Abstract
Background
It is not known whether preclinical cognitive decline is associated with fibrillar β-amyloid (Aβ) deposition irrespective of Apolipoprotein E (APOE) ε4 status.
Methods
From a prospective observational study of 623 cognitively normal individuals, we identified all subjects who showed preclinical decline of at least 2 standard deviations beyond the decline of the entire group in memory or executive function. Fourteen decliners were matched by APOE ε4 gene dose, age, sex, and education with 14 nondecliners. Dynamic Pittsburgh compound B (PiB) positron emission tomography (PET) scans, the Logan method, statistical parametric mapping, and automatically labeled regions of interest were used to characterize and compare cerebral-to-cerebellar PiB distribution volume ratios (DVR), reflecting fibrillar Aβ burden.
Results
At P<.005 (uncorrected), decliners had significantly greater DVR’s in comparison to nondecliners.
Conclusions
Asymptomatic longitudinal neuropsychological decline is associated with subsequent increased fibrillar amyloid deposition, even when controlling for APOE ε4 genotype.
Keywords: preclinical, Alzheimer’s disease, amyloid imaging, cognitive decline, Apolipoprotein E
Introduction
There is a growing interest in identifying the earliest preclinical measurements in order to diagnose Alzheimer’s disease (AD) when the disease may not yet be irreversibly established and most amenable to treatment and prevention.1 We previously demonstrated abnormally declining memory scores on longitudinal neuropsychological tests preceding symptoms of memory loss in a group of healthy volunteers, and found that APOE ε4 genotype—a known AD susceptibility gene—influenced the age of onset and pattern of this asymptomatic, preclinical decline.2, 3 Furthermore, even when controlling for APOE ε4 genotype, individuals with preclinical memory decline showed significantly greater correlations between cerebral hypometabolism in AD-affected brain regions, as measured by a baseline fluorodeoxyglucose (FDG) positron emission tomography (PET), and subsequent verbal memory decline than nondecliners.4
Fibrillar Aβ imaging, most notably with [11C] benzothiazole radiotracer Pittsburgh Compound-B(PiB) PET 5 and more recently with the fluorine-18-labeled tracers such as Florbetapir,6 has emerged as a potential biomarker for preclinical AD. Evidence suggests that increases in fibrillar Aβ deposition precede neuronal injury7, 8 and fibrillar amyloid deposition is a potential predictor of later symptomatic cognitive decline.9 In this study, we were interested in validating the value of asymptomatic longitudinal neuropsychological test score-change as a marker for preclinical AD by associating it with PiB retention. We predicted that in cognitively normal middle aged to elderly persons, despite the relationship between preclinical fibrillar Aβ burden and APOE gene dose,10, 11 the cohort with greater preclinical cognitive decline on neuropsychological testing would show increased PiB retention relative to the non-decliners even when matched for APOE ε4 genotype, age, sex, and education.
Methods
Study Participants
Participants in this study were drawn from the Arizona APOE Cohort, a group of healthy individuals who are from Maricopa County (Arizona) selected on the basis of APOE genotype and who undergo longitudinal neuropsychological assessment every 2 years.12 They were mostly 50 to 69 years old at entry and recruited through local newspaper advertisements that requested healthy individuals who had a first-degree relative with AD. Genetic determination of APOE allelic status was performed using a polymerase chain reaction–based assay.13 Screening tests for the longitudinal study included medical history, neurologic examination, Folstein Mini-Mental State Examination, Hamilton Depression Rating Scale, and the Structured Psychiatric Interview for Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised). None met criteria for mild cognitive impairment (MCI), 14 AD, 15 any other form of dementia, or major depressive disorder 16 at entry. Every two years, subjects completed a 4-hour battery of neuropsychological tests, divided into five cognitive domains with 4 different tests per domain.
