Abstract
Large rotator cuff tears (supraspinatus and infraspinatus) are common in patients that perform overhead activities (laborers, athletes). In addition, following large cuff tears, these patients commonly attempt to return to pre-injury activity levels. However, there is a limited understanding of the damaging effects on the uninjured joint tissues when doing so. Therefore, the objective of this study was to investigate the effect of returning to overuse activity following a supraspinatus and infraspinatus tear on shoulder function and the structural and biological properties of the intact tendons and glenoid cartilage. Forty rats underwent four weeks of overuse followed by detachment of the supraspinatus and infraspinatus tendons and were then randomized into two groups: return to overuse or cage activity. Ambulatory measurements were performed over time and structural and biologic properties of the adjacent tendons and cartilage were evaluated. Results demonstrated that animals returning to overuse activity did not have altered shoulder function but despite this, did have altered cartilage and tendon properties. These mechanical changes corresponded to altered transcriptional regulation of chondrogenic genes within cartilage and tendon. This study helps define the mechanical and biologic mechanisms leading to joint damage and provides a framework for treating active cuff tear patients.
Keywords: rotator cuff, animal model, overuse injury, chondrogenic phenotype
Introduction
Rotator cuff tendon tears are common injuries which can lead to significant pain and dysfunction. If untreated, rotator cuff tears may lead to injury in the surrounding uninjured tissues of the shoulder (Peltz et al., 2009; Perry et al., 2009a; Reuther et al., 2013). Mechanical stress, caused by altered or excessive joint loading, may disrupt shoulder joint homeostasis leading to the initiation and progression of joint damage. However, these mechanical mechanisms are poorly understood and clinicians often rely on limited evidence to recommend treatment options without clearly understanding the potentially damaging effects on the remaining shoulder joint tissues.
Large rotator cuff tendon tears involving both the supraspinatus and infraspinatus tendons are common and are likely to affect daily activities. These two-tendon tears may disrupt the force balance provided by the subscapularis anteriorly and infraspinatus posteriorly (Burkhart, 1991). This force balance disruption may lead to humeral head translations (Keener et al., 2009; Oh et al., 2011) and therefore increase the patient’s risk of developing secondary joint damage, such as tear propagation, long head of the biceps (LHB) degeneration (Lakemeier et al., 2010a), articular cartilage degeneration (Hsu et al., 2003), or cuff tear arthropathy (Neer et al., 1983). Clinical investigations have shown a correlation between two-tendon rotator cuff tears and secondary joint pathology (Keener et al., 2009), such as impaired joint function and LHB pain(Lakemeier et al., 2010a; Lakemeier et al., 2010b). Additionally, previous studies in a rat model have shown that in the presence of a two-tendon rotator cuff tear, joint function is altered (Perry et al., 2009b; Sarver et al., 2010) and the LHB tendon (Peltz et al., 2009), subscapularis tendon (Perry et al., 2009a), and glenoid articular cartilage (Reuther et al., 2012) display decreased mechanical properties.
Rotator cuff tears are common in individuals who perform repeated overhead activities. This is likely a result of repetitive compression of the rotator cuff under the acromial arch (Neer, 1983), leading to tendon degeneration and tendon tears. Unfortunately, many individuals (particularly laborers and professional overhead athletes) are required to continue high levels of overuse activity, despite the onset of rotator cuff tears. Repetitive overuse activity in the presence of two-tendon rotator cuff tears may place these patients at higher risk for development of secondary joint damage.
Previous studies have shown that returning to overuse activity following an isolated supraspinatus tear alters LHB tendon and glenoid articular cartilage properties, but does not alter shoulder function or the adjacent intact infraspinatus or subscapularis tendons (Reuther et al., 2013). However, the consequences associated with two-tendon rotator cuff tears (altered joint loading) and overuse activity (excessive joint loading) are not understood. Therefore, the objective of this study was to investigate the effect of returning to overuse activity following tears of both the supraspinatus and infraspinatus tendons on shoulder function, the remaining intact tendons, and glenoid cartilage mechanical and biological properties. We hypothesized that overuse activity following two-tendon rotator cuff tears would alter shoulder function and lead to structural and biological tissue damage (indicated by decreased mechanical properties and increased production of degenerative factors, inflammatory markers, and chondrogenic markers) in the remaining intact tendons (LHB and subscapularis tendons) and the glenoid articular cartilage.
Methods
Study Design
Following a 2 week training period, forty adult male Sprague-Dawley rats (400–450 grams) underwent 4 weeks of overuse (downhill,10° treadmill running at 17 m/min for 1 hr/day, 5 days/wk) (Soslowsky et al., 2000) in order to induce a tendinopathic condition in the supraspinatus tendon. Subsequently, the animals underwent unilateral detachment of the left supraspinatus and infraspinatus tendons (IACUC approved), as described (Thomopoulos et al., 2003).
Following tendon detachment, animals were randomized into two activity groups: cage activity (CA) or return to overuse (OV). Post-surgery, the OV group was subject to one week of cage activity before gradually returning to the overuse protocol over a period of 2 weeks, and subsequently completing an additional 5 weeks of overuse activity. All animals were sacrificed 8 weeks following surgical tendon detachment. For gene expression (N=6) and histology (N=4), tissues were flash frozen immediately after harvest and stored at −80°C or fixed in formalin, respectively. The remaining animals (N=10) designated for mechanical testing were stored intact at −20°C.
Quantitative Ambulatory Assessment
To assess joint function (Sarver et al., 2010), forelimb gait and ground reaction forces were recorded using an instrumented walkway one day prior to detachment surgery (baseline) and at 3, 7, 14, 28, 42, and 56 days post tendon detachment. Ground reaction force data, including medial/lateral, braking, propulsion, and vertical forces, were collected for each walk. Temporal spatial parameters were used to calculate step length and width. Parameters were averaged across walks on a given day for each animal and normalized to body weight.
Tendon Mechanical Testing
The animals were thawed, and the scapula and humerus were dissected with the LHB and subscapularis tendons intact. Tendon testing was performed as described (Peltz et al., 2009; Thomas et al.). Briefly, stain lines, for local optical strain measurement (at insertion and mid-substance), were placed on the LHB and upper and lower bands of the subscapularis tendons using Verhoeff’s stain. Cross-sectional area was measured using a custom laser device. The scapula and humerus were embedded in a holding fixture using PMMA, gripped with cyanoacrylate annealed sand paper, and immersed in PBS at 37°C. Tensile testing was performed as follows: preload to 0.08 N, preconditioning (10 cycles of 0.1–0.5 N at a rate of 1% strain/s), stress relaxation to 4% (LHB) or 5% (subscapularis) strain at a rate of 5% strain/s for 600s, and ramp to failure at 0.3% strain/s. Stress was calculated as force divided by initial area, and 2D Lagrangian strain was determined from stain line displacements using texture tracking software.
