Abstract
Objective
We tested the hypothesis that first-trimester metabolic biomarkers offered a unique profile in women with preeclampsia (PE) in the second half of pregnancy, compared to controls.
Method
We conducted a nested-case control study within a prospective cohort of pregnant women followed from the first-trimester to delivery. Cases were those who developed PEat any gestational age and these were compared with a control group without adverse pregnancy outcome, matched for gestational age within three days. We analyzed maternal blood obtained at 11–14 weeks’ gestation for 40 acylcarnitine species (C2-C18 saturated, unsaturated, and hydroxylated) and 32 amino acids by LC tandem mass spectrometry. Logistic regression modeling estimated the association of each metabolite with development ofPE.
Results
We compared 41 cases with preeclampsia with 41 controls, and found four metabolites (Hydroxyhexanoylcarnitine, alanine, phenylalanine, and glutamate) that were significantly higher in the cases withPE. The area under the curve (AUC) using these metabolites individually to predict PE varied from 0.77–0.80; and when combined, the AUC improved to 0.82(95% CI 0.80–0.85) for all cases of PEand 0.85 (95% CI 0.76–0.91) for early onsetPE.
Conclusion
Our findings suggest a potential role for first-trimester metabolomics in screening for PE.
Preeclampsia (PE) affects 5% of pregnancies and is a significant contributor to maternal mortality and morbidity. The effects of PE extend beyond pregnancy, with associated risk of type II diabetes, hypertensive disorders and coronary artery disease in later life (Bellamy L 2007).
Multiple pathological pathways contribute to the final phenotype of PE. These include poor placental perfusion, defective remodeling of utero-placental vessels in early pregnancy, maternal genetic predisposition and vascular endothelial dysfunction (Sibai et al 2005; Levine et al 2004; Redman and Sargent 2005).
While several attempts at identifying reliable biomarkers in early pregnancy to predict PEare ongoing, none of the suggested markers have demonstrated sufficient sensitivity and specificity to allow identification of pregnancies that would benefit from increased surveillance or early preventive therapies (Spencer et al 2008, Zhong et al 2010).
Metabolomic profiling has been proposed as a strategy for discovering low molecular weight chemicals present in the metabolome of a cell or tissue (Goodacre and Dell 2003). This approach identifies the final downstream products of gene expression, which allows detection of high-resolution multifactorial phenotypic signatures of the disease of interest (Oresic et al 2008; Kenny et al 2005, Kenny et al 2008).
There are limited studies on metabolomic profiling of PE and none of these are in the first-trimester (Kenny et al, 2010). We tested the hypothesis that first-trimester metabolic biomarkers offered a unique profile in women withPE in the second half of pregnancy, compared to controls without adverse pregnancy events.
Methods
This is a nested-case control study within a prospective cohort study of pregnant women followed from the first-trimester to delivery as part of a first-trimester screening study for adverse pregnancy outcomes.
Approval for the study was obtained from the institutional review board of Washington University School of Medicine in St Louis, MO and all women gave written, informed consent. Women with singleton pregnancies between 11 – 14 weeks’ gestation attending for first-trimester aneuploidy screening were approached to participate in the study from December 2009 to March 2011. Gestational age was calculated from the last menstrual period and confirmed by crown-rump length measurement.
Cases were defined as patients who developed PEat any gestational age. PEwas defined using guidelines from the American College of Obstetricians and Gynecologist (ACOG, 2002). These were compared with a control group without any adverse pregnancy outcome followed over the same period, matched for gestational age ±3 days.
Maternal serum analytes
Each patient provided approximately 10 cc of maternal blood which was drawn by venipuncture into non-heparinized tubes. The blood samples were allowed to clot, and centrifuged at 1,500g for 15 minutes. The serum was then removed, and aliquots were stored at −80°C until analyzed. 40 acylcarnitine species (C2-C18 saturated, unsaturated, and hydroxylated) were analyzed as butyl esters by direct flow-injection and precursor ion scanning on an API 3200 LC-MS/MS system (Applied Biosystems, Foster City, CA USA). Quantitation was achieved using a cocktail of 8 deuterated internal standards. Concentrations of 32 amino acids were assessed as butyl esters using C8 reversed-phase chromatography coupled to an API 3000 LC-MS/MS system (Applied Biosystems, Foster City, CA USA). Quantitation was performed using 32 distinct precursor-product ion combinations (MRM mode) employing 11 deuterated internal standards. Methodological details and analytic characteristics of this technique were previously reported by Dietzen et al (Dietzen et al, 2008). Internal standards for both techniques were obtained from Cambridge Isotope Laboratories, Andover, MA USA).
