Abstract
Background
Cutaneous thermal injuries (i.e. burns) remain a common form of debilitating trauma and outcomes are often worsened by wound infection with environmental bacteria, chiefly Pseudomonas aeruginosa.
Materials and Methods
We tested the effects of early administration of a single dose of azithromycin, with or without subsequent anti-pseudomonal antibiotics, in a mouse model of standardized thermal injury infected with P. aeruginosa on both wound site and systemic infection. We also tested the antimicrobial effects of these antibiotics alone or combined in comparative biofilm and planktonic cultures in vitro.
Results
In our model, early azithromycin administration significantly reduced wound and systemic infection without altering wound site or circulating neutrophil activity. The antimicrobial effect of azithromycin was additive with ciprofloxacin but significantly reduced the antimicrobial effect of tobramycin. This pattern was reproduced in biofilm cultures and not observed in planktonic cultures of P. aeruginosa.
Conclusion
these data suggest that early administration of azithromycin following burn-related trauma and infection may reduce P. aeruginosa infection and potential interactions with other antibiotics should be considered when designing future studies.
Keywords: Burn, Burn wound, Pseudomonas aeruginosa, Thermal injury, Azithromycin, Tobramycin, Ciprofloxacin, Biofilm, Drug-drug interaction, Cystic fibrosis
Introduction
Cutaneous thermal injury (i.e. skin burn) is one of the most common and debilitating forms of trauma. Each year there are over 1.3 million fires in the United States, and approximately 45,000 of these involve human injury severe enough to require hospitalization1. Burns continue to be a common cause of combat related trauma and often the majority of those injured are civilian rather than military personnel2. Though burn trauma critical care and outcomes have improved, there are still greater than 3,000 deaths annually1. After reaching the hospital, outcomes are often worsened by an acquired state of immunosuppression complicated by opportunistic infection. In patients with >40% of total body surface area (TBSA) burn, 75% of deaths are now secondary to infectious complication (e.g. pneumonia, sepsis) or inhalation injury rather than shock.3–6 Infection is an even greater cause of death from burn trauma in military personnel than in the general population7. This disparity may be related to challenges with rapid access to advanced medical care.
Despite aggressive local and systemic treatment to minimize infection, severe burn wounds continue to become infected with environmental and nosocomial pathogens at relatively high rates. Among these, Pseudomonas aeruginosa is paramount, accounting for over half of all severe burn infections8. This gram negative bacteria is well adapted to the environment, utilizing biofilm colony growth which provides a tremendous survival advantage for the pathogen and effectively prevents eradication by the host immune system or antimicrobial drug treatment. A recent review of burn trauma patients that acquired secondary infection with P. aeruginosa reported that mortality was approximately four fold greater than those without P. aeruginosa, with an average of 23 ventilator assisted days in P. aeruginosa infected patients9. Historically, mortality in burn patients with P. aeruginosa bacteremia has been as high as 77% over a 25 year period10. In light of such high incidence of pulmonary infection and morbidity in severe burn related trauma, interventions capable of limiting systemic spread to the lung may be useful adjuncts to current therapy.
Excessive neutrophil accumulation, combined with impaired clearance of the dead and dying leukocytes, has been shown to worsen tissue damage at injured sites. Recent studies also find that neutrophil products can accelerate P. aeruginosa biofilm formation, a key feature of infected burn wounds11–13. As neutrophils undergo necrosis, long strands of DNA and F-actin are released into the inflammatory milieu and polymerize through covalent attraction. P. aeruginosa can exploit the neutrophil-rich environment by utilizing these polymers as scaffolding, significantly enhancing early biofilm development11–13. Therefore, early and excessive neutrophil recruitment to the site of injury may by a therapeutic target when trying to minimize wound infection.
The pathological confluence of altered immune function, neutrophilic inflammation, and biofilm-enhanced P. aeruginosa infection present in thermal injury is also central to airway diseases such as cystic fibrosis (CF) and diffuse panbronchiolitis. In these chronic pulmonary conditions, macrolide therapy can effectively reduce neutrophilic inflammation and improve longterm outcomes14–17. The mechanism by which this occurs is multifactorial and not completely understood, as numerous antimicrobial and anti-inflammatory or immunomodulatory properties have been reported for azithromycin therapy16,18–21. Given the apparent efficacy of macrolide therapy in CF and other diseases, we hypothesized that azithromycin would reduce P. aeruginosa infection and systemic spread when administered early in a model of cutaneous burn with P. aeruginosa wound inoculation. Our data support this hypothesis. We also sought to test the impact of early azithromycin administration on more conventional anti-pseudomonal antibiotics including ciprofloxacin and tobramycin. Our data indicate that this macrolide may inhibit the antimicrobial effect of tobramycin against P. aeruginosa, particularly in a biofilm growth state.
Materials and Methods
Animals
Eight-week-old sex matched C57BL/6J mice ranging in weight from 17g–25g were obtained from Jackson Laboratories (Bar Harbor, ME). Animal care and use were in accordance with the Institutional Animal Care and Use Committee (IACUC) and with the permission of National Jewish Health. Animals were housed in microisolator cages within a clean, pathogen-free animal facility and fed irradiated chow to minimize the risk of bacterial contamination. All animals undergoing thermal injury were anesthetized with a single intraperitoneal (i.p.) injection of 0.1 mL of 0.1% Xylazine – 1% Ketamine solution. Three days before injury, hair was removed over the dorsum using an electric shaver and depilation cream (Surgi-Cream; Church & Dwight co., Inc.) to expose the skin surface as described22.
Thermal injuries
A ten percent total body surface area (TBSA), partial-thickness, third-degree burn was made on the exposed skin of anesthetized mice as previously described22–24. Our modified technique employed a uniform thermal injury by exposing the depilated area for 5 seconds to a round brass probe (diameter: 28 mm) heated to thermal equilibration with boiling tap water23. Sterile saline (2 mL i.p.) was administered to support fluid balance during recovery. Mice were then placed under a warming light and supervised until full recovery. Control mice were shaved but no thermal injury was performed.
Infectious Challenge
A derivative of P. aeruginosa strain PAO1 was obtained from the Pseudomonas Genetic Stock Center (East Carolina University). Bacteria was grown overnight in 2% heat-inactivated platelet poor pooled human plasma (HIPP) RPMI liquid media at 37 C with shaking and adjusted to an optical density at 600 nm (OD600) of 0.30 (corresponding to 5×108 cfu/ml) before dilution. Viable bacterial counts were performed by serial dilutions and plating on solid P. aeruginosa selective media to determine the exact stock titer on the day of each experiment. Before the bacterial challenge, the depilated skin surface of all the anesthetized mice was abraided with an 18G needle to promote infection after bacterial inoculation. Control mice without thermal injury received the same abrasion injury. Two hours following thermal injury, P. aeruginosa suspension (100 μL) containing 1×106 cfu in pre-sterilized saline was placed on the wound site and remained in place while the mice recovered from anesthesia. Body weights were recorded at the time of injury and daily thereafter.