Consistent with our previous definition of preclinical decline,2, 4 we identified all individuals enrolled in our longitudinal study who had completed at least 2 consecutive epochs of testing, remained cognitively normal, and fulfilled our defined criteria for amnestic and/or executive preclinical decline. Annualized test-retest change was calculated for each score on each test in the memory and executive functioning domains between epochs. “Decline” was defined a priori as a score declining at least 2 standard deviations (SDs) beyond the decline of the entire group. Table 1 provides detailed information on the decline criterion for each test score. For example, on the Rey Complex Figure Test recall, the sample as a whole demonstrated an average improvement in score of 0.27 (SD = 2.69) points per year. Thus, to be labeled as having declined on that particular test score, the subject would have to demonstrate a decline in test score of 5.11 points per year (mean change – 2SD or 0.27 – 5.38). “Decliners” were individuals who evidenced decline in scores on 2 different memory tests and/or 2 different executive function tests. Table 2 shows on which tests and scores he/she declined and what his/her specific amount of per year decline was on that test score. “Non-decliners” were defined as individuals who did not experience such per year decline. Memory domain measures included Auditory Verbal Learning Test (short-term memory; long-term memory; or percent recall), Selective Reminding Test free recall, Complex Figure Test recall, and Visual Retention Test total correct. Executive domain measures included Wechsler Adult Intelligence Scale-Revised Freedom from distractibility, Controlled Oral Word Association Test, Wisconsin Card Sorting Test (categories completed, total errors, or perseverative errors), and Paced Auditory Serial Attention Task (3- and 2-second administration).
TABLE 1.
Criteria for decline on each cognitive measure
| Test score | Mean change (SD)* | Mean – 2.0SD** |
|---|---|---|
| Memory measures | ||
| CFT recall | 0.27 (2.69) | −5.11 |
| AVLT STM | −0.001 (1.26) | −2.26 |
| AVLT LTM | −0.0009 (1.27) | −2.54 |
| AVLT %recall | −0.10 (9.77) | −19.64 |
| VRT | 0.0006 (0.88) | −1.76 |
| SRT Free | 0.63 (4.03) | −7.42 |
| Executive functioning measures | ||
| WAIS FFD | 0.14 (4.17) | −8.19 |
| COWA | 0.45 (4.12) | −7.79 |
| WCST cat | −0.10 (0.79) | −1.68 |
| WCST err | 0.79 (8.30) | 17.39^ |
| WCST per | 0.33 (4.58) | 9.49^ |
| PASAT 3 | 0.47 (4.15) | −7.84 |
| PASAT 2 | 1.23 (4.38) | −7.54 |
Mean (SD) amount of per year change in test score for the entire sample.
Amount of change in test score that is 2.0SD below the mean and therefore amount of change required to label as decline on that test score.
These are positive scores because higher scores indicate more errors and therefore a poorer performance
CFT recall = Rey Complex Figure Test delayed recall; AVLT STM = Rey Auditory Verbal Learning Test short term recall; AVLT LTM = AVLT long term recall; AVLT long term percent retention; VRT = Benton Visual Retention Test; SRT free = Selective Reminding Test free recall; WAIS FFD = Wechsler Adult Intelligence Scale Freedom From Distractibility; COWA = Controlled Oral Word Association Test; WCST cat = Wisconsin Card Sorting Test categories completed; WCST err = WCST errors; WCST per = perseverative errors; PASAT 3 = Paced Auditory Serial Attention Test 3 second trial; PASAT 2 = PASAT 2 second trial.
TABLE 2.
