Abstract
Sepsis is caused by an overwhelming immune response to bacterial infection. The discovery of high mobility group box 1 (HMGB1) as a late mediator of lethal sepsis has prompted investigation into the development of new therapeutics which specifically target this protein. Here, we show that chloroquine, an anti-malarial drug, prevents lethality in mice with established endotoxemia or sepsis. This effect is still observed even if administration of chloroquine is delayed. The protective effects of chloroquine were mediated through inhibition of HMGB1 release in macrophages, monocytes, and endothelial cells, thereby preventing its cytokine-like activities. As an inhibitor of autophagy, chloroquine specifically inhibited HMGB1-induced Iκ-B degradation and NF-κB activation. These findings define a novel mechanism for the anti-inflammatory effects of chloroquine and also suggest a new potential clinical use for this drug in the setting of sepsis.
Keywords: HMGB1, chloroquine, sepsis, autophagy, NF-κB, Beclin 1
1. Introduction
Sepsis, also known as systemic inflammatory response syndrome, is a life threatening condition and the most common cause of death in intensive care units [1]. Bacterial infections are the most common cause of sepsis. Sepsis is pathologically mediated by the release of multiple proinflammatory cytokines such as tumor necrosis factor (TNF) and high mobility group box 1 (HMGB1) from immune cells. TNF is released early in the development of sepsis [2], and anti-TNF-α neutralizing antibodies, although efficacious, appear to have a narrow therapeutic window in human patients [3]. HMGB1 functions as a late mediator of lethal endotoxemia and circulating HMGB1 is elevated in a delayed fashion in septic mice [4]. Once released, HMGB1 binds to cell-surface receptors (e.g.TLR4 and RAGE) and mediates various cellular responses, including activation of the NF-κB signaling pathway and subsequent translation and release of additional proinflammatory cytokines such as TNF and IL-6 [5]. Mice treated with anti-HMGB1 antibodies or HMGB1 antagonists are protected against lethal endotoxemia and sepsis [4, 6–9]. In addition, a high concentration of HMGB1 in the plasma of patients with severe sepsis correlates with an increased likelihood of mortality [10]. Therefore, HMGB1 is a molecular target which provides an opportunity for clinical intervention in sepsis and perhaps other inflammatory diseases [11, 12].
Chloroquine is a 4-aminoquinoline drug long used in the treatment or prevention of malaria [13] and is also a widely used inhibitor of autophagy being evaluated for usefulness in cancer therapy regimens [14]. Autophagy is a process of “self-eating”, in which cytoplasmic material (e.g. organelles and proteins) is delivered to lysosomes for degradation [15, 16]. Recent developments reveal an important role for autophagy in immunity and inflammation [17, 18]. Chloroquine raises the lysosomal pH and inhibits lysosome-autophagosome fusion [19]. Chloroquine also has been used as a non-steroidal anti-inflammatory drug to treat rheumatoid arthritis, lupus erythematosus, and multiple sclerosis [20–22]. Moreover, one study showed that chloroquine protects from sepsis-induced acute kidney injury and pro-inflammatory cytokine TNF release by decreasing TLR9 protein level [23]. However, it was previously unknown if chloroquine could inhibit HMGB1 signaling in experimental sepsis. Here, we show that chloroquine prevents fatality in mice with established lethal sepsis and systemic inflammation. Inhibition of autophagy with chloroquine reduced lipopolysaccharide (LPS)-mediated release of HMGB1, as well as HMGB1-mediated pro-inflammatory signaling responses in immune cells by inhibition of NF-κB activation.
2. Materials and methods
2.1 Reagents
The antibodies to LC3 (#NB100-2220), beclin 1 (#NB500-249), and ULK1 (#NB110-74844) were obtained from Novus (Woburn, MA, USA). The antibodies to Iκ-B (#4814), p65 (#4764), tubulin (#2128), and actin (#3700) were obtained from Cell Signaling Technology (Danvers, MA, USA). The antibodies to HMGB1 (#ab18256) and fibrillarin (ab4566) came from Abcam (Cambridge, MA, USA). LPS (Escherichia coli LPS 0111:B4; #L4391), chloroquine (#C6628), and hydroxychloroquine (#H0915) were obtained from Sigma (St. Louis, MO, USA). Recombinant HMGB1 protein with endotoxin content ≤ 200 pg/mg was kindly provided by Dr. Kevin J. Tracey (The Feinstein Institute for Medical Research, Manhasset, NY 11030) [24]. NE-PER Nuclear and Cytoplasmic Extraction Kits (#78835) were obtained from Thermo Fisher Scientific (Rockford, IL, USA).