Statistical Analysis
The metabolite levels for the cases and the control groups were compared using Student’s t test, Mann-Whitney test, Chi-squared or Fischer exact test, as appropriate. Logistic regression modeling was used to estimate the association between each metabolite and PE, adjusting for possible confounders identified from the univariate analysis. Receiver-operating characteristic curves and the area under the curves (AUC) were derived from the regression models for each metabolite. A final model was created to estimate the impact of combining all significant metabolites on the prediction of PE. Prediction scores for PE from the model combining all positive metabolites were generated. The same analysis was performed for the subgroup of cases with early onset PE requiring delivery under 34 weeks compared with the control group. A p-value <0.05 was considered significant for all analyses. Statistical analyses were performed using STATA version 10.0 (Stata Corp., College Station, TX).
Results
We identified 41 cases with PE and these were matched with 41 controls. The demographic characteristics for the study population stratified by cases and controls are displayed in Table 1. Cases with PE were more likely to be African-American and have higher body mass indices (BMI) when compared with the control group. In addition the PE cases were delivered at an earlier gestational age and had lower infant birth weight compared with the control group.
Table 1.
Demographics of study population.
| Control (n=41) | Preeclampsia (n=41) | p-value | |
|---|---|---|---|
| Mean Maternal age (SD) | 32.9 (5.5) | 30.5 (6.2) | 0.07 |
| Nulliparous (%) | 12 (29.3) | 20 (48.8) | 0.07 |
| Mean GA at recruitment (weeks, SD) | 12.1 (0.6) | 12.1 (0.7) | 0.87 |
| White (%) | 25 (61.0) | 17 (41.5) | 0.08 |
| Black (%) | 9 (21.9) | 20 (48.8) | 0.01 |
| Smoking (%) | 5 (12.2) | 8 (19.5) | 0.36 |
| Mean BMI (SD) | 27.7 (7.2) | 33.8 (8.8) | 0.001 |
| Pregestational diabetes (%) | 0 (0) | 9 (21.9) | 0.001 |
| Mean GA at delivery (weeks, SD) | 39.0 (2.8) | 35.3 (4.1) | <0.0001 |
| Mean birth weight (g, SD) | 3365.9 (357.8) | 2565.0 (870.3) | <0.0001 |
Of 40 acyl carnitine and 32 amino acids evaluated, 7 metabolites were significant higher in the cases with PE compared to the control (Table 2). Two of these, hydroxyisovalerylcarnitine and hydroxyhexanoylcarnitine (C5OH and C6OH) are carnitine esters and 5 are amino acids. The AUC for using these metabolites individually to predict PE varied from 0.67–0.73. When combined, the AUC improved to 0.84 for all cases of PE and 0.85 for early onset PE. Due to the significant differences in BMI, ethnicity and pregestational diabetes between the cases with preeclampsia and the control group, we used logistic regression modeling to adjust for these potential confounders. The results of the adjusted analysis are shown in Table 3. Following the adjustment, C5OH, arginine and hydroxyproline were no longer found to be significant. Subsequent analysis were limited to the four significant metabolites.
Table 2.
Significant metabolites identified in first-trimester maternal serum (unadjusted).
| Metabolite | Class | β- coefficient (95% CI) |
p-value | AUC (95% CI) |
Up/down in Preeclampsia |
|---|---|---|---|---|---|
| C5OH (Hydroxyisovalerylcarnitine/Hydroxymethybutyrylcarnitine | Acyl carnitine | 30.9 (6.8–55.0) | 0.012 | 0.67 (0.57–0.76) | Increased |
| C6OH (Hydroxyhexanoylcarnitine) | Acyl carnitine | 34.3 (4.5–64.0) | 0.024 | 0.67(0.57–0.76) | Increased |
| Phenylalanine | Amino acid | 0.06 (0.02–0.10) | 0.006 | 0.68 (0.58–0.77) | Increased |
| Glutamate | Amino acid | 0.03 (0.01–0.04) | 0.002 | 0.73 (0.63–0.81) | Increased |
| Arginine | Amino acid | 0.02 (0.01–0.03) | 0.004 | 0.70 (0.60–0.79) | Increased |
| Alanine | Amino acid | 0.02 (0.01–0.03) | 0.009 | 0.68 (0.58–0.77) | Increased |
| Hydroxyproline | Amino acid | 0.11 (0.03–0.20) | 0.012 | 0.68(0.58–0.77) | Increased |
| Combined | 0.0001 | 0.84 (0.75–0.91) |
Table 3.