Test score declines for each Decliner subject
| Decliner | Test | Amount of Decline | Decline cutoff (Table 1) | Subsequent epoch decline |
|---|---|---|---|---|
| Memory Decliner 1 | no | |||
| CFT recall | −6.65 | −5.11 | ||
| VRT | −2.66 | −1.76 | ||
| Memory Decline 2 | no | |||
| AVLT STM | −2.87 | −2.26 | ||
| VRT | −1.92 | −1.76 | ||
| Memory Decliner 3 | no | |||
| AVLT STM | −2.99 | −2.26 | ||
| SRT free | −7.58 | −7.42 | ||
| Memory Decliner 4 | no | |||
| AVLT % ret | −20.14 | −19.64 | ||
| VRT | −2.18 | −1.76 | ||
| Memory Decliner 5 | no | |||
| AVLT STM | −3.19 | −2.26 | ||
| AVLT LTM | −3.19 | −2.54 | ||
| AVLT % ret | −19.96 | −19.64 | ||
| VRT | −2.18 | −1.76 | ||
| Memory Decliner 6 | yes | |||
| AVLT LTM | −3.27 | −2.54 | ||
| VRT | −2.76 | −1.76 | ||
| Memory Decliner 7 | yes | |||
| CFT recall | −5.13 | −5.11 | ||
| AVLT LTM | −2.85 | −2.54 | ||
| Memory Decliner 8 | yes | |||
| CFT recall | −5.74 | −5.11 | ||
| SRT free | −8.98 | −7.42 | ||
| Memory Decliner 9 | no* | |||
| AVLT STM | −2.99 | −2.26 | ||
| VRT | −2.00 | −1.76 | ||
| Memory and Executive Decliner 1 | yes* | |||
| AVLT STM | −4.79 | −2.26 | ||
| SRT free | −13.77 | −7.42 | ||
| COWA | −8.87 | −7.79 | ||
| WCST cat | −2.99 | −1.68 | ||
| WCST per | 17.96 | 9.49 | ||
| WCST err | 30.54 | 17.39 | ||
| Memory and Executive Decliner 2 | no | |||
| AVLT STM | −2.88 | −2.26 | ||
| AVLT LTM | −3.84 | −2.54 | ||
| AVLT % ret | −22.08 | −19.64 | ||
| VRT | −1.92 | −1.76 | ||
| WAIS FFD | −10.91 | −8.19 | ||
| COWA | −8.50 | −7.79 | ||
| PASAT 3 | −13.44 | −7.84 | ||
| Executive Decliner 1 | no | |||
| COWA | −9.48 | −7.79 | ||
| WCST cat | −2.49 | −1.68 | ||
| WCST err | 28.44 | 17.39 | ||
| WCST per | 10.48 | 9.49 | ||
| Executive Decliner 2 | yes | |||
| WCST cat | −4.62 | −1.68 | ||
| WCST per | 10.15 | 9.49 | ||
| WCST err | 42.46 | 17.39 | ||
| PASAT 2 | −9.23 | −7.54 | ||
| Executive Decliner 3 | unknown* | |||
| WCST cat | −2.25 | −1.68 | ||
| PASAT 3 | −17.21 | −7.84 | ||
mean cortical PiB retention > 1.25
From the group of 623 cognitively normal participants, we identified an initial list of 30 individuals who fit our definition of decline. Of these, 16 either refused participation, had medical contraindications to participation, or had since moved away. The remaining 14 individuals either previously had consented for the longitudinal study or prospectively agreed for this study to undergo PiB PET scanning, and each of these decliners was then matched by APOE genotype, age, sex, and education to an individual in the non-decliner group who also consented to PiB PET scanning (14 with amnestic and/or executive preclinical decline and 14 nondecliners). All 28 individuals (6 ε4 homozygotes, 6 ε4 heterozygotes, and 16 ε4 noncarriers) gave their written informed consent according to the Declaration of Helsinki, and the study was approved by the Mayo Clinic and Banner Health Institutional Review Boards.
Fibrillar Aβ PET
Fibrillar Aβ PET was performed at the Banner Alzheimer’s Institute/Banner Good Samaritan Regional Medical Center using a Siemens HR+ scanner in the 3-dimensional mode, a transmission scan, the i.v. injection of 15 mCi of 11C-PiB, and a 90-min dynamic sequence of emission scans. The Logan method and an automatically labeled cerebellar region-of-interest were used to compute parametric brain images of the PiB Distribution Volume Ratio (DVR), a measure of fibrillar Aβ burden, in the decliners and non-decliners. All of the scans were acquired at or subsequent to the epoch(s) in which decline occurred.