2.2 Cell culture
Murine macrophage-like RAW 264.7 cells and human umbilical vein endothelial cells (HUVECs) were obtained from American Type Culture and were cultured in RPMI medium 1640 or DMEM supplemented with 10% heat-inactivated fetal bovine serum and 2 mM glutamine. Human peripheral blood mononuclear cells (HUPBMCs) were isolated from the blood of healthy donors by density gradient centrifugation through Ficoll and cultured in RPMI 1640 supplemented with 10% heat-inactivated human serum and 2 mM glutamine, as previously described [25].
2.3 Animal model of endotoxemia and sepsis
Endotoxemia was induced in Balb/C mice (male, 7–8 weeks old, 20–25 g weight) by intraperitoneal (i.p.) injection of bacterial endotoxin (LPS, 5 mg/kg), as previously described [4, 8]. Sepsis was induced in male Balb/C mice (male, 7–8 week old, 20–25 g weight) by cecal ligation and puncture was performed as previously described [4, 8]. Chloroquine was administered i.p. in mice at indicated doses and time points. Blood was collected at indicated time points, allowed to clot for two hours at room temperature, and then centrifuged for 15 minutes at 1,500×g. Serum samples were stored at 20°C before analysis. Mortality was recorded for up to two to three weeks after injection to ensure that no additional late deaths occurred. All animal experiments strictly followed the guidelines of the Institutional Review Board.
2.4 Cytokine measurements
Commercially available enzyme linked immunosorbant assay (ELISA) kits were used to measure the concentrations of HMGB1 (Shino Test Corporation, Tokyo, Japan, #ST51011), TNF (R&D Systems, Minneapolis, MN, USA, #DRT100, #MRT20), and IL-6 (R&D Systems, #D6050, #M6000B) in serum or the culture medium according to the manufacturer’s instructions.
2.5 RNAi
Transfection with beclin 1-shRNA (SHCLNG-NM_019584), ULK1- shRNA (SHCLNG-NM_009469), and control shRNA (SHC001) from Sigma, were performed using the FuGENE® HD Transfection Reagent (Roche Applied Science, 04709705001) according the manufacturer’s instructions.
2.6 Western blot analysis
Proteins in cell lysates was first resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, then transferred to nitrocellulose membrane and subsequently incubated with the primary antibody, as previously described [26]. After incubation with peroxidase-conjugated secondary antibodies, the signals were visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA, # 32106) according to the manufacturer’s instructions. Relative band intensity was quantified using the Gel-pro Analyzer® software (Media Cybernetics, Bethesda, MD, USA).
2.7 NF-κB activation assay
Cells were transiently transfected in a 12-well plate with an NF-κB luciferase reporter plasmid or control empty plasmid using the Lipofectamine 2000 reagent, as previously described [27]. After 24–48 hours, the cells were exposed to various agents. Luciferase activity was determined using the luciferase assay system with the reporter lysis buffer (#E2000) obtained from Promega (Madison, WI, USA). The results are expressed as relative NF-κB activity after normalizing to the control empty plasmid.
2.8 Quantitative real time polymerase chain reaction
cDNA from various cell samples were amplified by real-time quantitative polymerase chain reaction (PCR) with specific primers from SABiosciences (Frederick, MD, USA) as previously described [28]. The control group was set as 100%.
2.9 Statistical analysis
Data are expressed as means ± SEM of two or three independent experiments. Significance of differences between groups was determined by analysis of variance (ANOVA) least significant difference (LSD) test. The Kaplan-Meier survival analysis with Logrank significance test was used to compare the differences in mortality rates between groups. A p-value < 0.05 was considered statistically significant.