Cartilage Mechanical Testing
Following LHB testing, the glenoid was prepared for mechanical testing by sharply detaching the LHB at its insertion on the superior rim of the glenoid. The glenoid was then preserved by wrapping in soft tissue and frozen (−20°C).
For cartilage thickness measurement (Reuther et al., 2012), each scapula was thawed and immersed in PBS containing protease inhibitors (5 mM Benz-HCl, 1mM PMSF, 1 M NEM) at room temperature. Specimens were scanned at 0.25 mm increments using a 55 MHz ultrasound probe (Visualsonics, Inc) in plane with the scapula. Captured B-mode images of each scan were segmented by selecting the cartilage and bony surfaces of the glenoid. The 3D positions of these surfaces were reconstructed and used to determine cartilage thickness maps. Each map was divided into six regions (center (C), posterior-superior (PS), posterior-inferior (PI), anterior-superior (AS), anterior-inferior (AI), and superior (S)) and an average thickness was computed for each region. Following ultrasound scanning, specimens were wrapped in soft tissue and frozen (−20°C) until mechanical testing.
For cartilage mechanical testing (Reuther et al., 2012), each scapula was thawed and immersed in PBS containing the protease inhibitor cocktail at room temperature. Utilizing a 0.5 mm diameter, non-porous spherical indentor, cartilage indentation testing was performed. Briefly, a preload (0.005 N) was followed by 8 step-wise stress relaxation tests (8 µm ramp at 2 µm/s followed by a 300 second hold). The scapula was repositioned for each localized region such that the indenter tip was perpendicular to the cartilage surface in each region. Cartilage thickness for indentation testing was determined by identifying the indentation location on each thickness map. Equilibrium elastic modulus was calculated, as described (Hayes et al., 1972), at 20% indentation and assuming Poisson’s ratio (v=0.30).
RNA Extraction
Tissues were harvested from the insertion site (0–2 mm) of the subscapularis and LHB and five regions (excluding the superior region) of the glenoid articular cartilage using a 0.75 mm diameter biopsy punch. Immediately following harvest, specimens were placed in liquid nitrogen and stored at −80°C. RNA was isolated using a TRIspin method (Reno et al., 1997). Briefly, specimens were pulverized into a powder and TRIzol extracted (Life Technologies Inc.). Following phase separation, the aqueous phase was processed via fractionation on RNA Clean & Concentrator™-5 columns (Zymo Research).
Reverse Transcription and Amplification
The extracted total RNA was measured for purity on a NanoDrop1000 spectrophotometer and quantified using the Quant-iT™ RiboGreen® RNA Assay Kit (Life Technologies Inc). Per manufacturer’s protocol, equal amounts of RNA from each sample were reverse transcribed, amplified, and cleaned-up using the Ovation® Pico System V2 (NuGEN Technologies, Inc.) and DNA Clean & Concentrator™-25 (Zymo Research).
qPCR
Target genes for chondrogenesis, osteogenesis, degeneration, inflammation, angiogenesis, and apoptosis were examined. GAPDH was used for normalization. The investigated genes of interest, accession numbers, and designed primer pairs are in Table I. Real-time quantitative PCR reactions were set-up in triplicate for each biological sample using Power SYBR Green chemistry (Life Technologies Inc.). A StepOnePlus Real-Time PCR System (Applied Biosystems) was used to perform 40 PCR amplification cycles and subsequent melt curve analysis. Due to low RNA yield in some tissues, sample size was reduced to N=3 or 4.
Table 1.
qPCR Targets and Primers
| Biological Significance | Gene | RefSeq Accesion Number | Primer sequences |
|---|---|---|---|
| Reference | GAPDH | NM_017008.4 | F: 5’-ctcccattcttccacctttg-3’ R: 5’-ccagggtttcttactccttgg-3’ |
| Angiogenesis | VEGFA | NM_031836.2 | F: 5’-tcaccaaagccagcacatag-3’ R: 5’-ttgaccctttccctttcctc-3’ |
| Apoptosis | CASP3 | NM_012922.2 | F: 5’-ttggaacgaacggacctg-3’ R: 5’-tccactgtctgtctcaataccg-3’ |
| Chondrogenesis | ACAN | NM_022190.1 | F: 5’-acccaaacagcagaaacagc-3’ R: 5’-aaagtgtccaaggcatccac-3’ |
| COL2A1 | NM_012929.1 | F: 5’-tcaagtcgctgaacaaccag-3’ R: 5’-ggttgggatcaatccagtagtc-3’ |
|
| SOX9 | AB073720 | F: 5’-cgagccggatctgaagaag-3’ | |
| XM_003750950.1 | R: 5’-tgacgtgtggcttgttcttg-3’ | ||
| Degradation | ADAMTS4 | NM_023959.1 | F: 5’-tggacaatggctatggacac-3’ R: 5’-tggacaatggcttgagtcag-3’ |
| ADAMTS5 | NM_198761.1 | F: 5’-cgaccctcaagaacttttgc-3’ R: 5’-gggtgtcacatgaatgatgc-3’ |
|
| MMP3 | NM_133523.2 | F: 5’-ttgtccttcgatgcagtcag-3’ R: 5’-gagacggccaaaatgaagag-3’ |
|
| MMP13 | NM_133530.1 | F: 5’-aggccttcagaaaagccttc-3’ R: 5’-aaggcccatcaaatgggtag-3’ |
|
| Inflammation | IL1B | NM_031512.2 | F: 5’-tccacctcaatggacagaac-3’ R: 5’-tgtgccgtctttcatcacac-3’ |
| TNFA | NM_012675.3 | F: 5’-ggaggagaagttcccaaatg-3’ R: 5’-gcttggtggtttgctacgac-3’ |
|
| Osteogenesis | RUNX2 | NM_053470.2 | F: 5’-tctgccgagctacgaaatg-3’ R: 5’-tggggaggatttgtgaagac-3’ |
mRNA transcripts evaluated and primers used by qPCR.