Antibiotic treatments
The timing of antibiotic administration was designed to test the effect of an intervention that could be easily administered outside of a medical setting and conventional antibiotics commonly provided in an advanced medical care setting. Antibiotics were obtained from the National Jewish Health pharmacy (Denver, CO) and prepared in sterile saline. Azithromycin was administered as a single dose (20mg/kg i.p.) injection 6 hours following thermal injury (4 hours following inoculation with P. aeruginosa). Tobramycin was administered beginning 24 hours after thermal injury and once daily (10 mg/kg/dose i.p.) for a total of 3 doses. Ciprofloxacin was administered beginning 24 hours after thermal injury and twice daily at 20 mg/kg/dose i.p. through 72 hours. Equal volume of sterile saline was administered i.p. as a control intervention for mice not receiving antibiotics at each time point.
Tissue Collection
Mice were euthanized 72 hours after the thermal injury using CO2 exposure per IACUC approved methods. After confirming death, skin from the injured region, lungs and spleen were collected and tissue surface was decontaminated with 95% ethanol. Blood samples were obtained from the retro-orbital plexus of mice for quantification of circulating leukocytes by H/E staining on glass slides.
Viable bacterial counts
Using a sterilized 5mm biopsy punch (Miltex, Inc., York, PA), 2 skin samples from each euthanized animal were obtained and suspended in 1mL of sterile PBS containing 0.01% Triton X-100. Skin specimens were incubated for 1hr at 37°C with rotat ion. Lungs and spleen were collected and separately suspended in 1mL of sterile PBS. All tissue samples (skin, lung and spleen) were homogenized with a blend of stainless steel beads ranging from 0.9–2.0 mm in a Bullet Blender tissue homogenizer (Next Advance, Inc., Averill Park, NY) per manufacturer instructions. Homogenates were diluted with sterile saline and serially plated on pseudomonas isolation agar (PIA) plates (Becton, Dickinson and Company, Franklin Lakes, NJ). Plates were incubated overnight at 37°C and P. aeruginosa colonies were counted.
Myeloperoxidase (MPO) assay
MPO assay was performed on skin samples to measure neutrophil accumulation at the wound site. Briefly, 2 biopsies were obtained as above, weighed, transferred to microcentrifuge tubes and flash frozen in liquid nitrogen. The tissue was suspended in HTAB buffer (0.5% w/v) (Sigma-Aldrich, St. Louis, MO) and pulverized in a tissue grinder (Kimble Chase, Vineland, NJ). Samples were centrifuged at max speed for 15min at 4C. The resultant supernatant was assayed for MPO activity using a spectrophotometric reaction with O-dianisidine hydrochloride (Sigma-Aldrich, St. Louis, MO) at 460 nm25.
In vitro flow-cell biofilm culture
A second derivative of P. aeruginosa strain PAO1 was obtained from M. Vasil (University of Colorado Health Sciences Center). This strain was transformed with plasmid pMF230 to express GFP (M. Franklin, Center for Biofilm Engineering at Montana State University, Bozeman, MT), and was grown in modified SCFM medium26 with one-tenth the concentration of amino acids (0.1× SCFM). Stock solutions of azithromycin (APP Pharmaceuticals, Schaumburg, IL) and tobramycin (X-GEN Pharmaceuticals, Inc., Northport, NY) were prepared in sterile water, aliquoted, and stored at −80°C. Ciprofloxacin solution (Hospira, Inc., Lake Forest, IL) was aliquoted and stored at −80°C. Drug aliquots were diluted in 0.1× SCFM prior to use.
Biofilms of PAO1 pMF230 were grown in three-channel flow cells with individual channels 1 × 4 × 40 mm (Technical University of Denmark, Lyngby). Each flow cell was covered with a glass coverslip (50 ×24 mm; Fisher Scientific, Pittsburgh, PA). The flow cell system was prepared and assembled as described27. The system was flushed with 0.1× SCFM at 37°C for 2 h prior to inoculation. An overnight culture of PAO1 pMF230 was grown at 37°C with aeration, diluted to an OD600 of 0.05, and 250 μL injected into each flow cell channel. To allow attachment of bacteria to the substratum, flow cells were incubated without flow at 37°C for 1 h. After resumption of flow (0.1× SCFM), biofilms were grown at 37°C for 64 h, then exposed to the same medium containing azithromycin (20 μg/mL), tobramycin (40 μg/mL), or ciprofloxacin (4 μg/mL), alone or in combination, for an additional 22 h. Propidium iodide (15 μM) was also added to the medium to stain dead cells.
Biofilms were imaged on an LSM 700 confocal scanning laser microscope (CSLM) with a Plan-Neofluor 40× / 0.75 objective (Carl Zeiss, Jena, Germany). Solid-state diode lasers (488 nm and 555 nm) and appropriate filter sets were used for excitation and detection of GFP and propidium iodide. Image stacks were acquired at three random positions in the center region of each flow cell lane at a distance of 5–10 mm from the inlet with z-stack intervals of 1.5 μm28. COMSTAT image analysis software was used to quantify live and dead biomass volume29. The change in mean % decrease in live biomass volume provided an estimate of the anti-biofilm effects of each drug regimen, and the SD provided an estimate of the variance of the mean.
In vitro Planktonic Culture in Antibiotics
To test the effect of azithromycin addition to tobramycin or ciprofloxacin in a non-biofilm growth condition, the same strain of P. aeruginosa used in the biofilm culture experiments was cultured in 5 mL of Mueller Hinton broth at 37 C with continuous shaking in aerobic liquid bacterial culture vials for 24 hours. Minimal inhibitory concentration (MIC) break points were determined by visual inspection over a log-2 scale of antibiotic concentration for ciprofloxacin and tobramycin separately.
Simultaneous P. aeruginosa cultures were then repeated for 24 hours over a log-2 scale with each antibiotic with or without the addition of 20 mcg/mL azithromycin to test the effect of azithromycin in this system. This is the concentration of azithromycin used in the biofilm flow-cell experiments. The planktonic culture experiments were replicated 3 times to ensure consistent break point data.
Statistical Analysis
Normative data are represented by mean value +/− standard error of the mean (SEM). Non-normative data (i.e. CFU data) are represented as mean with 95% confidence intervals (CI). Kuskal-Wallis test was used for multiple comparisons in nonparametric analysis, non-matched conditions. Wilcoxon test was used in matched pairs, nonparametric analysis. Friedman test was used for multiple comparisons in matched, non-parametric, multiple comparisons. Combination antibiotic testing in vivo used Mann-Whitney unpaired t-testing and in vitro studies used unpaired T-tests with Welch correction.