3. Results
3.1 Pretreatment with chloroquine/hydroxychloroquine protects mice against lethal endotoxemia and inhibits release of TNF and HMGB1
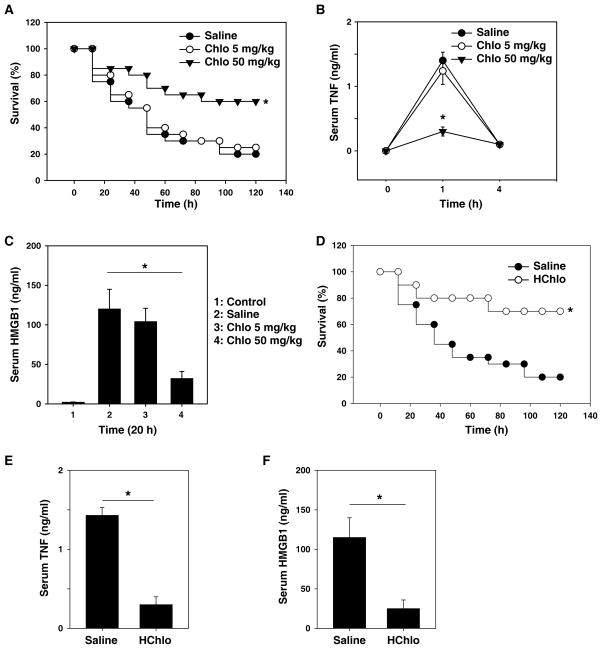
We evaluated the protective effect of chloroquine in a standard murine model of endotoxemia. Administration of a single dose of the chloroquine (50 mg/kg, i.p., or 5 mg/kg, i.p.) was followed 30 minutes later by an injection of LPS (5 mg/kg LPS, i.p.). Pretreatment with chloroquine at 50 mg/kg conferred significant protection from lethal endotoxemia (survival in chloroquine-treated mice = 12/20; survival in saline-treated mice = 4/20; P < 0.05) (Fig. 1A). All of these mice were observed for at least two weeks, and no late deaths occurred, indicating that chloroquine does not merely delay the onset of LPS lethality, but provides long-lasting protection. To gain insight into the protective mechanism of chloroquine against lethal endotoxemia, we evaluated its effects on the systemic accumulation of TNF-α (peaking between one to two hours) and HMGB1 (peaking between 16–32 hours) [2, 4]. Pretreatment of endotoxemic mice with chloroquine (50 mg/kg, i.p.) significantly attenuated the serum levels of both TNF-α at one hour (Fig. 1B) and HMGB1 at 20 hours (Fig. 1C) after LPS treatment, indicating that it prevented endotoxemia by attenuating the release of early and late cytokine release. Since hydroxychloroquine is far less toxic to the retina in clinic, we examined the protective effects of hydroxychloroquine in lethal endotoxemia. Like chloroquine, its derivative, hydroxychloroquine, protects mice against lethal endotoxemia (Fig. 1D) and inhibits release of TNF-α (Fig. 1E) and HMGB1 (Fig. 1F).
Figure 1. Chloroquine/hydroxychloroquine pretreatment prevents endotoxin lethality by attenuating TNF and HMGB1 release in vivo.
(A) Mice (n=20 per group) were injected with a single dose of chloroquine (“Chlo”) as indicated, followed 30 minutes later by a lethal infusion of endotoxin (LPS, 5 mg/kg, i.p.). The Kaplan-Meyer method was used to compare the differences in survival rates between groups. *, P<0.05 versus saline group. (B, C) In parallel, serum levels of TNF at 1–4 hours (B) and HMGB1 at 20 hours (C) were measured by ELISA (n=4 animals per group, values are mean ± SEM, *, P<0.05). (D) Mice (n=20 per group) were injected with hydroxychloroquine (“HChlo”, 100 mg/kg), followed 30 minutes later by a lethal infusion of endotoxin (LPS, 5 mg/kg, i.p.). The Kaplan-Meyer method was used to compare the differences in survival rates between groups. *, P<0.05 versus saline group. (E, F) In parallel, serum levels of TNF-α at 1 hour (E) and HMGB1 at 20 hours (F) were measured by ELISA (n=4 animals per group, values are mean ± SEM, *, P<0.05).