A brain mapping algorithm (SPM8, http://www.fil.ion.ucl.ac.uk/spm/) and the subjects’ spatial information from their 3.5-to-10 min emission frames were used to spatially normalize the images according to the coordinates of a standard brain atlas17 and the spatial normalization parameters were applied to the subjects’ DVR images. Voxel-wise general linear model (GLM) was used together with uncorrected p=0.005 as type-I error threshold to generate statistical parametric maps of significantly greater cerebral-to-cerebellar PiB DVR in preclinical decliner subjects than the matched non-decliner controls, as well as in the opposite direction. In order to evaluate the effect of APOE status on fibrillar Aβ burden in this sample, we also examined statistical parametric maps of significantly greater cerebral-to-cerebellar PiB DVR in APOE ε4 carriers than non ε4 carriers, irrespective of decliner/nondecliner status.
Finally, we secondarily examined pre-defined region of interests (ROIs), thought to be associated with AD and based on a prior study,10 independent of the current analysis. ROIs included a mean cortical (consisting of frontal, posterior cingulate-precuneus, and lateral temporal lobes) ROI, as well as frontal, posterior cingulate-precuneus, lateral temporal, lateral parietal, basal ganglia, medial temporal, and occipital ROIs. Given the hypothesis that decliners would have higher PiB retention than the nondecliners, we considered a one-tailed t-test p-value of less than 0.05 to be significant for the ROI analysis. P values reported are based on unpaired t-tests; however, given our careful matching design we additionally performed, at hoc, paired t-test analyses for comparisons of decliners and nondecliners. On all analyses for which we did not have an a priori hypothesis, we used standard unpaired 2-tailed t-tests.
Results
Each group of 3 e4 homozygotes, 3 e4 heterozygotes, 8 e4 noncarriers had 5 males, 9 females, an average age of 66.5 years (range 59 to 79 years), and an average of 16 years of education (range 12 to 20 years). There were no differences in length of follow-up (average 12 years follow-up for each group, decliners ranged 8 to 17 years, nondecliners ranged 8 to 14 years, P=0.75), MMSE (range 28 to 30, P=0.23), diabetes (none in any participants), Hamilton Depression Scores (all <10), hypertension, cigarette smoking, hyperlipidemia, or family history of AD (85.7% with a first degree relative, P=1.0). Likewise, when the groups were divided by APOE status rather than decliner/non-decliner status, there remained no differences in any of the demographic characteristics.
Among the 14 decliners, there were 9 with memory decline, 3 with executive functioning decline, and 2 with both memory and executive functioning decline. All showed evidence of decline at or before PiB PET imaging, ranging from 0 to 4 epochs (mean 1.4 epochs, SD 1.3 epochs) prior to the scan. All subjects were cognitively and functionally normal at the time of the scan. Although by definition there was significant longitudinal decline in certain neuropsychological test scores at or prior to the scan for each individual in the decliner group, cross-sectionally at the time of the scan the only statistically significant group differences (without controlling for multiple comparisons) between decliner and and nondecliner were in the Rey Complex Figure Test (CFT) recall and Benton Visual Retention Test (VRT) (Table 3). At baseline there were no significant differences between the decliner and nondecliner groups on any of the tests.
TABLE 3.
Neuropsychological Test Scores at time of PiB PET scan
| Test | Decliner | Non-Decliner | P-value |
|---|---|---|---|
| Memory measures | |||
| AVLT STM | 8.7 (2–15) | 9.2 (2–13) | 0.69 |
| AVLT LTM | 8.4 (2–14) | 9.3 (4–13) | 0.47 |
| AVLT % recall | 71.6 (25–100) | 81.4 (50–100) | 0.18 |
| SRT free recall | 88.8 (58–107) | 93.5 (55–106) | 0.43 |
| CFT recall | 18.0 (10–27.5) | 24.9 (11.5–32) | 0.02 |
| VRT total correct | 5.7 (3–9) | 7.2 (2–10) | 0.03 |
| Executive functioning measures | |||
| WAIS-R FDD | 107.8 (89–134) | 111.6 (97–125) | 0.36 |
| COWAT | 46.0 (30–62) | 47.9 (31–73) | 0.60 |
| WCST cat complete | 4.1 (0–6) | 4.9 (1–6) | 0.34 |
| WCST total errors | 32.5 (14–66) | 27.1 (12–62) | 0.41 |
| WCST pers errors | 14.4 (4–32) | 9.1 (4–21) | 0.07 |
| PASAT-3 sec | 45.1 (25–60) | 51.5 (25–60) | 0.19 |
| PASAT-2 sec | 49.6 (16–58) | 45.5 (33–57) | 0.18 |
Values are means with ranges in parentheses. P values were calculated using unpaired 2-tailed t-tests.