Results
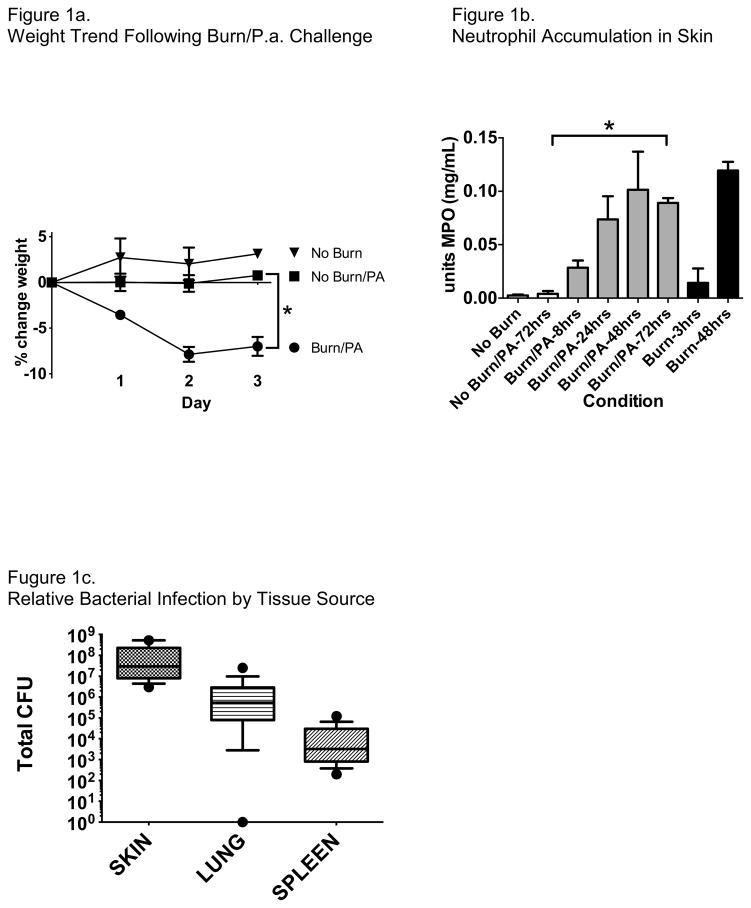
In our experimental model of cutaneous thermal injury and wound infection with P. aeruginosa, we find that both neutrophil recruitment to the injury site and weight loss in the animals are highly dependent on thermal injury rather than bacterial inoculation (Figure 1). Host inflammatory response appears to peak at 48 hours after injury. As expected, the burden of bacterial infection measured 72 hours after wound inoculation was greatest at the wound site but consistently spread to systemic organs as measured in the lung and spleen (Figure 1c). Thus, we believe that this experimental design sufficiently models both local and systemic infection of P. aeruginosa originating from a burn wound and allows us to test the impact of antibiotics either alone or in combination.
Figure 1.
Mice challenged with combined cutaneous burn and infection with PAO1 had significantly greater weight loss compared with mice not burned, with or without PAO1 challenge (Figure 1a, mean +/− SEM, *P<0.01). Weight loss was maximal at 48 hours after challenge. Skin tissue myeloperoxidase content, reflecting neutrophil accumulation, steadily increased in mice challenged with burn and PAO1, peaking by 48 hours (Figure 1b, mean +/− SEM). Mice without thermal injury but challenged with PAO1 did not have increased MPO content in the skin through 72 hours, indicating that there was no significant neutrophil recruitment to the wound without thermal injury. *P<0.001. Burden of bacterial infection was significantly greater at the skin wound and inoculation site, but consistently had spread to both the lungs and spleen by 72 hours after inoculation (Figure 1c, mean +/− 95% CI).
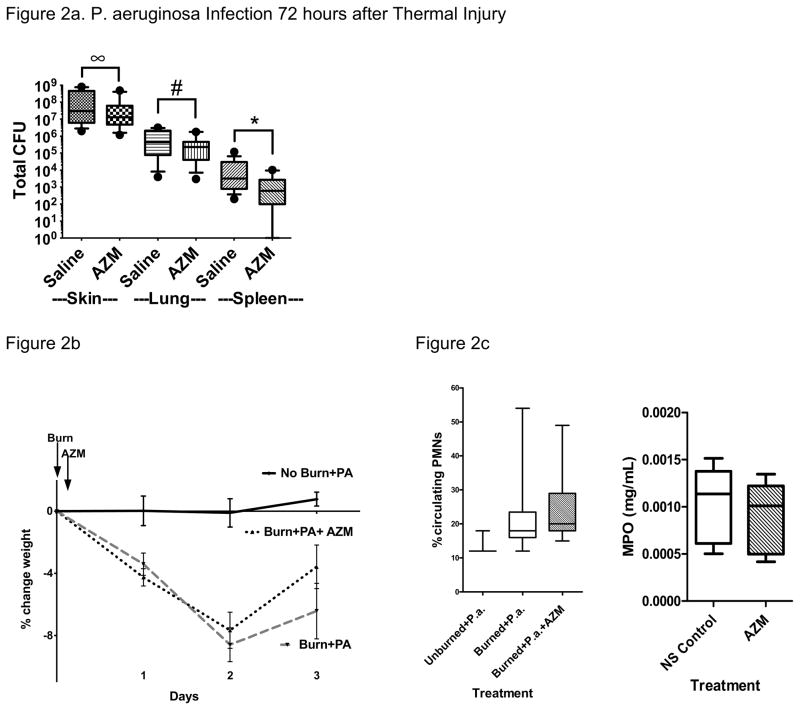
Our first analysis was the impact of a single, physiologically relevant dose of azithromycin administered intraperitoneally (IP) 2 hours following thermal injury. We found modest yet significant decreases in wound site and systemic bacterial loads with this intervention alone (Figure 2). We also observed a non-significant trend toward better weight recovery in animals treated with azithromycin compared with placebo. We did not detect a change in wound site or circulating neutrophil concentration in response to azithromycin in our model (Figure 2c).
Figure 2. Effect of Azithromycin on Local and Systemic Bacterial Infection.
A single administration of azithromycin (20 mg/mL i.p.) 4 hours following inoculation of the burn wound significantly reduced P. aeruginosa infection both at the burn site and systemically in the lung and spleen (Figure 2a, mean +/− 95% CI. ∞P=0.05, #P<0.001, *Spleen P<0.05). Graphs reflect N=15 from 5 replicate experiments. (NS, normal saline control; AZM, azithromycin; *P<0.05, #P<0.01).
Azithromycin group trended toward greater weight recovery at 72 hours following challenge but this was not statistically significant (Figure 2b). Azithromycin did not significantly affect the number of circulating neutrophils in the peripheral blood or neutrophil accumulation at the site of injury (2c) measured at 72 hours.