Histology
Rotator cuff samples were left intact as bone-tendon-muscle units. For the scapula, the bony glenoid origin of the LHB was resected, and the bone–tendon–muscle units of the LHB were pinned and processed separately from the remaining glenoid. All samples were processed, longitudinal sections (7 um) were collected, and tendon samples stained with hematoxylin–eosin (H&E) while cartilage samples were stained with Safranin O, Fast Green, and Iron Hematoxylin. H&E stained tendon sections were imaged at the insertion site and mid-substance of each tendon at 200X magnification. For the LHB, the mid-substance region was further subdivided (intra-articular space, proximal groove, and distal groove). Cell density (cells per mm2) and cell shape (aspect ratio; 0–1 with 1 being a circle) were quantified using a bioquantification software system (Bioquant Osteo II). Cartilage sections were imaged at 200X magnification in five regions (C, PS, PI, AS, and AI) corresponding to the indentation locations and graded using a Modified Mankin Score (including scores for cellularity, structure, matrix staining). Due to the presence of an intact tidemark in all images, scoring for this category was removed. Cartilage histology scoring was performed by three blinded investigators. Cartilage cell density was also quantified.
Statistics
For the ambulatory assessment, significance was assessed using a 2-way ANOVA with repeated measures on time with follow-up t-tests between groups at each time point. In this data set, points were occasionally absent (~7%) for a specific animal on a specific day. Therefore, multiple imputations were conducted on the ambulation data to allow for a repeated measures analysis. Tissue mechanics and tendon histology were assessed using a one-tailed and two-tailed t-test, respectively. Data from gene expression experiments were analyzed using REST software based on the Pfaffl method, which adjusts for experimentally determined assay efficiencies in determining relative expression levels of the transcripts of interest (Pfaffl et al., 2002). For cartilage thickness and histology scoring, median grades were compared between groups using a Mann Whitney test. Significance was set at p<0.05 and trends at p<0.1.
Results
Ambulatory Data
Overuse activity following the rotator cuff tendon detachments did not demonstrate a significant group X time interaction effect or main effect for group (ground reaction forces, spatial, and temporal parameters) (S-Table I).
Tendon Properties
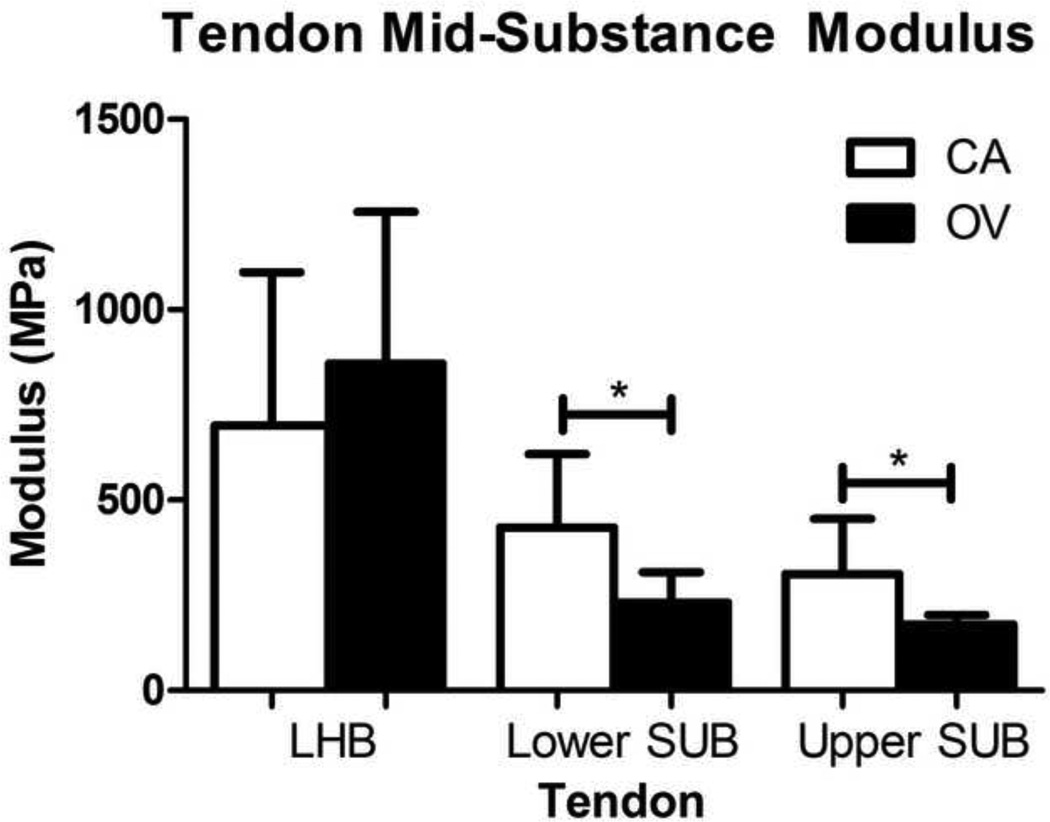
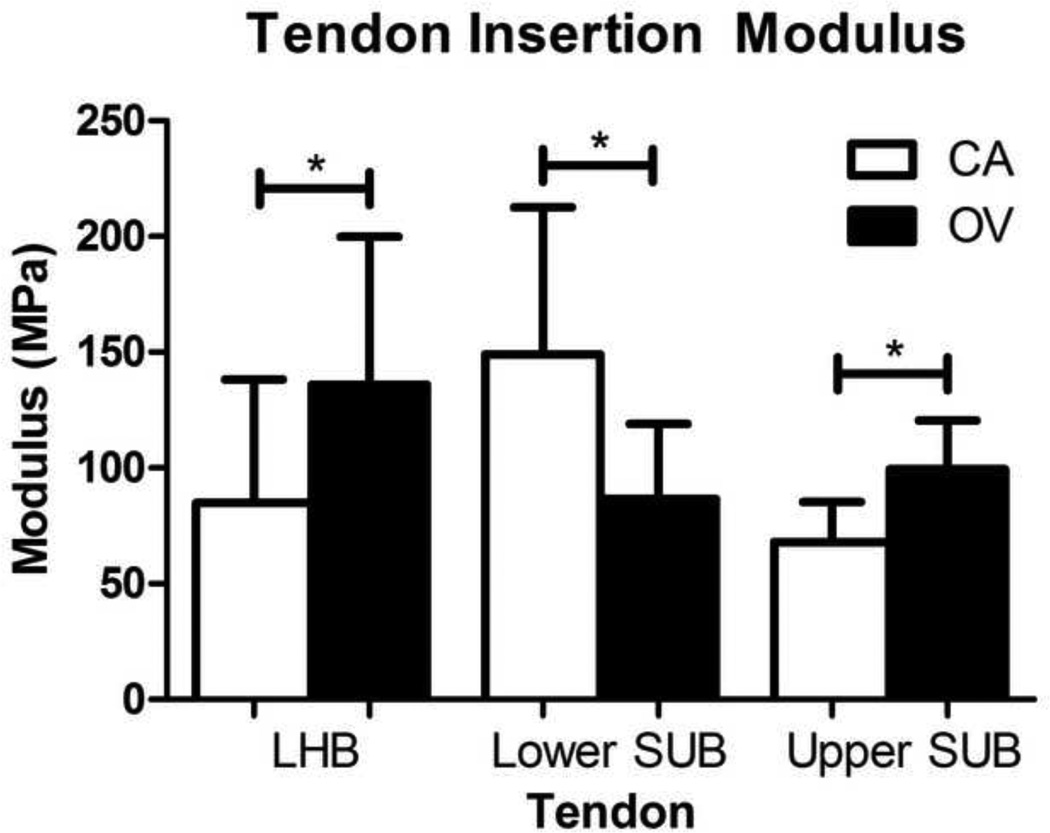
Tendon elastic modulus decreased in both the lower and upper subscapularis mid-substance and the lower subscapularis insertion site following OV compared to CA (Figure 1 and 2). In addition, tendon elastic modulus increased with OV in the insertion site of the LHB and the upper band of the subscapularis (Figure 2). No differences in cross-sectional area were observed in any tendon (S-Table II). Histology results demonstrated that both the upper and lower subscapularis tendons of the OV group had a less rounded cell shape at the insertion site (Table II, Figure 3). In addition, at the mid-substance of the lower subscapularis tendon, there was an increased cell density for the OV group. No other histological differences were observed between groups.
