. doi:10.1098/rspb.2012.0683. PubMed: 22628470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meredith RW, Janečka JE, Gatesy J, Ryder OA, Fisher CA et al. (2011) Impacts of the Cretaceous Terrestrial Revolution and KPg extinction on mammal diversification. Science 334: 521-524. doi:10.1126/science.1211028. PubMed: 21940861. [DOI] [PubMed] [Google Scholar]
- 45. Steiper ME, Young NM (2006) Primate molecular divergence dates. Mol Phylogenet Evol 41: 384-394. doi:10.1016/j.ympev.2006.05.021. PubMed: 16815047. [DOI] [PubMed] [Google Scholar]
- 46. Goodman M, Porter CA, Czelusniak J, Page SL, Schneider H et al. (1998) Toward a phylogenetic classification of primates based on DNA evidence complemented by fossil evidence. Mol Phylogenet Evol 9: 585-598. doi:10.1006/mpev.1998.0495. PubMed: 9668008. [DOI] [PubMed] [Google Scholar]
- 47. Tavaré S, Marshall CR, Will O, Soligo C, Martin RD (2002) Using the fossil record to estimate the age of the last common ancestor of extant primates. Nature 416: 726-729. doi:10.1038/416726a. PubMed: 11961552. [DOI] [PubMed] [Google Scholar]
- 48. Smith T, Rose KD, Gingerich PD (2006) Rapid Asia-Europe-North America geographic dispersal of earliest Eocene primate Teilhardina during the Paleocene-Eocene Thermal Maximum. Proc Natl Acad Sci U S A 103: 11223-11227. doi:10.1073/pnas.0511296103. PubMed: 16847264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Williams BA, Kay RF, Kirk EC (2010) New perspectives on anthropoid origins. Proc Natl Acad Sci U S A 107: 4797-4804. doi:10.1073/pnas.0908320107. PubMed: 20212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Steiper ME, Seiffert ER (2012) Evidence for a convergent slowdown in primate molecular rates and its implications for the timing of early primate evolution. Proc Natl Acad Sci U S A 109: 6006-6011. doi:10.1073/pnas.1119506109. PubMed: 22474376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yoder AD (2003) The phylogenetic position of genus Tarsius: whose side are you on? In: Gursky S. Tarsiers: past, present, and future. New Jersey: Rutgers University Press; pp. 161-175. [Google Scholar]
- 52. Shekelle M, Meier R, Wahyu I, Ting N (2010) Molecular phylogenetics and chronometrics of Tarsiidae based on 12S mtDNA haplotypes: evidence for Miocene origins of crown tarsiers and numerous species within the Sulawesian clade. Int J Primatol 31: 1083-1106. doi:10.1007/s10764-010-9457-8. [Google Scholar]
- 53. Horvath JE, Weisrock DW, Embry SL, Fiorentino I, Balhoff JP et al. (2008) Development and application of a phylogenomic toolkit: resolving the evolutionary history of Madagascar’s lemurs. Genome Res 18: 489-499. doi:10.1101/gr.7265208. PubMed: 18245770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eizirik E, Murphy WJ, Springer MS, O’Brien SJ (2004) Molecular phylogeny and dating of early primate divergences. In: Ross C, Kay RF. Anthropoid origins: new visions. New York: Kluwer Academic/Plenum Publishers. pp. 45-64. [Google Scholar]
- 55. Yoder AD, Yang Z (2004) Divergence dates for Malagasy lemurs estimated from multiple gene loci: geological and evolutionary context. Mol Ecol 13: 757-773. doi:10.1046/j.1365-294X.2004.02106.x. PubMed: 15012754. [DOI] [PubMed] [Google Scholar]
- 56. Opazo JC, Wildman DE, Prychitko T, Johnson RM, Goodman M (2006) Phylogenetic relationships and divergence times among New World monkeys (Platyrrhini, Primates). Mol Phylogenet Evol 40: 274-280. doi:10.1016/j.ympev.2005.11.015. PubMed: 16698289. [DOI] [PubMed] [Google Scholar]