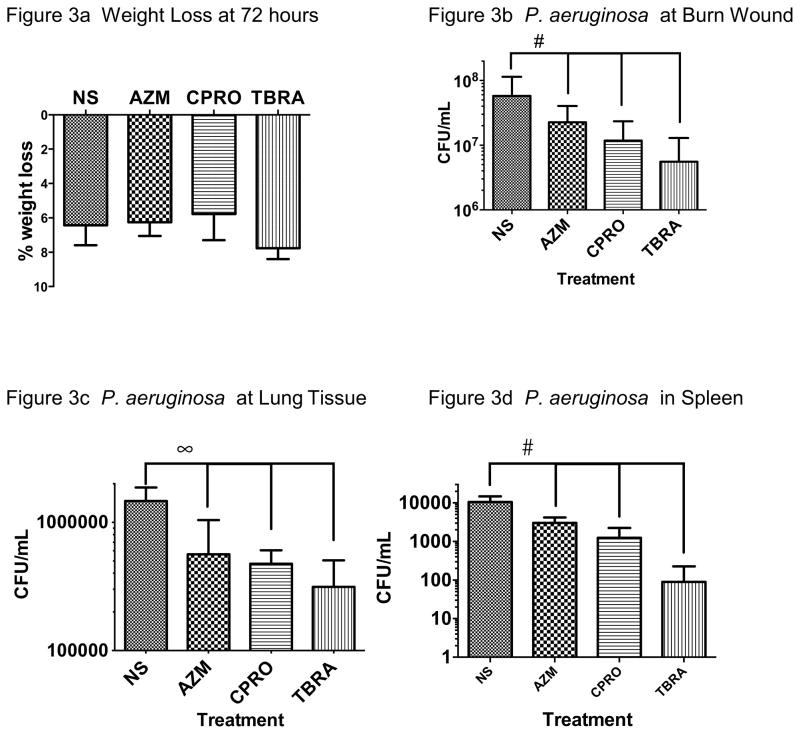
We next compared the effects of monotherapy with azithromycin (single dose at 4 hours), ciprofloxacin, or tobramycin (each provided from 24 to 72 hours). In our model, there was no significant effect on weight loss but we observed reduced bacterial load at the wound site, lung and spleen with each antibiotic compared with normal saline (NS) (Figure 3). Tobramycin was significantly more effective than azithromycin (P<0.02) but no other significant differences between antibiotics were seen.
Figure 3. Effect of Antibiotic Monotherapy.
Mice treated with each of the three antibiotics tested as monotherapy showed similar weight loss to saline control at 72 hours after challenge (Figure 3a, mean +/− SEM, N=10–16). Azithromycin, ciprofloxacin, or tobramycin administration each reduced both wound site (Figure 3b, #P<0.01) and systemic infection of PAO1 (Figures 3c, 3d, ∞P<0.001, #P<0.01) when compared with saline control.
The comparison with saline control was statistically significant and tobramycin appeared to be superior as monotherapy in concentrations tested, but no statistically significant difference was observed between antibiotics. NS, normal saline; AZM, azithromycin; CPRO, ciprofloxacin; TBRA, tobramycin.
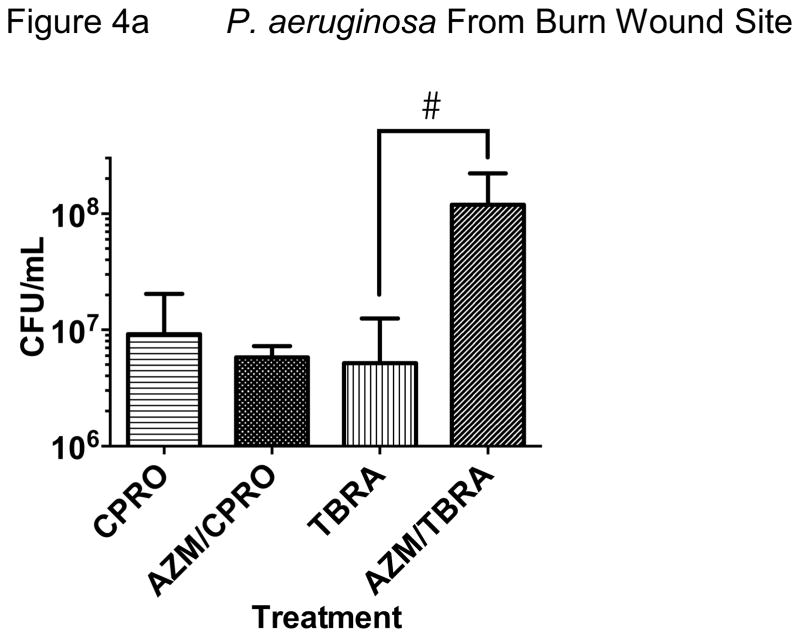
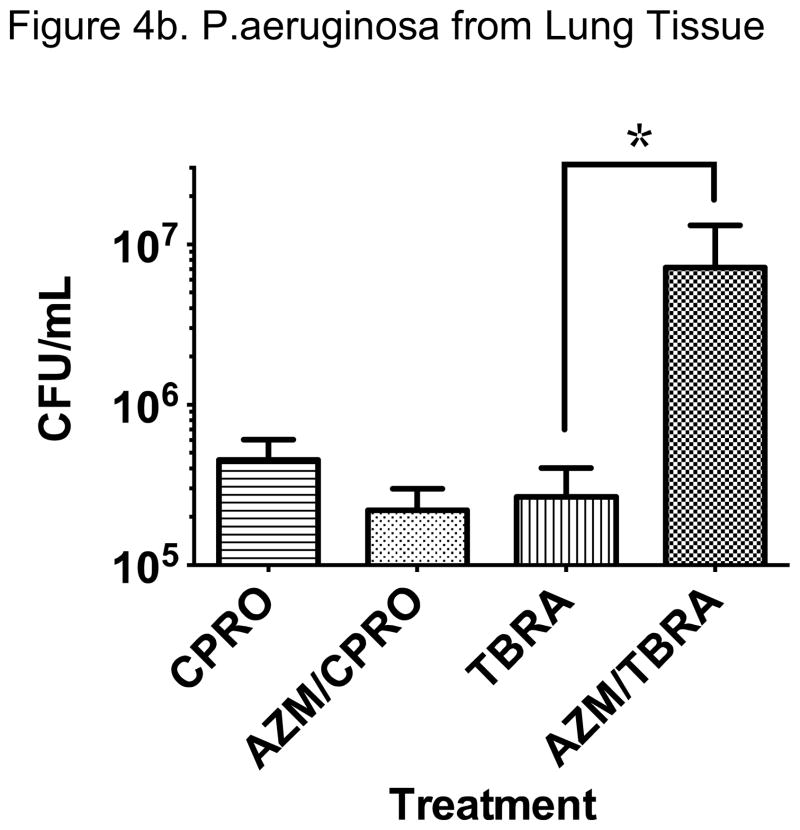
We next tested the effect of early azithromycin administration on the antibacterial properties of subsequent ciprofloxacin or tobramycin, based on our protocol. We observed that azithromycin trended toward less bacterial growth at the wound site and lung when compared with ciprofloxacin alone (Figure 4). The combination of azithromycin and ciprofloxacin was not statistically superior to ciprofloxacin alone in our model but did result in the least P. aeruginosa growth of any of the antibiotic regimens tested. Importantly, we found much greater bacterial density at the wound site and lung in animals treated with azithromycin and tobramycin when compared with tobramycin alone, suggesting antagonism of the antibacterial properties of tobramycin against P. aeruginosa (Figure 4). These data were replicated in repeat experiments with consistent effects in our model.