Metabolites identified in first-trimester maternal serum adjusted for maternal BMI, diabetes and ethnicity.
| Metabolite | Class | Adjusted β- coefficient (95% CI) |
Adjusted p-value |
AUC (95% CI) |
|---|---|---|---|---|
| C5OH (Hydroxyisovalerylcarnitine/Hydroxymethybutyrylcarnitine | Acyl carnitine | 22.9 (−2.8–48.6) | 0.081 | 0.77 (0.67–0.85) |
| C6OH (Hydroxyhexanoylcarnitine) | Acyl carnitine | 42.2 (7.07–77.7) | 0.019 | 0.78 (0.69–0.86) |
| Phenylalanine | Amino acid | 0.084 (0.027–0.140) | 0.004 | 0.80 (0.70–0.87) |
| Glutamate | Amino acid | 0.022 (0.005–0.039) | 0.011 | 0.79 (0.77–0.82) |
| Arginine | Amino acid | 0.013 (0.008–0.027) | 0.050 | 0.77 (0.74–0.80) |
| Alanine | Amino acid | 0.018 (0.005–0.031) | 0.008 | 0.78 (0.75–0.81) |
| Hydroxyproline | Amino acid | 0.089 (−.002–0.182) | 0.058 | 0.76 (0.66–0.84) |
| Combination of four significant metabolites | 0.0001 | 0.82 (0.80–0.85) |
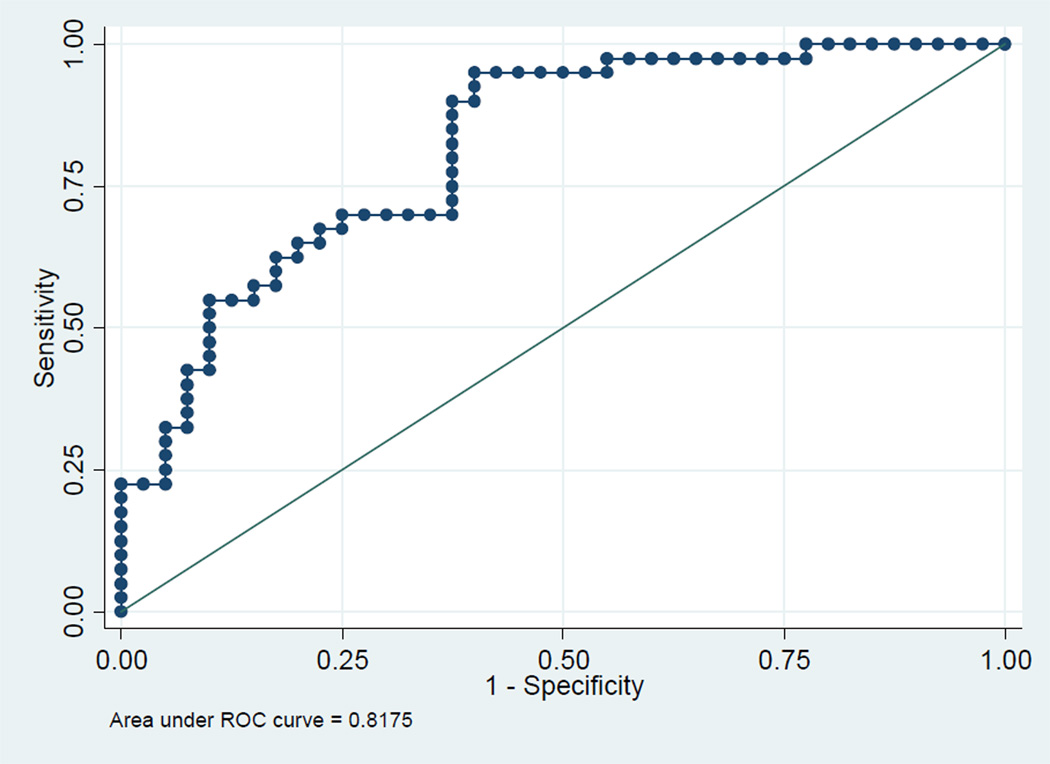
The median metabolomic prediction scores for PE using the combined significant metabolites are shown for all PE and early onset PE in figures 1A and 2A, respectively. The ROC curves for all PE and early onset PE are shown in figures 1B and 2B, respectively.
Fig. 1.