- 57. Schrago CG (2007) On the time scale of New World primate diversification. Am J Phys Anthropol 132: 344-354. doi:10.1002/ajpa.20459. PubMed: 17133436. [DOI] [PubMed] [Google Scholar]
- 58. Tosi AJ, Detwiler KM, Disotell TR (2005) X-chromosomal window into the evolutionary history of the guenons (Primates: Cercopithecini). Mol Phylogenet Evol 36: 58-66. doi:10.1016/j.ympev.2005.01.009. PubMed: 15904856. [DOI] [PubMed] [Google Scholar]
- 59. Tosi AJ, Melnick DJ, Disotell TR (2004) Sex chromosome phylogenetics indicate a single transition to terrestriality in the guenons (tribe Cercopithecini). J Hum Evol 46: 223-237. doi:10.1016/j.jhevol.2003.11.006. PubMed: 14871564. [DOI] [PubMed] [Google Scholar]
- 60. Ting N, Tosi AJ, Li Y, Zhang YP, Disotell TR (2008) Phylogenetic incongruence between nuclear and mitochondrial markers in the Asian colobines and the evolution of the langurs and leaf monkeys. Mol Phylogenet Evol 46: 466-474. doi:10.1016/j.ympev.2007.11.008. PubMed: 18180172. [DOI] [PubMed] [Google Scholar]
- 61. Wang XP, Yu L, Roos C, Ting N, Chen CP et al. (2012) Phylogenetic relationships among the colobine monkeys revisited: new insights from analyses of complete mt genomes and 44 nuclear non-coding markers. PLOS ONE 7: e36274. doi:10.1371/journal.pone.0036274. PubMed: 22558416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hasegawa M, Thorne JL, Kishino H (2003) Time scale of eutherian evolution estimated without assuming a constant rate of molecular evolution. Genes Genet Syst 78: 267-283. doi:10.1266/ggs.78.267. PubMed: 14532706. [DOI] [PubMed] [Google Scholar]
- 63. Israfil H, Zehr SM, Mootnick AR, Ruvolo M, Steiper ME (2011) Unresolved molecular phylogenies of gibbons and siamangs (Family: Hylobatidae) based on mitochondrial, Y-linked, and X-linked loci indicate a rapid Miocene radiation or sudden vicariance event. Mol Phylogenet Evol 58: 447-455. doi:10.1016/j.ympev.2010.11.005. PubMed: 21074627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thinh VN, Mootnick AR, Geissmann T, Li M, Ziegler T et al. (2010) Mitochondrial evidence for multiple radiations in the evolutionary history of small apes. BMC Evol Biol 10: 74. doi:10.1186/1471-2148-10-74. PubMed: 20226039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Roos C, Geissmann T (2001) Molecular phylogeny of the major hylobatid divisions. Mol Phylogenet Evol 19: 486-494. doi:10.1006/mpev.2001.0939. PubMed: 11399155. [DOI] [PubMed] [Google Scholar]
- 66. Yoder AD, Yang Z (2000) Estimation of primate speciation dates using local molecular clocks. Mol Biol Evol 17: 1081-1090. doi:10.1093/oxfordjournals.molbev.a026389. PubMed: 10889221. [DOI] [PubMed] [Google Scholar]
- 67. Zinner D, Arnold ML, Roos C (2011) The strange blood: natural hybridization in primates. Evol Anthropol 20: 96-103. doi:10.1002/evan.20301. PubMed: 22034167. [DOI] [PubMed] [Google Scholar]
- 68. Rumpler Y, Hauwy M, Fausser JL, Roos C, Zaramody A et al. (2011) Comparing chromosomal and mitochondrial phylogenies of the Indriidae (Primates, Lemuriformes). Chromosome Res 19: 209-224. doi:10.1007/s10577-011-9188-5. PubMed: 21336668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rumpler Y, Warter S, Hauwy M, Fausser JL, Roos C et al. (2008) Comparing chromosomal and mitochondrial phylogenies of sportive lemurs (Genus Lepilemur, Primates). Chromosome Res 16: 1143-1158. doi:10.1007/s10577-008-1265-z. PubMed: 19067195. [DOI] [PubMed] [Google Scholar]