Figure 1.
Tendon mid-substance modulus for the adjacent intact tendons (LHB = long head of the biceps, upper sub = upper subscapularis, and lower sub = lower subscapularis) of both the cage activity (CA) and overuse (OV) groups. The upper and lower sub in the OV group had a significant decrease in modulus compared to the CA group (p<0.05). No other group differences were observed in any tendon. Data is shown as mean ± SD.
Figure 2.
Tendon insertion site modulus for the adjacent intact tendons (LHB = long head of the biceps, upper sub = upper subscapularis, and lower sub = lower subscapularis) of both the cage activity (CA) and overuse (OV) groups. The LHB and the upper sub in the OV group had a significant increase while the lower sub had a significant decrease in modulus compared to the CA group (p<0.05). Data is shown as mean ± SD.
Table 2.
Tendon Histology
| Tendon | Location | Cell Shape | p-value | Cell Density | p-value | ||
|---|---|---|---|---|---|---|---|
| CA | OV | CA | OV | ||||
| Long Head of the Biceps | INS | 0.11 ± 0.63 | 0.66 ± 0.09 | 0.72 | 2,137 ± 291 | 2,537 ± 1,677 | 0.61 |
| INTRA | 0.47 ± 0.03 | 0.51 ± 0.08 | 0.32 | 1,690 ± 303 | 1,270 ± 864 | 0.34 | |
| PROX | 0.43 ± 0.05 | 0.48 ± 0.09 | 0.38 | 1,484 ± 327 | 1,795 ± 1,066 | 0.55 | |
| DIS | 0.32 ± 0.1 | 0.39 ± 0.11 | 0.33 | 1,695 ± 560 | 1,489 ± 788 | 0.66 | |
| Upper Subscapularis | INS | 0.67 ± 0.1 | 0.52 ± 0.05 | 0.03* | 1,763 ± 734 | 1,736 ± 288 | 0.95 |
| MID | 0.39 ± 0.06 | 0.44 ± 0.04 | 0.24 | 1,654 ± 489 | 1,859 ± 352 | 0.52 | |
| Lower Subscapularis | INS | 0.68 ± 0.06 | 0.51 ± 0.03 | 0.006* | 1,419 ± 370 | 2,883 ± 1,590 | 0.13 |
| MID | 0.38 ± 0.06 | 0.35 ± 0.04 | 0.58 | 914 ± 202 | 2,772 ± 928 | 0.01* | |
Tendon histology was analyzed for differences in cell shape (aspect ratio; 0–1 with 1 being a circle) and cell density (cells per mm2) of the adjacent intact tendons. A significantly (*p<0.05) less rounded cell shape at the upper and lower subscapularis insertion site and an increased cell density at the mid-substance of the lower subscapularis tendon were observed with overuse (OV) compared to cage activity (CA). Data is shown as mean ± SD.
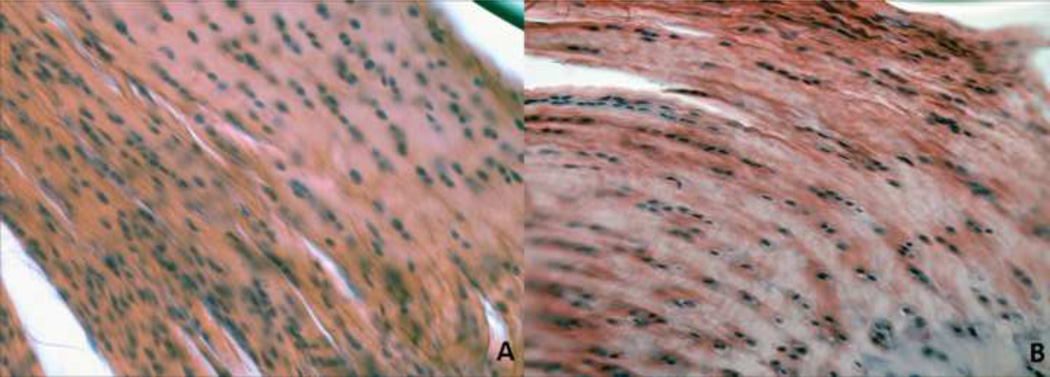
Figure 3.
Tendon histology (subscapularis and biceps) was quantified for cell shape and cell density at the insertion and mid-substance locations. A representative image for the upper subscapularis insertion is displayed. Cell shape was significantly more rounded (*p<0.05) in the cage activity group (A) compared to the overuse group (B). Note: Grading performed on original images [S-Figure I]. For publication, filters applied to images [A-B]: auto-tone, auto-contrast, auto-color (all three to each individually).