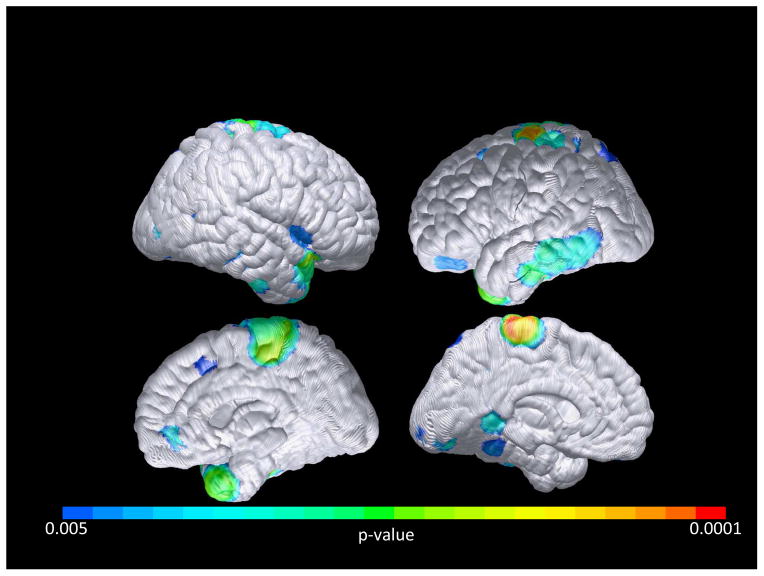
In our whole brain contrasts, at p< 0.005(uncorrected), increased DVR was found in the decliners compared to non-decliners at several areas, particularly the temporal pole, paracentral lobule, and occipital region (Figure 1), with the strongest effect (p<0.001) in the right temporal pole (52, 22, −32 [x, y, z]; T = 4.15) and the left occipital lobe (−24, −66, 32 [x, y, z]; T = 4.13). Paired t-test results were similar but with more significant differences. Importantly and at the given p=0.005 level, there were only 5 voxels over the whole brain volume where non-decliners had higher DVR compared to decliners. In contrast, there were total of 2782 voxels in the opposite direction. Using Monte-Carlo simulation we estimated that the type-1 error of observing as many as 2782 voxels in one direction and as few as 5 in the opposite direction is p<0.001. Our whole brain contrast based on APOE status (p<.005, uncorrected) showed increased DVR in the APOE ε4 carriers compared to ε4 non-carriers, with the strongest effect (p<.005) in the right putamen (34, 2, −4 [x, y, z]; T = 3.7) and inferior frontal lobe (36, 62, −2 [x, y, z]; T = 3.12). However, the total number of voxels where APOE ε4 carriers had greater PiB DVRs than ε4 non-carriers was 301, which is significantly less than the number of voxels in which decliners had greater PiB DVRs than nondecliners (p<0.001).
FIGURE 1. Cortical Pittsburgh Compound B (PIB) retention, Cognitively Normal Decliners > Nondecliners.
Voxelwise analysis demonstrates preclinical cognitive decline associated with PiB retention in the temporal, paracentral, and occipital lobe association cortices (p<0.005, uncorrected). Occipital areas with the greatest significant differences are unable to be fully visualized in this cortical projection.