Figure 4. Divergent Effects of Azithromycin when Added to Ciprofloxacin or Tobramycin.
We observed a difference in effect of early administration of azithromycin with subsequent ciprofloxacin or tobramycin on P. aeruginosa infection at the skin. A trend toward additive antimicrobial effect with ciprofloxacin was noted while apparent antagonism with tobramycin was observed. Significantly greater bacteria were cultured from the wound when azithromycin preceded tobramycin administration compared with tobramycin administration alone (Figure 4a; #P<0.01, N=8–10 from 3 replicate experiments).
Early administration of azithromycin appeared to increase bacterial spread to the lung in animals treated with tobramycin compared with tobramycin alone (*P<0.02, N=12–15). This was not observed with azithromycin and ciprofloxacin where the combination resulted in a reduction in lung bacterial density that was not statistically significant compared to ciprofloxacin alone. Splenic P. aeruginosa growth showed similar trends but was relatively low in all antibiotic treatment groups and no significant differences were observed (data not shown).
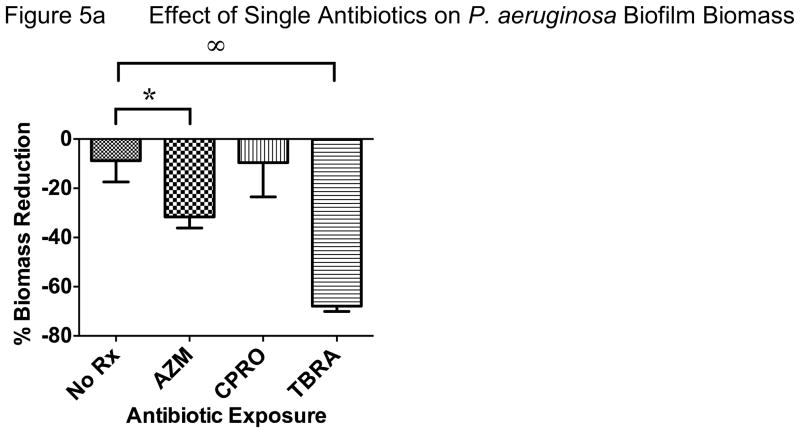
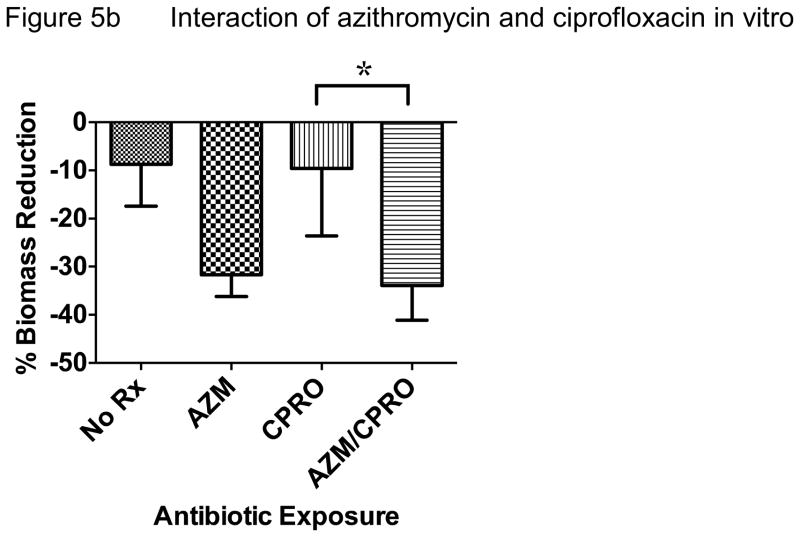
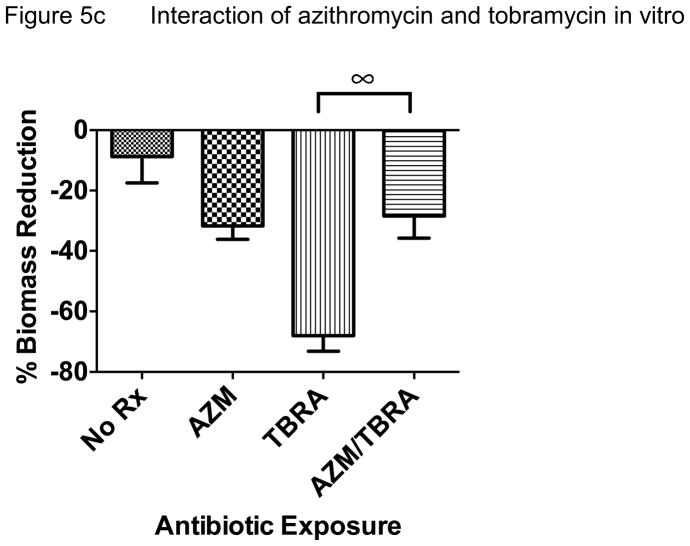
In an effort to reproduce these data in another model system and test if host responses are critical to the apparent interactions between antibiotics, we performed in vitro bacterial culture experiments without host tissue and compared biofilm vs. planktonic growth conditions. When P. aeruginosa was grown as a biofilm and exposed to antibiotic regimens tested in vivo, we observed very similar effects. Azithromycin and tobramycin both significantly reduced biomass, a common outcome measure of biofilm bacterial density (Figure 5a). Tobramycin was more effective than azithromycin and ciprofloxacin did not demonstrate antibacterial effects compared with no antibiotic control in this model. However, when azithromycin was added to ciprofloxacin, this combination was significantly better than ciprofloxacin alone, though not significantly better than azithromycin alone (Figure 5b). Consistent with our in vivo experimental data, the addition of azithromycin significantly reduced the antibacterial effect of tobramycin (Figure 5c). Overall, we observed that azithromycin improved the antibacterial effect of ciprofloxacin while inhibiting this effect for tobramycin when tested against P. aeruginosa. Much of the improvement observed with ciprofloxacin may be due to azithromycin alone, given similar reduction in biomass observed with azithromycin alone.
Figure 5. In Vitro Biofilm Culture.
P. aeruginosa biomass reductions (i.e. antimicrobial effect) after 22 hours flow compared with baseline were: no antibiotic 9%, azithromycin 32%, ciprofloxacin 10%, tobramycin 68%. Ciprofloxacin (4 mcg/mL) had no statistically significant effect on P. aeruginosa biofilm biomass when compared with no antibiotic exposure. Azithromycin and especially tobramycin (40 mcg/mL) were more effective than no treatment or the other antibiotics tested (*P<0.05, ∞P <0.001).