Cartilage Properties
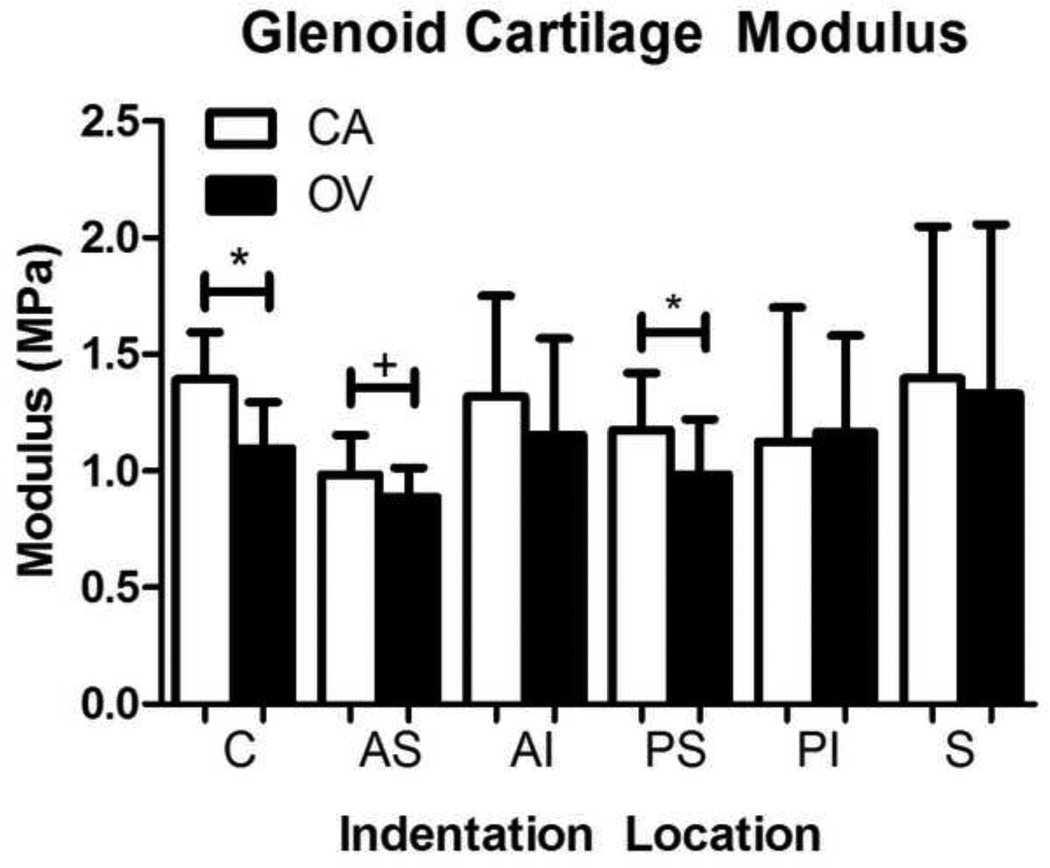
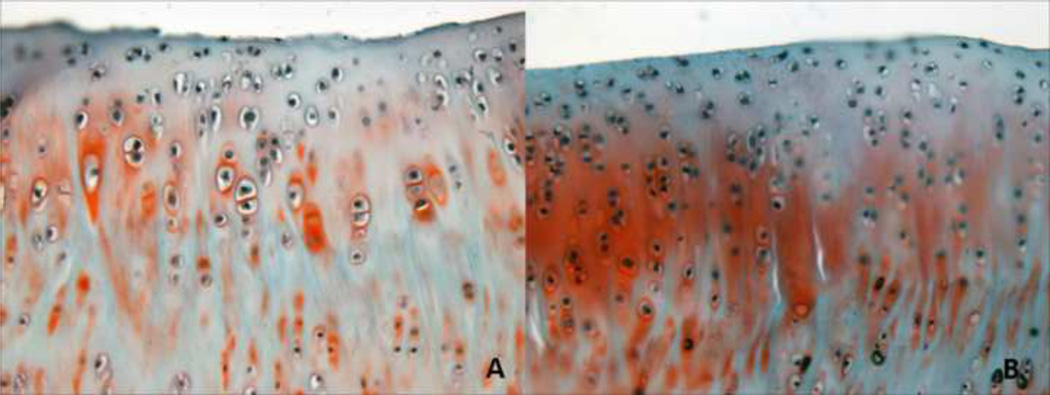
The OV group had a significantly decreased equilibrium elastic modulus in the PS and C regions of the glenoid cartilage compared to the CA group (Figure 4). A trend toward decreased modulus was also observed in the AS region. No group differences in cartilage thickness were observed in any region (S-Table III). Modified Mankin scoring showed a trend toward higher scores in the PI region of the CA group compared to the OV group (S-Table IV). Additionally, a significant increase in cell density was observed in the PS region, with only a trend in the AI region (Table III, Figure 5).
Figure 4.
Glenoid cartilage modulus at 6 glenoid regions (center (C), anterior-superior (AS), anterior-inferior (AI), posterior-superior (PS), posterior-inferior (PI), and superior (S)). Modulus values were significantly lower (*p<0.05) in the overuse (OV) group at the C and PS regions, with a similar trend (#p<0.1) in the AS region, compared to cage activity (CA) group. Data is shown as mean ± SD.
Table 3.
Cartilage Cell Density
| Glenoid Region | Cell Density | p-value | |
|---|---|---|---|
| CA | OV | ||
| Anterior-Inferior | 560±32# | 410±11 | 0.09# |
| Anterior-Superior | 580±70 | 530±12 | 0.56 |
| Center | 556±12 | 530±76 | 0.79 |
| Posterior-Inferior | 467±17 | 490±173 | 0.89 |
| Posterior-Superior | 373±44* | 574±103 | 0.04* |
Cartilage histology was analyzed for cell density (cells per mm2) in 5 glenoid regions (center, anterior-superior, anterior-inferior, posterior-superior, posterior-inferior). A significantly increased (*p<0.05) cell density was observed in the posterior-superior region with overuse (OV) compared to cage activity (CA). No other group differences were observed. Data is shown as mean ± SD.
Figure 5.
Cartilage histology was quantified for cell density at five regions (center (C), anterior-superior (AS), anterior-inferior (AI), posterior-superior (PS), posterior-inferior (PI)). A representative image for the posterior-superior glenoid cartilage is displayed. Cell density was significantly increased (*p<0.05) in the overuse group (B) compared to the cage activity group (A). Note: Grading performed on original images [S-Figure II]. For publication, filters applied to images [A–B]: auto-tone, auto-contrast, auto-color (all three to each individually).