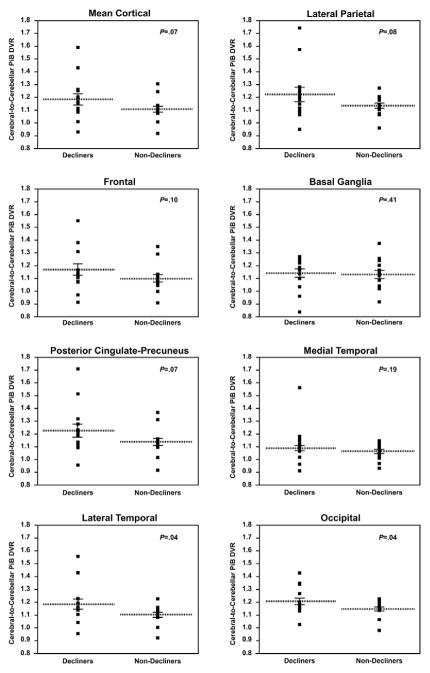
Analysis based on our a priori ROI’s showed significantly greater PiB DVRs for the lateral temporal ROI (mean DVR of decliners=1.19 +/− SD of 0.15; mean DVR of nondecliners=1.11 +/− SD of 0.07; P =0.04, one tailed unpaired t-test; P =0.03 paired t-test) and occipital ROI ( mean DVR of decliners=1.25 +/− SD of 0.12; mean DVR of nondecliners=1.19 +/− 0.07; P=0.04, one tailed unpaired t-test; P =0.03 paired t-test), and there was a trend for greater mean cortical PiB DVR for the decliners than the non-decliners (mean DVR of decliners=1.19 +/− SD of 0.17; mean DVR of nondecliners=1.11 +/− SD of 0.09; P=0.07, one tailed unpaired t-test; P =0.05) (Figure 2). In contrast, when divided by APOE status, ε4 carriers in this sample did not show statistically significant increases in PiB binding compared to the ε 4 non-carriers in any of the ROIs.
FIGURE 2. Regional FibrillarAβ burden in cognitively normal decliners and nondecliners.
Data generated from previously defined ROIs.10 P values correspond to the association between PiB retention and group status (1-tailed unpaired t-test) for each ROI (mean cortical, occipital, frontal, posterior cingulate-precuneus, lateral temporal, lateral parietal, basal ganglia, and medial temporal).
Discussion
Our results show that a neuropsychological construct of preclinical cognitive decline correlates with an imaging endophenotype of AD. We previously demonstrated that cerebral FDG PET hypometabolism correlated with subsequent verbal memory decline in Alzheimer disease-affected brain regions even when controlling for APOE status.4 We now have evidence that preclinical cognitive decline correlates with an increased measure of fibrillar Aβ deposition and that this effect is independent of APOE status. Indeed, in this particular sample, the decliners showed a stronger association with increased PiB retention than did the APOE ε4 carriers, which is consistent with a recent study showing a greater association of Aβ load with memory decline in healthy older adults than with APOE status.18
The type of decline defined in this study is subtle and all subjects reported in this study have so far remained asymptomatic. Further, about half of the subjects identified as decliners did not continue to meet our definition of decline in the subsequent epoch due to some improvement in one or both test scores (Table 2), and we do not yet know which of these individuals will eventually be diagnosed with MCI or AD. The four decliners with the highest mean cortical PiB retention included two who improved in subsequent epochs, one who remained a decliner in subsequent epochs, and one for whom we do not yet have follow-up neuropsychological data (though this subject had previously shown consistent decline such that scores were on the cusp of an MCI diagnosis). Given the association of this subtle cognitive decline with increased PiB retention, however, it is possible that such variability in scores is a harbinger of subsequent clinically significant impairment.
The correlation of subtle decline with increased PiB retention is consistent with other studies showing intra-individual preclinical memory decline to be associated with elevated cortical amyloid burden18–20 and supports the potential value of serial neuropsychological testing as a way of monitoring risk. Indeed, others have suggested that subtle memory decline may precede the development of fibrillar Aβ plaques.21 Ongoing longitudinal monitoring of our participants will confirm whether preclinical decline as we have defined it is a marker for future disease. Whether the combination of increased Aβ load and preclinical cognitive decline is a stronger predictor of future disease than either one alone is the subject of ongoing investigation.