The addition of azithromycin to ciprofloxacin was significantly more effective at reducing P. aeruginosa biomass than ciprofloxacin alone (*P<0.05). This combined treatment resulted in 34% P. aeruginosa biomass reduction compared with 10% observed with ciprofloxacin alone.
When azithromycin was added to tobramycin, the effective biomass reduction was lost (68% to 28%, P<0.001) and was no longer statistically significant when compared with no treatment or azithromycin alone.
To determine if interactions between antibiotics observed in our animal model of burn wound were likely dependent on a bacterial biofilm growth state, we repeated the in vitro P. aeruginosa culture experiments using the same strain of bacteria and concentration of azithromycin under planktonic growth conditions. We observed that azithromycin slightly improved the antibacterial properties of both ciprofloxacin and tobramycin (Table 1). This small reduction in MIC is unlikely to be significant but suggests that the ability of azithromycin to inhibit tobramycin may depend on the growth state of P. aeruginosa. Appreciating that biofilms are widely recognized as a critical element of burn wound infection with P. aeruginosa, these data also indirectly support the validity of our animal model.
Table 1.
Effect of Azithromycin in Planktonic Growth Conditions
| MIC without azithromycin | MIC with azithromycin | |
|---|---|---|
| tobramycin | 1.0 mcg/mL | 0.5 mcg/mL |
| ciprofloxacin | 1.0 mcg/mL | 0.5 mcg/mL |
Addition of azithromycin to tobramycin or ciprofloxacin had no significant effect on the minimal inhibitory concentration (MIC) of these antibiotics after 24 hours in a shaking liquid culture at 37 C (Table 1). The slight reduction in MIC for both ciprofloxacin and tobramycin is unlikely to be a significant change but clearly does not indicate antagonism of tobramycin during planktonic bacterial growth conditions. These experiments used the same P. aeruginosa bacterial strain and concentration of azithromycin as that used in the biofilm culture model above. Data were replicated 3 times to ensure consistency. Azithromycin alone did not provide an MIC break point through 20 mcg/mL indicating a lack of antimicrobial effect as monotherapy (data not shown).
Discussion
Cutaneous thermal injury continues to be a major cause of morbidity and mortality. In the setting of severe burn, wound infections (e.g. P. aeruginosa) and systemic spread are critical determinants of outcome. Therefore, anti-infective strategies are central to medical care in the burn setting. The opportunity to intervene early after thermal injury may also be important, particularly if advanced care may be delayed such as in combat-related trauma.
In an animal model of skin burn wound similar to that published by other researchers22,24,30, we find that a single systemic administration of the macrolide antibiotic azithromycin shortly after thermal injury significantly reduced local and particularly systemic pulmonary infection with P. aeruginosa. The magnitude of treatment effect was similar to that observed with ciprofloxacin or tobramycin monotherapy commenced 24 hours after injury and continued for an additional 48 hours. Azithromycin did not alter local or systemic neutrophil recruitment in this model. Numerous antimicrobial and immunomodulatory properties have been reported for azithromycin when used to target P. aeruginosa infection7,8,16,18,19. These prior investigations have largely been limited to studies of airway infection—commonly modeling airway diseases such as cystic fibrosis. Though precise mechanisms of action were not explored in our experiments modeling thermal injury, azithromycin may have conferred antimicrobial effects against P. aeruginosa rather than immunomodulatory effects in the host animals. Azithromycin administered shortly after wound inoculation may also have inhibited bacterial biofilm formation. This effect was not directly tested in our experiments but has been previously reported19,31,32. Reductions in the burden of bacterial infection were similar with exposure to azithromycin, ciprofloxacin, or tobramycin alone at the predetermined times and concentrations tested. Clearly the earlier timing for azithromycin administration compared with the other more conventional anti-pseudomonal antibiotics may have biased the data but the study was designed to test the effect of a treatment that could be easily administered outside of a medical setting.
One of the most interesting observations was the divergent effect of azithromycin when administered in combination with either ciprofloxacin or tobramycin. Azithromycin treatment with subsequent ciprofloxacin generally provided greater antibacterial effect, and was the most successful of any treatment protocol tested in vivo. This was particularly true when testing systemic spread to the lung. Conversely, administering azithromycin and subsequent tobramycin resulted in significant loss of antimicrobial effect observed with tobramycin alone. These animals had P. aeruginosa infection at both the wound site and systemic organs that were no better than that of saline control.
Follow up in vitro experiments employing biofilm culture techniques with constant flow antibiotic exposure were consistent with the in vivo findings. These data demonstrated that azithromycin improved the antimicrobial effect of ciprofloxacin while significantly inhibiting the effectiveness of tobramycin. This culture method does not incorporate tissue or leukocyte elements of bacterial clearance and therefore these data also suggest that the observed interactions between antibiotics are likely independent of any immunomodulatory effects. In vitro experiments employing planktonic culture conditions and the same strain of bacteria did not show an effect of azithromycin added to ciprofloxacin or tobramycin. Though unlikely significant, azithromycin at the same concentration as that used in the biofilm culture model, resulted in a slight decrease in tobramycin MIC under planktonic growth conditions. Combined, these in vitro data suggest that an apparent interaction between azithromycin and tobramycin may depend largely on the state of bacterial growth. Greater mechanistic detail of these interactions is clearly needed and a topic of ongoing investigation. Appreciating that azithromycin greatly concentrates in leukocytes, these data may be clinically relevant to diseases with significant and ongoing neutrophilic inflammation, such as CF airway disease.
Other laboratories have identified a potential antagonism between azithromycin and tobramycin in vitro using more traditional biofilm culture techniques and other strains of P. a.33,34. Whether or not the apparent drug-drug interaction is specific to certain bacterial strains or species is unknown but similar findings from multiple laboratories using different bacterial strains raises concern that this effect may be significant. Azithromycin has a shared dicationic structure with aminoglycosides (i.e. tobramycin) and these chemicals may competitively interact in self-promoted uptake mechanisms when targeting gram negative bacteria35. Ciprofloxacin does not utilize this mechanism to target gram negative bacteria, which may help explain why its antimicrobial effects were not inhibited by azithromycin in our models. Other macrolides are not dicationic and it has been hypothesized that this may explain both the extended gram negative activity observed for azithromycin and also the lack of antagonism observed between clarithromycin and tobramycin in vitro36. Azithromycin and tobramycin both target the ribosome in bacteria, though at different subunit sites, and it is unknown if this relates to the observations in our study.