Gene Expression
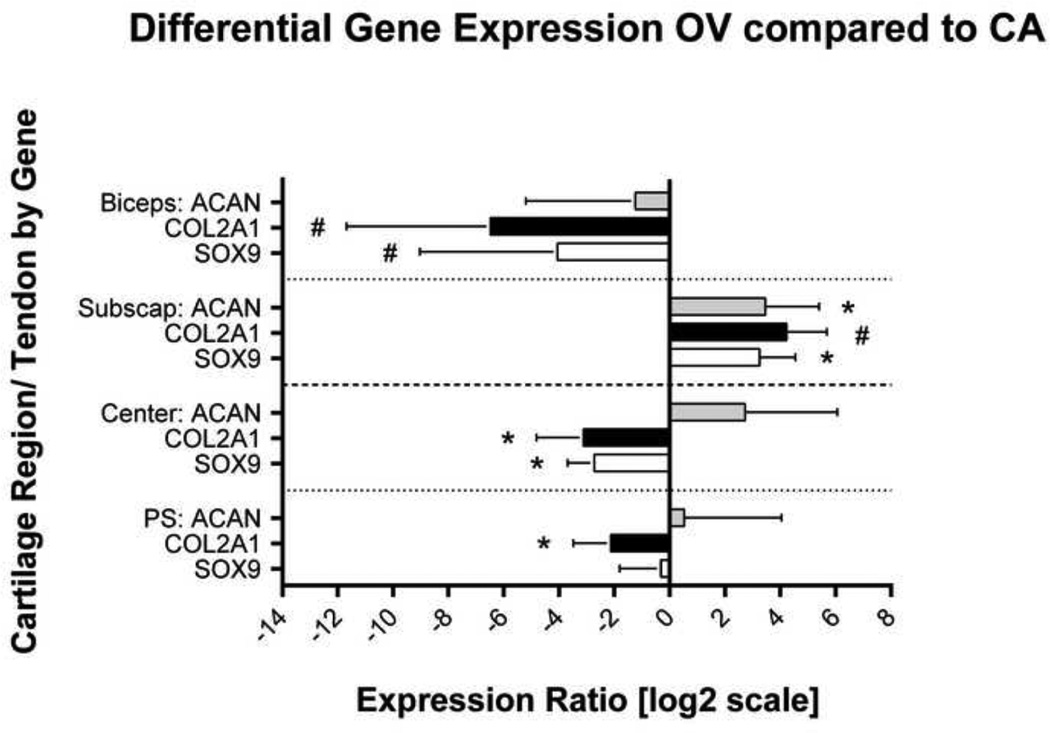
For tendon, the subscapularis demonstrated a significant upregulation of chondrogenic genes, ACAN and SOX9, 10.8 and 9.4-fold, respectively, in the OV group compared to the CA group (Figure 6). Although not significant, a similar trend was observed with Col II (upregulated 15.6-fold) and VEGF (downregulation 1.6-fold) was observed with OV. Additionally, the LHB demonstrated a trend toward downregulation of the chondrogenic genes, SOX9 and Col II, 90.6 and 16.8-fold, respectively. All other target genes did not demonstrate differences between groups (S-Table V). For cartilage, qPCR was performed in 2 regions (C and PS), due to the presence of significant mechanical alterations. Specifically, the PS and C regions demonstrated that OV generated a significant downregulation of Col II, 8.7 and 3.45-fold, respectively, when compared to the CA group. The C region had an additional downregulation of SOX9, 6.6-fold, when compared to the CA group (Figure 6). All other target genes did not demonstrate significant differences in these regions (S-Table V).
Figure 6.
Gene expression of chondrogenic markers is shown in the overuse (OV) group, relative to the cage activity (CA) group. The relative ratio of each target is normalized by GAPDH and corrected to CA transcript level (equal to log[1]=0). Chondrogenic genes were downregulated in the biceps tendon, upregulated in the subscapularis tendon, and downregulated in both cartilage regions (C and PS). Data is represented as expression ratio ± SD of each target mRNA in the OV group.
Discussion
Results demonstrate that 8 weeks of overuse in the presence of two-tendon rotator cuff tears leads to alterations in the adjacent uninjured tendons (LHB, subscapularis) and regions of the glenoid articular cartilage (C, AS, and PS) without altering shoulder joint function.
Alterations in shoulder function following a two-tendon tear have been observed both clinically (Nakajima et al.) and in this animal model (Perry et al., 2009b; Sarver et al., 2010). However, shoulder function has never been examined with the addition of overuse activity in the presence of a two-tendon rotator cuff tear. Specifically, we hypothesized that following 8 weeks of overuse in the presence of a two-tendon tear, shoulder joint function would be altered. However, there were no differences in any of the joint forces or temporal spatial parameters between the OV and CA groups. It has been suggested that a tear of both the supraspinatus and infraspinatus tendon will cause an imbalance of the anterior-posterior forces, therefore diminishing dynamic restraint and overall shoulder function (Burkhart, 1991; Reuther et al., 2013). Previous studies in this animal model have demonstrated that disruption of the anterior-posterior force balance does diminish shoulder function (Hsu et al., 2011; Perry et al., 2009b). However, results from this study suggest that the addition of overuse does not further exacerbate the altered joint mechanics. It is likely that 8 weeks of overuse activity did not diminish the dynamic stability of the intact musculotendinous units (i.e., LHB, subscapularis, teres minor, deltoid), preserving joint function. Future investigations will determine if these results exist at longer time points.
Clinically, shoulder joint function is often used as a metric to determine the overall integrity of joint structures. However, injured tissues may compensate to maintain normal shoulder function, masking any underlying structural alterations. Therefore, we examined the properties of the remaining uninjured tendons (LHB and upper and lower bands of the subscapularis). Results demonstrated that following a return to overuse, the tendon modulus decreased at the insertion site of the lower subscapularis and at the mid-substance of both the upper and lower bands of the subscapularis. In addition, there was an increase in tendon modulus at the insertion site of the LHB and upper subscapularis. Previously, when examining the effect of overuse following an isolated supraspinatus tear, there were no changes to the subscapularis tendon (Reuther et al., 2013). This may be due to the presence of an intact anterior-posterior force balance between the subscapularis and infraspinatus.