The temporal lobe had the largest group difference and spatial effect for the decliner group relative to the nondecliner group, which has been previously associated with preclinical memory impairment in a group of healthy and MCI individuals.22 Our sample also showed significantly greater DVR in occipital region, which is preferentially affected in amyloid angiopathy.23 Since the groups were matched by APOE status, and since ε4 carriers have greater amyloidosis especially frontotemporally,10, 11 the occipital group differences in our sample may in part reflect the cancelling out of the APOE effect.
PiB retention only reflects fibrillar beta-amyloid deposition, not all cortical amyloid associated with AD and other diseases. Furthermore, diseases other than AD can have associated amyloid pathology with PiB retention, e.g., vascular amyloid deposition, or diffuse amyloid pathology in Dementia with Lewy Bodies.24 Limitations of our study include the relatively small sample and the lack of follow-up data regarding future development of MCI or AD. Our definition of preclinical decline is admittedly cumbersome in that it requires multiple tests to decline over several years and so is not easily translated to clinical practice. The significance of asymptomatic decline on longitudinal neuropsychological tests is not always clear, and we currently lack a universally accepted objective standard for abnormal decline. However, having shown that the neuropsychological indicators of presymptomatic cognitive decline are related to an AD-related pattern of cerebral hypometabolism using FDG PET, and an indicator of fibrillar Abeta burden using PiB PET, there is a greater sense of urgency to develop a strategy of monitoring cognitive decline that is more clinically practical—perhaps such as the use of “reliable change indices” for individual neuropsychological tests.25, 26 Additional studies are needed to determine the extent to which it is associated with other AD biomarkers, including MRI measurements of brain atrophy and cerebrospinal fluid biomarkers of beta-amyloid and tau pathology. Preclinical neuropsychological test decline combined with biomarker evidence of disease may be one strategy to recognize the individuals at highest risk for subsequent disease.
Conclusion
The correlation of increased amyloidosis with preclinical cognitive decline suggests that longitudinal neuropsychological testing may be a particularly sensitive early marker for future disease.
Acknowledgments
The authors thank Bruce Henslin and Jacqueline Esque for study coordination, and Cole Reschke for help with data management and configuration. Study funded by Mayo Foundation (grant to C.M.S.), National Institute on Aging R01MH57899-01 (grant to E.M.R.), State of Arizona (grant to E.M.R. and R.J.C.).
Footnotes
Previous Presentation: 64th American Academy of Neurology Annual Meeting, April 25, 2012
Disclosures: Dr. Stonnington, Dr. Chen, Dr. Caselli, Ms. Liu, Dr. Locke, Dr. Dueck, Mr. Roontiva, and Ms. Lee report no disclosures. Dr. Fleisher serves on advisory boards for Eli Lilly and Avid radiopharmaceuticals, is a DSMB member for Merck, and is a paid speaker for Siemens. He has received grant funding from Avid Radio Pharmaceuticals. He is a site investigator for GE, Lilly, Avid, Merck, Roche, Janssen, Pfizer, Wyeth, Genentech, and Targacept. Dr. Reiman is a scientific advisor for Sygnis, AstraZeneca, Bayer, Eisai, Elan, Eli Lilly, GlaxoSmithKline, Intellect, Link Medicine, Novartis, Siemens, and Takeda. He has research contracts with AstraZeneca and Avid. He has a patent pending (through Banner Health) for biomarker strategy for the evaluation of presymptomatic AD treatments.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011 May;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caselli RJ, Reiman EM, Locke DE, et al. Cognitive domain decline in healthy apolipoprotein E epsilon4 homozygotes before the diagnosis of mild cognitive impairment. Arch Neurol. 2007 Sep;64(9):1306–1311. doi: 10.1001/archneur.64.9.1306. [DOI] [PubMed] [Google Scholar]