A Metabolomic prediction score for all preeclampsia from model combining all 4 significant metabolites
B ROC curve of full model with all 4 significant metabolites
Fig. 2.
A Metabolomic prediction score for early onset preeclampsia (delivered <34 weeks).
B ROC curve using all 4 analytes to predict early onset Preeclampsia (requiring delivery <34 weeks).
The detection rates for all PE at a 10% false positive (FPR) using the combination of significant metabolites is 50%; and 60% at a 20% FPR. For early onset PEthe combined model had a detection rate of 50% at a 10% FPR. The detection rate for early onset PE increased to 70% at a 20% FPR.
Because the acyl carnitine levels were only weakly significantly different between cases with PE and the control group, we evaluated a reduced model containing only the three significant amino acids listed in Table 3. The AUC for this limited model was 0.81 (95% CI, 0.72–0.88) with a similar detection rate for all PE at the different FPRs (Figure 3).
Fig. 3.
ROC curve for model containing only three significant amino acids.
Discussion
The data show that there are seven organic molecules elevated in maternal blood above controls in the first trimester of pregnancies that ultimately developed preeclampsia in the second half of pregnancy. Two of these were hydroxylated carnitine esters while 5 were amino acids. Following adjustment for potential confounders, four metabolites remained significant. These findings lay a foundation for prospective trials that use metabolomic signature markers in early pregnancy screening for PE.
Our study is novel as we targeted the maternal blood metabolome in the first-trimester. Interestingly Kenny et al (Kenny et al, 2011) evaluated maternal blood obtained at 15 weeks gestation in 60 women with PE compared with 60 matched controls. The fourteen metabolites significantly associated with PE were then validated in 39 other women who developed PE, compared with 40 controls. The AUC reported by the later study for their discovery and validation populations (0.94 and 0.90, respectively) are slightly higher than those in the current study. Importantly, the amino acids and acyl carnitine metabolites evaluated in that study are different from those in the current study. This underscores the need for standardization of protocols for future studies of the metabolome.
There is a great interest in identifying reliable markers that predict PE early in pregnancy. The aim of such efforts is to identify those pregnancies that could benefit from increased surveillance or preventive interventions (Cuckle, H 2011). The AUC and detection rates for PE seen in this study are similar to those reported in other studies evaluating the use of other serum markers in the first-trimester (Poon et al, 2010; Dugoff et al 2004; Goetzinger et al, 2010). For example, Goetzinger et al reported an AUC of 0.70 using first-trimester PAPP-A in predicting PE. Similar to reports from using other first-trimester serum markers, the metabolites appear to have better discriminating ability in detecting early onset PE.
Our study is not without limitations. The sample size is relatively small and therefore many metabolites that are not significant in this study may be found to be significant in future larger studies. For example, a post hoc power analysis showed that we had only 64% power to detect a difference in mean levels in some of the negatively-charged amino acids such as leucine and valine. We would require 50 cases of preeclampsia and 50 controls to have 80% power. Importantly, there are studies suggesting that the level of these metabolites can be affected by maternal obesity, type 2 diabetes and ethnicity (Adams et al, 2009; Mihalik et al, 2010). Due to the sample size limitation, we could not perform stratified analysis using a control group limited to obese or diabetic women. We therefore used logistic regression analysis to adjust for these confounders. Future validation studies should be matched for these potential confounders.
In addition we limited our data driven search for significant metabolites to two main groups of organic molecules, acyl carnitine and amino acids. Work is ongoing evaluating other possible metabolites. For example, quantitation of symmetric and asymmetric dimethylarginine may shed more light on the role of nitric oxide synthase in PE because these molecules are key regulators of nitric oxide synthase. Other predictors of PE may be derived from the circulating lipidome which contains more than 500 distinct molecular entities. (Quehenberger et al, 2010). The lipidome contains key mediators of a) vascular tone (sphingosine phosphates), b) inflammation (prostaglandins), c) insulin sensitivity (free fatty acids), and d) liver function (lipoproteins). Analysis of such additional biomarkers promises to further improve early detection of PE.
Our findings raises the possibility of using these metabolite signatures in designing future screening tests for PE and also may provide further insights into the pathogenesis of PE. Larger studies are needed to validate these findings. If such studies employ a similar design to the current study, the cases and control groups should be matched for the potential confounders discussed above.