In this study, we disrupted this balance by detaching both the supraspinatus and infraspinatus tendons. The combination of overuse (treadmill running) and overload (force balance disruption) did result in mechanical changes along the length of both the upper and lower bands of the subscapularis tendon and at the insertion site of the LHB. Histologic changes were also observed at upper and lower insertions of the subscapularis and mid-substance of the lower subscapularis. Gene expression modifications also provide support for the mechanical changes. The insertion site of the subscapularis (including upper and lower bands) demonstrated increased expression of chondrogenic markers. Altered regulation of chondrogenic markers within overused tendons has also been identified by others (Archambault et al., 2007; Attia et al.; Attia et al., 2012) and is believed to contribute to a chronic tendinopathic condition (Archambault et al., 2007; Xu and Murrell, 2008). A shift from normal tendon phenotype to a cartilage phenotype may be due to a change in tendon mechanical loading (Attia et al., 2013). Specifically, previous studies have demonstrated that when exposed to compressive loads, tenocytes respond by producing an extra-cellular matrix containing type II collagen (Thomopoulos et al.; Wren et al., 2000). It is likely that both tendons (biceps and subscapularis) experience subacromial compressive loading due to humeral head migration. If the tendons are continually exposed to these altered loading patterns with overuse, these mechanical and biologic changes may worsen, leading to pain, dysfunction, and even additional tears.
Mechanical changes were also observed in the LHB with overuse. This is similar to our recent study examining the effect of overuse following a supraspinatus only tear (Reuther et al., 2013). In the presence of an isolated supraspinatus tear, mechanical changes were observed in the LHB mid-substance. However, in the current study, the mechanical changes were localized to the LHB insertion. These location differences may be a result of differences in magnitude, type (i.e., compressive, shear), and/or direction of altered joint loads between the two injury models. To support the LHB mechanical changes observed, gene expression from the insertion site demonstrated decreased expression of chondrogenic markers. Specifically, this shift away from the cartilage phenotype may have improved the tensile mechanics of the tissue.
Mechanical alterations were also observed in the articular cartilage. Specifically, cartilage mechanics decreased in three regions (C, AS, and PS). These changes are likely a result of increased humeral head translations and abnormal joint loading. Overuse following an isolated supraspinatus tear has also been shown to lead to mechanical alterations in cartilage; specifically, within the superior and inferior regions of the glenoid. The different locations of mechanical changes observed is likely a result of differences in humeral head translations due to disruption of the anterior-posterior force balance and loss of concavity compression of the humeral head on the glenoid. Results also demonstrated a significant increase in cell density in the PS region of the OV group compared to the CA group. This may be indicative of increased cell activity due to overuse and is consistent with mechanical changes observed in this region. Additionally, the mechanical changes observed corresponded to decreased Col II gene expression in the PS and C regions. Type II collagen is the predominant collagen found in articular cartilage. Decreased Col II gene expression may indicate decreased collagen production, which may manifest as compromised mechanical properties and decreased tissue integrity. Previous studies have demonstrated decreased cartilage stiffness in experimental models of osteoarthritis (Herzog et al., 1998) and in human osteoarthritic cartilage (Kleemann et al., 2005; Setton et al., 1999).
This study has several limitations. Although the rat shoulder has similar anatomy and has been widely used as a model of rotator cuff injury, the use of a quadruped animal does not exactly replicate the human condition. However, the rat shoulder produces large amounts of glenohumeral forward flexion which, combined with supraspinatus acromial impingement, and replicates repetitive overhead activity in the human (Soslowsky et al., 1996). Additionally, for tendon gene expression, tissue was obtained from the insertion site only and for the subscapularis, the upper and lower bands were combined. As a result, direct comparisons to regional mechanical data were not always possible and the magnitude of altered local gene expression changes may have been suppressed. Despite these limitations, results clearly demonstrate that mechanical consequences are localized to the subscapularis and LHB tendons and cartilage after returning to overuse following a two-tendon tear.
Taken together, these results indicate that returning to overuse activity following detachment of the supraspinatus and infraspinatus does not alter shoulder function but does alter the regulation of chondrogenic genes within tendon and cartilage and compromises tissue mechanics. This study suggests that patients returning to a high level of activity following a two-tendon rotator cuff tear may place the joint at higher risk for subscapularis, LHB, and cartilage injury. Additionally, this study helps to define the mechanical and biological processes by which overuse following large rotator cuff tears lead to joint damage. Understanding the mechanical and biological signals that influence chondrogenic differentiation and/or phenotypic switching of tenocytes may be a key factor in prevention and treatment of tendinopathy. This information may provide a framework to determine optimal long-term treatment strategies for patients with rotator cuff tears that are required or desire to return to pre-injury overuse activity levels. Future studies will examine earlier time points to understand the progression of joint changes and an additional scenario of a combined supraspinatus-infraspinatus-LHB tear to further elucidate joint changes in the presence of overuse.
Supplementary Material
Acknowledgements
This study was funded by NIH/NIAMS (R01AR056658, T32AR007132) and the Penn Center for Musculoskeletal Disorders (P30AR050950).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None of the authors have any relationships or conflicts of interest to disclose.