To our knowledge, these data are the first in vivo demonstration of anti-Pseudomonal effects of azithromycin in a burn wound model, and the first in vivo demonstration of an apparent interaction with tobramycin when targeting P. aeruginosa infection. In our model, the combined thermal injury and bacterial infection developed pronounced and yet variable tissue destruction at the wound site. We believe that this variability in wound severity may contribute to sampling error and inherent imprecision when quantifying skin bacterial burden. However, measuring the level of systemic spread to the lung is more uniform and this model and captures the entire pulmonary tissue source, a site remote from tissue destruction or antibiotic administration. Consistent findings from the in vitro biofilm culture model strengthens our confidence in these data. Together, these studies identify a potential antagonism between azithromycin and tobramycin when targeting P. aeruginosa infection—particularly in a biofilm growth state. The relationship with ciprofloxacin in both models, though not as strong, was often additive and clearly not antagonistic.
Azithromycin is not commonly prescribed to burn patients, but is used as chronic therapy for more than 70% of patients with cystic fibrosis (CF) who have persistent pulmonary infection with P. aeruginosa. Increasingly, CF patients are being prescribed chronic azithromycin before acquisition of Pseudomonas for its purported immunomodulatory benefit. This is relevant as the common eradications strategy to clear early P. aeruginosa in the airway, supported by a large clinical trial in CF, is inhaled tobramycin37. Tobramycin is also the most frequently used systemic antibiotic when treating patients with CF during acute pulmonary exacerbations, and therefore potential antagonism between these drugs should be further explored. In published subgroup analysis of data from the original clinical trial of chronic azithromycin in CF, subjects using inhaled tobramycin had 1/3 the benefit (i.e. improved lung function) when compared to subjects not using tobramycin38. The effect in subjects using both inhaled tobramycin and oral azithromycin, though positive, was not statistically significant when compared to placebo. In vitro data indicate that this interaction may be specific to azithromycin and not to other macrolides, which have also shown some of the beneficial properties for which this therapy was originally tested in CF.33 However, a recent clinical trial of clarithromycin in CF patients failed to show clinical improvement39.
We believe that the significant antimicrobial effects of a single dose of azithromycin given shortly after injury are also potentially important to medical care for burn-related trauma, including combat-related injury. There are 128 burn centers in the US, but only 25% of the population live within 1 hour of a center, and only 46% live within 2 hours40. More so, military and combat related burns are often triaged and managed outside of burn centers, with severe cases being later transported to higher levels of care2. Therefore, when trying to minimize morbidity and systemic spread of infection, a safe, widely available that could be self-administered or given by medical personnel prior to transfer to a regional burn center would be a useful adjuvant to current therapy. Further studies of this effect, including the determination of specificity for azithromycin and alternative dosing regimens, may be warranted. Many critically ill burn patients are treated with tobramycin, and this should be considered in the design of any future study design.
There are limitations to these studies and these data cannot be extrapolated to directly inform human disease and therapy. Both the burn and infection models are artificial and standardized, and do not adequately represent the breadth or complexity of injury seen in medical burn centers. In our model, the burn site was abraded, similar to many combat related traumas, but this may not sufficiently recapitulate the type of tissue injury often seen. Likewise, repeated bacterial contamination and polymicrobial infections are frequently encountered in clinical practice and were not explored here. In this study we have focused on what appears to be the most common bacterial pathogen encountered but recognize that any investigation of antibiotic effectiveness must consider the greater complexity of trauma-related infections. We have constrained our study to a strain of P. a. that was originally collected from a burn wound site (PAO1). As indicated above, others have reported similar patterns of drug interaction in vitro using alternative strains of P. aeruginosa and we are now expanding this research to multiple CF clinical isolates from the airway. In the present experiments, we tested limited concentrations of the antibiotics in vivo and in vitro, taken from published literature review and at doses considered relevant to human diagnostic testing and medical care. This limitation may explain some of the minor differences observed in vivo compared with in vitro—particularly where the in vitro efficacy of azithromycin alone was not statistically significant. Alternatively, antimicrobial effects observed with azithromycin as monotherapy may require biofilm growth conditions and/or immunomodulatory effects on the host. Despite these limitations, our data raise both promise for early azithromycin use and concern over potential interactions with commonly used anti-pseudomonal agents—namely tobramycin.
Acknowledgments
This works was supported by the US Department of Defense DR080371 to JAN and DPN, by NIH/NCATS Colorado CTSI Grant Number KL2 TR000156 to DPN, and NIAID R01AI067653 to SMM. Support was also provided by the National Institutes of Health (1R01HL090991), the Rebecca Runyon Bryan Chair for Cystic Fibrosis, and the Cystic Fibrosis Foundation. Contents are the authors’ sole responsibility and do not necessarily represent official views of the sponsors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burn incidence and treatment in the united states: 2011 Fact Sheet. American burn association; 2011. [Google Scholar]
- 2.Roeder RA, Schulman CI. An Overview of war-related thermal injuries. J Craniofac Surg. 2010;21:971–975. doi: 10.1097/SCS.0b013e3181e1e802. [DOI] [PubMed] [Google Scholar]
- 3.Mayhall CG. The epidemiology of burn wound infections: then and now. Clin Infect Dis. 2003;37(4):543–550. doi: 10.1086/376993. [DOI] [PubMed] [Google Scholar]
- 4.Baker CC, Miller CL, Trunkey DD. Predicting fatal sepsis in burn patients. J Trauma. 1979;19(9):641–648. doi: 10.1097/00005373-197909000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Barrow RE, Spies M, Barrow LN, Herndon DN. Influence of demographics and inhalation injury on burn mortality in children. Burns. 2004;30(1):72–77. doi: 10.1016/j.burns.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Fitzwater J, Purdue GF, Hunt JL, O’Keefe GE. The risk factors and time course of sepsis and organ dysfunction after burn trauma. J Trauma. 2003;54(5):959–966. doi: 10.1097/01.TA.0000029382.26295.AB. [DOI] [PubMed] [Google Scholar]
- 7.Gomez R, Murray CK, Hospenthal DR, et al. Causes of mortality by autopsy findings of combat casualties and civilian patienta admitted to a burn unit. J Am Coll Surg. 2009;208:348–354. doi: 10.1016/j.jamcollsurg.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Hodle AE, Richter KP, Thompson RM. Infection control practices in US burn units. J Burn Care and Research. 2006;27(2):142–151. doi: 10.1097/01.BCR.0000203493.31642.79. [DOI] [PubMed] [Google Scholar]