Acknowledgement
Dr. Goetzinger is supported by a training grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5 T32 HD055172-02) and from a NIH/NCRR Washington University ICTS grant (UL1 RR024992). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
References
- Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139:1073–1081. doi: 10.3945/jn.108.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. Diagnosis and management of preeclampsia and eclampsia. Washington, DC: The College; 2002. ACOG practice bulletin no. 33. [Google Scholar]
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuckle HS. Screening for pre-eclampsia--lessons from aneuploidy screening. Placenta. 2011 Feb;32(Suppl):S42–S48. doi: 10.1016/j.placenta.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Dietzen DJ, Weindel AL, Carayannopoulos MO, Landt M, Normansell ET, Reimschisel TE, Smith CH. Rapid comprehensive amino acid analysis by liquid chromatography/tandem mass spectrometry: comparison to cation exchange with post-column ninhydrin detection. Rapid Commun Mass Spectrom. 2008;22:3481–3488. doi: 10.1002/rcm.3754. [DOI] [PubMed] [Google Scholar]
- Dugoff L, Hobbins JC, Malone FD, Porter TF, Luthy D, Comstock CH, Hankins G, Berkowitz RL, Merkatz I, Craigo SD, Timor-Tritsch IE, Carr SR, Wolfe HM, Vidaver J, D'Alton ME. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial) Am J Obstet Gynecol. 2004 Oct;191(4):1446–1451. doi: 10.1016/j.ajog.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Goetzinger KR, Singla A, Gerkowicz S, Dicke JM, Gray DL, Odibo AO. Predicting the risk of pre-eclampsia between 11 and 13 weeks' gestation by combining maternal characteristics and serum analytes, PAPP-A and free β-hCG. Prenat Diagn. 2010 Dec;30(12–13):1138–1142. doi: 10.1002/pd.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodacre R, Kell DB. Evolutionary computation for the interpretation of metabolome data. In: Harrigan GG, Goodacre R, editors. Metabolic Profiling: Its Role in Biomarker Discovery and Gene Function. Boston, MA: Kluwer Academic Publishers; 2003. pp. 239–256. [Google Scholar]
- Kenny L, Dunn W, Ellis D, Myers J, Baker P, Consortium G, Kell D. Novel biomarkers for pre-eclampsia detected using metabolomics and machine learning. Metabolomics. 2005;1:227–234. [Google Scholar]
- Kenny LC, Broadhurst D, Brown M, Dunn WB, Redman CW, Kell DB, Baker PN. Detection and identification of novel metabolomic biomarkers in preeclampsia. Reprod Sci. 2008;15:591–597. doi: 10.1177/1933719108316908. [DOI] [PubMed] [Google Scholar]
- Kenny LC, Broadhurst DI, Dunn W, Brown M, North RA, McCowan L, Roberts C, Cooper GJ, Kell DB, Baker PN Screening for Pregnancy Endpoints Consortium. Robust early pregnancy prediction of later preeclampsia using metabolomic biomarkers. Hypertension. 2010 Oct;56(4):741–749. doi: 10.1161/HYPERTENSIONAHA.110.157297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, DeLany JP. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 2010;18:1695–1700. doi: 10.1038/oby.2009.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oresic M, Simell S, Sysi-Aho M, Nanto-Salonen K, Seppanen-Laakso T, Parikka V, Katajamaa M, Hekkala A, Mattila I, Keskinen P, Yetukuri L, Reinikainen A, Lahde J, Suortti T, Hakalax J, Simell T, Hyoty H, Veijola R, Ilonen J, Lahesmaa R, Knip M, Simell O. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J Exp Med. 2008;205:2975–2984. doi: 10.1084/jem.20081800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon LC, Akolekar R, Lachmann R, Beta J, Nicolaides KH. Hypertensive disorders in pregnancy: screening by biophysical and biochemical markers at 11–13 weeks. Ultrasound Obstet Gynecol. 2010 Jun;35(6):662–670. doi: 10.1002/uog.7628. [DOI] [PubMed] [Google Scholar]
- Quehenberger O, Armando AM, Brown AH, Milne SB, Myers JS, Merrill AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- SPENCER K, COWANS NJ, NICOLAIDES KH. Low levels of maternal serum PAPP-A in the first trimester and the risk of pre-eclampsia. Prenat Diagn. 2008;28:7–10. doi: 10.1002/pd.1890. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Tuuli M, Odibo AO. First-trimester assessment of placenta function and the prediction of preeclampsia and intrauterine growth restriction. Prenat Diagn. 2010 Apr;30(4):293–308. doi: 10.1002/pd.2475. [DOI] [PubMed] [Google Scholar]