References
- Archambault JM, Jelinsky SA, Lake SP, Hill AA, Glaser DL, Soslowsky LJ. Rat supraspinatus tendon expresses cartilage markers with overuse. J Orthop Res. 2007;25:617–624. doi: 10.1002/jor.20347. [DOI] [PubMed] [Google Scholar]
- Attia M, Huet E, Gossard C, Menashi S, Tassoni MC, Martelly I. Early Events of Overused Supraspinatus Tendons Involve Matrix Metalloproteinases and MMPRIN/CD147 in the Absence of Inflammation. Am J Sports Med. 41:908–917. doi: 10.1177/0363546512473817. [DOI] [PubMed] [Google Scholar]
- Attia M, Huet E, Gossard C, Menashi S, Tassoni MC, Martelly I. Early Events of Overused Supraspinatus Tendons Involve Matrix Metalloproteinases and EMMPRIN/CD147 in the Absence of Inflammation. Am J Sports Med. 2013;41:908–917. doi: 10.1177/0363546512473817. [DOI] [PubMed] [Google Scholar]
- Attia M, Scott A, Duchesnay A, Carpentier G, Soslowsky LJ, Huynh MB, Van Kuppevelt TH, Gossard C, Courty J, Tassoni MC, Martelly I. Alterations of overused supraspinatus tendon: a possible role of glycosaminoglycans and HARP/pleiotrophin in early tendon pathology. J Orthop Res. 2012;30:61–71. doi: 10.1002/jor.21479. [DOI] [PubMed] [Google Scholar]
- Burkhart SS. Arthroscopic treatment of massive rotator cuff tears. Clinical results and biomechanical rationale. Clin Orthop Relat Res. 1991:45–56. [PubMed] [Google Scholar]
- Hayes WC, Keer LM, Herrmann G, Mockros LF. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5:541–551. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- Herzog W, Diet S, Suter E, Mayzus P, Leonard TR, Muller C, Wu JZ, Epstein M. Material and functional properties of articular cartilage and patellofemoral contact mechanics in an experimental model of osteoarthritis. J Biomech. 1998;31:1137–1145. doi: 10.1016/s0021-9290(98)00136-5. [DOI] [PubMed] [Google Scholar]
- Hsu HC, Luo ZP, Stone JJ, Huang TH, An KN. Correlation between rotator cuff tear and glenohumeral degeneration. Acta Orthop Scand. 2003;74:89–94. doi: 10.1080/00016470310013725. [DOI] [PubMed] [Google Scholar]
- Hsu JE, Reuther KE, Sarver JJ, Lee CS, Thomas SJ, Glaser DL, Soslowsky LJ. Restoration of anterior-posterior rotator cuff force balance improves shoulder function in a rat model of chronic massive tears. J Orthop Res. 2011;29:1028–1033. doi: 10.1002/jor.21361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keener JD, Wei AS, Kim HM, Steger-May K, Yamaguchi K. Proximal humeral migration in shoulders with symptomatic and asymptomatic rotator cuff tears. J Bone Joint Surg Am. 2009;91:1405–1413. doi: 10.2106/JBJS.H.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemann RU, Krocker D, Cedraro A, Tuischer J, Duda GN. Altered cartilage mechanics and histology in knee osteoarthritis: relation to clinical assessment (ICRS Grade) Osteoarthritis Cartilage. 2005;13:958–963. doi: 10.1016/j.joca.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Lakemeier S, Reichelt JJ, Timmesfeld N, Fuchs-Winkelmann S, Paletta JR, Schofer MD. The relevance of long head biceps degeneration in the presence of rotator cuff tears. BMC Musculoskelet Disord. 2010a;11:191. doi: 10.1186/1471-2474-11-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakemeier S, Schwuchow SA, Peterlein CD, Foelsch C, Fuchs-Winkelmann S, Archontidou-Aprin E, Paletta JR, Schofer MD. Expression of matrix metalloproteinases 1, 3, and 9 in degenerated long head biceps tendon in the presence of rotator cuff tears: an immunohistological study. BMC Musculoskelet Disord. 2010b;11:271. doi: 10.1186/1471-2474-11-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima D, Yamamoto A, Kobayashi T, Osawa T, Shitara H, Ichinose T, Takasawa E, Takagishi K. The effects of rotator cuff tears, including shoulders without pain, on activities of daily living in the general population. J Orthop Sci. 17:136–140. doi: 10.1007/s00776-011-0186-4. [DOI] [PubMed] [Google Scholar]
- Neer CS., 2nd Impingement lesions. Clin Orthop Relat Res. 1983:70–77. [PubMed] [Google Scholar]
- Neer CS, 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65:1232–1244. [PubMed] [Google Scholar]
- Oh JH, Jun BJ, McGarry MH, Lee TQ. Does a critical rotator cuff tear stage exist?: a biomechanical study of rotator cuff tear progression in human cadaver shoulders. J Bone Joint Surg Am. 2011;93:2100–2109. doi: 10.2106/JBJS.J.00032. [DOI] [PubMed] [Google Scholar]
- Peltz CD, Perry SM, Getz CL, Soslowsky LJ. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. J Orthop Res. 2009;27:416–420. doi: 10.1002/jor.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SM, Getz CL, Soslowsky LJ. After rotator cuff tears, the remaining (intact) tendons are mechanically altered. J Shoulder Elbow Surg. 2009a;18:52–57. doi: 10.1016/j.jse.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SM, Getz CL, Soslowsky LJ. Alterations in function after rotator cuff tears in an animal model. J Shoulder Elbow Surg. 2009b;18:296–304. doi: 10.1016/j.jse.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reno C, Marchuk L, Sciore P, Frank CB, Hart DA. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques. 1997;22:1082–1086. doi: 10.2144/97226bm16. [DOI] [PubMed] [Google Scholar]
- Reuther KE, Sarver JJ, Schultz SM, Lee CS, Sehgal CM, Glaser DL, Soslowsky LJ. Glenoid cartilage mechanical properties decrease after rotator cuff tears in a rat model. J Orthop Res. 2012;30:1435–1439. doi: 10.1002/jor.22100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuther KE, Thomas SJ, Sarver JJ, Tucker JJ, Lee CS, Gray CF, Glaser DL, Soslowsky LJ. Effect of return to overuse activity following an isolated supraspinatus tendon tear on adjacent intact tendons and glenoid cartilage in a rat model. J Orthop Res. 2013;31:710–715. doi: 10.1002/jor.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver JJ, Dishowitz MI, Kim SY, Soslowsky LJ. Transient decreases in forelimb gait and ground reaction forces following rotator cuff injury and repair in a rat model. J Biomech. 2010;43:778–782. doi: 10.1016/j.jbiomech.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setton LA, Elliott DM, Mow VC. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage. 1999;7:2–14. doi: 10.1053/joca.1998.0170. [DOI] [PubMed] [Google Scholar]
- Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- Thomas SJ, Miller KS, Soslowsky LJ. The upper band of the subscapularis tendon in the rat has altered mechanical and histologic properties. J Shoulder Elbow Surg. 2012;21:23–31. doi: 10.1016/j.jse.2011.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S, Das R, Birman V, Smith L, Ku K, Elson EL, Pryse KM, Marquez JP, Genin GM. Fibrocartilage tissue engineering: the role of the stress environment on cell morphology and matrix expression. Tissue Eng Part A. 2011;17:1039–1053. doi: 10.1089/ten.tea.2009.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- Wren TA, Beaupre GS, Carter DR. Mechanobiology of tendon adaptation to compressive loading through fibrocartilaginous metaplasia. J Rehabil Res Dev. 2000;37:135–143. [PubMed] [Google Scholar]
- Xu Y, Murrell GA. The basic science of tendinopathy. Clin Orthop Relat Res. 2008;466:1528–1538. doi: 10.1007/s11999-008-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.