- 70. Meyer M, Stenzel U, Hofreiter M (2008) Parallel tagged sequencing on the 454 platform. Nat Protoc 3: 267-278. doi:10.1038/nprot.2007.520. PubMed: 18274529. [DOI] [PubMed] [Google Scholar]
- 71. Meyer M, Stenzel U, Myles S, Prüfer K, Hofreiter M (2007) Targeted high-throughput sequencing of tagged nucleic acid samples. Nucleic Acids Res 35: e97. doi:10.1093/nar/gkm380. PubMed: 17670798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wyman SK, Jansen RK, Boore JL (2004) Automatic annotation of organellar genomes with DOGMA. Bioinformatics 20: 3252-3255. doi:10.1093/bioinformatics/bth352. PubMed: 15180927. [DOI] [PubMed] [Google Scholar]
- 73. Katoh K, Asimenos G, Toh H (2009) Multiple alignment of DNA sequences with MAFFT. Methods Mol Biol 537: 39-64. doi:10.1007/978-1-59745-251-9_3. PubMed: 19378139. [DOI] [PubMed] [Google Scholar]
- 74. Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17: 540-552. doi:10.1093/oxfordjournals.molbev.a026334. PubMed: 10742046. [DOI] [PubMed] [Google Scholar]
- 75. Swofford DL (2003) PAUP*: phylogenetic analysis using parsimony (* and other methods), version 4. Sunderland: Sinauer Associates. [Google Scholar]
- 76. Maddison WP, Maddison DR (2001) Mesquite: a modular system for evolutionary analysis, version 2.75. Available: http://mesquiteproject.org. Accessed: 2012 March 15.
- 77. Zwickl DJ (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence data sets under the maximum likelihood criterion. Austin: the University of Texas at Austin. [Google Scholar]
- 78. Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP (2001) Bayesian inference of phylogeny and its impact on evolutionary biology. Science 294: 2310-2314. doi:10.1126/science.1065889. PubMed: 11743192. [DOI] [PubMed] [Google Scholar]
- 79. Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572-1574. doi:10.1093/bioinformatics/btg180. PubMed: 12912839. [DOI] [PubMed] [Google Scholar]
- 80. Posada D (2009) Selection of models of DNA evolution with jModelTest. Methods Mol Biol 537: 93-112. doi:10.1007/978-1-59745-251-9_5. PubMed: 19378141. [DOI] [PubMed] [Google Scholar]
- 81. Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7: 457-472. doi:10.1214/ss/1177011136. [Google Scholar]
- 82. Rambaut A, Drummond AJ (2007) Tracer: MCMC trace analysis tool, version 1.5. Available: http://tree.bio.ed.ac.uk/software/tracer/. Accessed: 2008 February 11.
- 83. Nylander JA, Wilgenbusch JC, Warren DL, Swofford DL (2008) AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24: 581-583. [DOI] [PubMed] [Google Scholar]
- 84. Kishino H, Hasegawa M (1989) Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in Hominoidea. J Mol Evol 29: 170-179. doi:10.1007/BF02100115. PubMed: 2509717. [DOI] [PubMed] [Google Scholar]
- 85. Shimodaira H, Hasegawa M (1999) Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol Biol Evol 16: 1114-1116. doi:10.1093/oxfordjournals.molbev.a026201. [Google Scholar]
- 86. Drummond AJ, Ho SY, Phillips MJ, Rambaut A (2006) Relaxed phylogenetics and dating with confidence. PLOS Biol 4: e88. doi:10.1371/journal.pbio.0040088. PubMed: 16683862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7: 214. doi:10.1186/1471-2148-7-214. PubMed: 17996036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rambaut A (2008) FigTree: Tree figure drawing tool, version 1.2.2. Available: http://tree.bio.ed.ac.uk/software/figtree/. Accessed: 2008 December 18.