- 9.Armour AD, Shankowsky HA, Swanson T, Lee J, Tredget EE. The impact of nosocomially-acquired resistant Pseudomonas in a burn unit. J Trauma. 2007;63:164–171. doi: 10.1097/01.ta.0000240175.18189.af. [DOI] [PubMed] [Google Scholar]
- 10.McManus AT, Mason AD, McManus WF, Pruitt BA. Twenty-five year review of Pseudomonas aeruginosa bacteremia in a burn center. Eur J Clin Microbiol Infect Dis. 1985;4:219–223. doi: 10.1007/BF02013601. [DOI] [PubMed] [Google Scholar]
- 11.Walker TS, Worthen GS, Poch KR, et al. Enhanced pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect Immun. 2005;73(6):3693–3701. doi: 10.1128/IAI.73.6.3693-3701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parks QM, Young RL, Poch KR, Malcolm KC, Vasil ML, Nick JA. Neutrophil enhancement of Pseudomonas aeruginosa biofilm development: human F-actin and DNA as targets for therapy. J Med Microbiol. 2009;58(Pt 4):492–502. doi: 10.1099/jmm.0.005728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson DM, Parks QM, Young RL, et al. Disruption of contact lens-associated Pseudomonas aeruginosa biofilms formed in the presence of neutrophils. Invest Ophthalmol Vis Sci. 2011;52(5):2844–2850. doi: 10.1167/iovs.10-6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai WC, Rodriguez ML, Young KS, et al. Azithromycin blocks neutrophil recruitment in pseudomonas endobronchial infection. Am J Respir Crit Care Med. 2004;170:1331–1339. doi: 10.1164/rccm.200402-200OC. [DOI] [PubMed] [Google Scholar]
- 15.Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290:1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 16.Verleden GM, Vanaudenaerde BM, Dupont LJ, VanRaemdonck DE. Azithromycin reduces airway neutrophilia and interleukin-8 in patients with bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2006;174:566–570. doi: 10.1164/rccm.200601-071OC. [DOI] [PubMed] [Google Scholar]
- 17.Florescu DF, Murphy PJ, Kalil AC. Effects of prolonged use of azithromycin in patients with cystic fibrosis: a meta-analysis. Pulm Pharmacol Ther. 2009;22(6):467–472. doi: 10.1016/j.pupt.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Culic O, Erakovic V, Cepelak I, et al. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur J Pharmacol. 2002;450:277–289. doi: 10.1016/s0014-2999(02)02042-3. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann N, Lee B, Hentzer M, et al. Azithromycin blocks quorum sensing and alginate polymer formation an dincreases the sensitivity to serum and stationary-growth-phase killing of pseudomonas aeruginosa and attenuates chronic p. aeruginosa lung infection in cftr(−/−) mice. Antimicrob Agents Chemother. 2007;51:3677–3687. doi: 10.1128/AAC.01011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavilanes X, Huaux F, Meyer M, et al. Azithromycin fails to reduce increased expression of neutrophil-related cytokines in primary-cultured epithelial cells from cystic fibrosis mice. J Cyst Fibros. 2009;8(3):203–210. doi: 10.1016/j.jcf.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro CM, Hurd H, Wu Y, et al. Azithromycin treatment alters gene expression in inflammatory, lipid metabolism, and cell cycle pathways in well-differentiated human airway epithelia. PLoS ONE. 2009;4(6):e5806. doi: 10.1371/journal.pone.0005806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Sullivan S, Lederer J, Horgan A, Chin D, Mannick J, Rodrick M. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Annals of Surgery. 1995;222:482–490. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinoshita M, Shinomiya N, Ono S, et al. Restoration of natural IgM production from liver B cells by exogenous IL-18 improves the survival of burn-injured mice infected with Pseudomonas aeruginosa. J Immunol. 2006;177:4627–4635. doi: 10.4049/jimmunol.177.7.4627. [DOI] [PubMed] [Google Scholar]
- 24.Suber FM, Carroll C, Moore FD., Jr Innate response to self-antigen signficantly exacerbates burn wound depth. Proc Natl Acad Sci USA. 2007;104(10):3973–3977. doi: 10.1073/pnas.0609026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley PP, Priebat DA, Christensen RD, Rothstein C. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. Journal of Investigative Dermatology. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 26.Palmer KL, Aye LM, Whiteley M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol. 2007;189:8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen BB, Sternberg C, Andersen JB, et al. Molecular tools for study of biofilm physiology. Methods Enzymol. 1999;310:20–42. doi: 10.1016/s0076-6879(99)10004-1. [DOI] [PubMed] [Google Scholar]
- 28.Heydorn A, Ersboll BK, Hentzer M, Parsek MR, Givskov M, Molin S. Experimental reproducibility in flow-chamber biofilms. Microbiology. 2000;146:2409–2415. doi: 10.1099/00221287-146-10-2409. [DOI] [PubMed] [Google Scholar]
- 29.Heydorn A, Nielsen AT, Hentzer M, et al. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- 30.Dai T, Gupta A, Huang YY, et al. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob Agents Chemother. 2012 Dec 21; doi: 10.1128/AAC.01652-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohler T, Dumas J, Van Delden C. Ribosome protection prevents azithromycin-mediated quorum -sensing modulation and stationary-phase killing of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2007;51:4243–4248. doi: 10.1128/AAC.00613-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moskowitz SM, Foster JM, Emerson J, Burns JL. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. Journal of clinical microbiology. 2004 May;42(5):1915–1922. doi: 10.1128/JCM.42.5.1915-1922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tre-Hardy M, Nagant C, El Manssouri F, et al. Efficacy of the combination of tobramycin and a macrolide in an in vitro pseudomonas aeurginosa mature biofilm model. Antimicrob Agents Chemother. 2010;54(10):4409–4415. doi: 10.1128/AAC.00372-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner T, Soong G, Sokol S, Saiman L, Prince A. Effects of azithromycin on clinical isolates of Pseudomonas aeruginosa from cystic fibrosis patients. Chest. 2005;128:912–919. doi: 10.1378/chest.128.2.912. [DOI] [PubMed] [Google Scholar]
- 35.Imamura Y, Higashiyama Y, Tomono K, et al. Azithromycin exhibits bactericidal effects on Pseudomonas aeruginosa through interaction with the outer membrane. Antimicrob Agents Chemother. 2005;49:1377–1380. doi: 10.1128/AAC.49.4.1377-1380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaara M. Outer membrane permeability barrier to azithromycin, clarithromycin, and roxithermycin in gram-negative enteric bacteria. Antimicrob Agents Chemother. 1993;37:354–356. doi: 10.1128/aac.37.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Treggisri MM, Retsch-Bogart G, Mayer-Hamblett N, et al. Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med. 2011;165(9):847–856. doi: 10.1001/archpediatrics.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saiman L, Mayer-Hamblett N, Campbell P, Marshall BC. Heterogeneity of treatment response to azithromycin in patients with cystic fibrosis. Am J Respir Crit Care Med. 2005;172:1008–1012. doi: 10.1164/rccm.200502-218OC. [DOI] [PubMed] [Google Scholar]
- 39.Robinson P, Schechter MS, Sly PD, et al. Clarithromycin therapy for patients with cystic fibrosis: a randomized controlled trial. Pediatr Pulmonol. 2012;47(6):551–557. doi: 10.1002/ppul.21613. [DOI] [PubMed] [Google Scholar]
- 40.Klein MBKC, Nelson J, Rivara FP, Bibran NS, Concannon T. Geographic access to burn center hospitals. JAMA. 2009;(302):1774–1781. doi: 10.1001/jama.2009.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]