- 3.Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009 Jul 16;361(3):255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caselli RJ, Chen K, Lee W, Alexander GE, Reiman EM. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Arch Neurol. 2008 Sep;65(9):1231–1236. doi: 10.1001/archneurol.2008.1. [DOI] [PubMed] [Google Scholar]
- 5.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004 Mar;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 6.Lister-James J, Pontecorvo MJ, Clark C, et al. Florbetapir f-18: a histopathologically validated Beta-amyloid positron emission tomography imaging agent. Semin Nucl Med. 2011 Jul;41(4):300–304. doi: 10.1053/j.semnuclmed.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol. 2011 Sep 12; doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012 Aug 30;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doraiswamy PM, Sperling RA, Coleman RE, et al. Amyloid-beta assessed by florbetapir F 18 PET and 18-month cognitive decline: A multicenter study. Neurology. 2012 Aug 1; doi: 10.1212/WNL.0b013e3182661f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantarci K, Lowe V, Przybelski SA, et al. APOE modifies the association between Abeta load and cognition in cognitively normal older adults. Neurology. 2012 Jan 24;78(4):232–240. doi: 10.1212/WNL.0b013e31824365ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caselli RJ, Reiman EM, Osborne D, et al. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004 Jun 8;62(11):1990–1995. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- 13.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. Journal of lipid research. 1990 Mar;31(3):545–548. [PubMed] [Google Scholar]
- 14.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001 May 8;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 15.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R, Patient Edition/Non-patient Edition,(SCID-P/SCID-NP) Washington, D.C: American Psychiatric Press, Inc; 1990. [Google Scholar]
- 17.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 18.Lim YY, Ellis KA, Pietrzak RH, et al. Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology. 2012 Oct 16;79(16):1645–1652. doi: 10.1212/WNL.0b013e31826e9ae6. [DOI] [PubMed] [Google Scholar]
- 19.Darby DG, Brodtmann A, Pietrzak RH, et al. Episodic memory decline predicts cortical amyloid status in community-dwelling older adults. J Alzheimers Dis. 2011;27(3):627–637. doi: 10.3233/JAD-2011-110818. [DOI] [PubMed] [Google Scholar]
- 20.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009 Dec;66(12):1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cairns NJ, Ikonomovic MD, Benzinger T, et al. Absence of Pittsburgh compound B detection of cerebral amyloid beta in a patient with clinical, cognitive, and cerebrospinal fluid markers of Alzheimer disease: a case report. Arch Neurol. 2009 Dec;66(12):1557–1562. doi: 10.1001/archneurol.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chetelat G, Villemagne VL, Pike KE, et al. Independent contribution of temporal beta-amyloid deposition to memory decline in the pre-dementia phase of Alzheimer’s disease. Brain : a journal of neurology. 2011 Mar;134(Pt 3):798–807. doi: 10.1093/brain/awq383. [DOI] [PubMed] [Google Scholar]
- 23.Ly JV, Donnan GA, Villemagne VL, et al. 11C-PIB binding is increased in patients with cerebral amyloid angiopathy-related hemorrhage. Neurology. 2010 Feb 9;74(6):487–493. doi: 10.1212/WNL.0b013e3181cef7e3. [DOI] [PubMed] [Google Scholar]
- 24.Quigley H, Colloby SJ, O’Brien JT. PET imaging of brain amyloid in dementia: a review. International journal of geriatric psychiatry. 2011 Oct;26(10):991–999. doi: 10.1002/gps.2640. [DOI] [PubMed] [Google Scholar]
- 25.Pedraza O, Smith GE, Ivnik RJ, et al. Reliable change on the Dementia Rating Scale. J Int Neuropsychol Soc. 2007 Jul;13(4):716–720. doi: 10.1017/S1355617707070920. [DOI] [PubMed] [Google Scholar]
- 26.Sachs BC, Lucas JA, Smith GE, et al. Reliable change on the Boston naming test. J Int Neuropsychol Soc. 2012 Mar;18(2):375–378. doi: 10.1017/S1355617711001810. [DOI] [PMC free article] [PubMed] [Google Scholar]