- 89. Seiffert ER, Simons EL, Attia Y (2003) Fossil evidence for an ancient divergence of lorises and galagos. Nature 422: 421-424. doi:10.1038/nature01489. PubMed: 12660781. [DOI] [PubMed] [Google Scholar]
- 90. Franzen JL, Gingerich PD, Habersetzer J, Hurum JH, von Koenigswald W et al. (2009) Complete primate skeleton from the Middle Eocene of Messel in Germany: morphology and paleobiology. PLOS ONE 4: e5723. doi:10.1371/journal.pone.0005723. PubMed: 19492084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Poux C, Douzery EJ (2004) Primate phylogeny, evolutionary rate variations, and divergence times: a contribution from the nuclear gene IRBP. Am J Phys Anthropol 124: 1-16. doi:10.1002/ajpa.10322. PubMed: 15085543. [DOI] [PubMed] [Google Scholar]
- 92. Zalmout IS, Sanders WJ, Maclatchy LM, Gunnell GF, Al-Mufarreh YA et al. (2010) New Oligocene primate from Saudi Arabia and the divergence of apes and Old World monkeys. Nature 466: 360-364. doi:10.1038/nature09094. PubMed: 20631798. [DOI] [PubMed] [Google Scholar]
- 93. Kay RF, Fleagle JG, Mitchell TR, Colbert M, Bown T et al. (2008) The anatomy of Dolichocebus gaimanensis, a stem platyrrhine monkey from Argentina. J Hum Evol 54: 323-382. doi:10.1016/j.jhevol.2007.09.002. PubMed: 18001820. [DOI] [PubMed] [Google Scholar]
- 94. Steiper ME, Young NM, Sukarna TY (2004) Genomic data support the hominoid slowdown and an Early Oligocene estimate for the hominoid-cercopithecoid divergence. Proc Natl Acad Sci U S A 101: 17021-17026. doi:10.1073/pnas.0407270101. PubMed: 15572456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vignaud P, Duringer P, Mackaye HT, Likius A, Blondel C et al. (2002) Geology and palaeontology of the Upper Miocene Toros-Menalla hominid locality, Chad. Nature 418: 152-155. doi:10.1038/nature00880. PubMed: 12110881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Information on the studied species including mt genome length along with accession numbers for GenBank and the European Nucleotide Archive.
(XLS)
Base composition of individual mt genomes and average base composition for the studied genera (in bold).
(XLS)
Estimated divergence ages and 95% credibility intervals (in parentheses) for datasets mtDNA1 and mtDNA3 based on 9 internal calibration points and comparable estimates from earlier studies ( [15,17,18]).
(XLS)
Results from alternative tree topology (Kishino-Hasegawa and Shimodaira-Hasegawa) tests for questionable relationships based on 1000 bootstraps. Shown are likelihoods and differences to the most probable topology. Significant (P<0.05) results are labeled with an asterisk.
(DOC)
Calibration points used for divergence time estimates.
(XLS)
Diagram showing the G/C content of the mt genomes of the studied genera.
(TIF)
Phylogram as obtained from dataset mtDNA2. Newly generated sequences are indicated in bold. Black dots on nodes indicate ML support and Bayesian posterior probabilities of 100% and 1.0, respectively. Values below are shown at the respective branches.
(TIF)
Phylogram as obtained from dataset mtDNA3. Newly generated sequences are indicated in bold. Black dots on nodes indicate ML support and Bayesian posterior probabilities of 100% and 1.0, respectively. Values below are shown at the respective branches.
(TIF)
Phylogram as obtained from dataset mtDNA4. Newly generated sequences are indicated in bold. Black dots on nodes indicate ML support and Bayesian posterior probabilities of 100% and 1.0, respectively. Values below are shown at the respective branches.
(TIF)
Original alignment of the 83 studied primate individuals and four outgroup taxa.
(FA)