3.2 Delayed administration of chloroquine protects mice against lethal endotoxemia and inhibits release of HMGB1
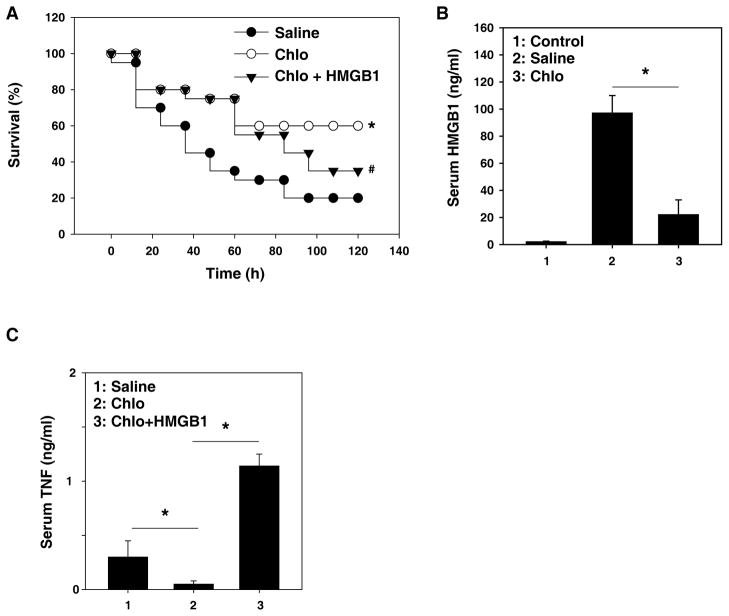
To investigate whether chloroquine treatment could be delayed until after administration of LPS and still maintain its protective effects, we injected chloroquine four hours after LPS (followed by additional doses at 12, 24, and 36 hours after LPS), a time at which the clinical manifestations of LPS-induced toxicity, including diarrhea, piloerection, and depressed spontaneous activity were evident. This delayed treatment conferred significant protection against lethal endotoxemia (Fig. 2A). All of these mice were observed for at least two weeks, and no late deaths occurred. Notably, treatment of endotoxemic mice with chloroquine beginning four hours after LPS injection significantly attenuated the systemic release of HMGB1 measured at 30 hours after the onset of endotoxemia (Fig. 2B). To demonstrate that the protective effect of chloroquine occurs via inhibition of HMGB1 release, we treated mice with HMGB1 protein after LPS insult and chloroquine treatment. The reduced lethality (Fig. 2A) and TNF-α production (Fig. 2C) in response to chloroquine is partially reversed. These findings indicate that delayed treatment with chloroquine confers significant protection partly by inhibition of HMGB1 release.
Figure 2. Delayed administration of chloroquine attenuates the lethality of endotoxemia and sepsis HMGB1.
(A) Mice (n=20 per group) received a lethal infusion of endotoxin (LPS, 5 mg/kg, i.p.) and were treated with chloroquine (“Chlo”, 50 mg/kg) 4, 12, 24, and 36 hours later with or without HMGB1 (100 μg per mouse, i.p., followed at 48 hours after LPS). The Kaplan-Meyer method was used to compare the differences in survival rates between groups. *, P<0.05 versus saline group; #, P<0.05 versus chloroquine group. (B, C) In parallel, serum levels of and HMGB1 at 30 hours and TNF-α at 50 hours was measured by ELISA (n=4 animals per group, values are mean ± SEM, *, P<0.05).
3.3 Delayed administration of chloroquine protects mice against lethal sepsis and inhibits release of HMGB1
Although endotoxemia provides a useful model for investigating complex cytokine cascades, more clinically relevant animal models are necessary to explore the therapeutic benefit of various agents in the treatment of human sepsis. One well-characterized, standardized animal model of sepsis is induced by surgical perforation of the cecum (CLP). After cecal ligation, the first dose of chloroquine was given 24 hours after the onset of sepsis and followed by additional doses at 48 and 72 hours post-sepsis. Compared to the saline-treated group, chloroquine at 50 mg/kg significantly increased the animal survival rate (Fig. 3A). ELISA analysis of serum prepared from blood obtained 30 hours after cecal puncture revealed that chloroquine significantly reduced circulating HMGB1 levels (Fig. 3B). These findings indicate that chloroquine confers significant protection in sepsis by inhibition of HMGB1 release.
Figure 3. Chloroquine protects mice against lethal sepsis and inhibits HMGB1 release.

(A) The cecal ligation and puncture technique was used to induce intraabdominal sepsis in mice (n = 20 per group). Repeated administration of indicated chloroquine at 24, 36, 48, 60, and 72 hours after cecal ligation and puncture significantly increased survival, as compared to the saline group (*, P<0.05), as measured by the Kaplan-Meyer test. (B) In parallel, serum levels and HMGB1 at 30 hours was measured by ELISA (n=5 animals per group, values are mean ± SEM, *, P<0.05).
3.4 Chloroquine inhibits the cytokine activity and release of HMGB1
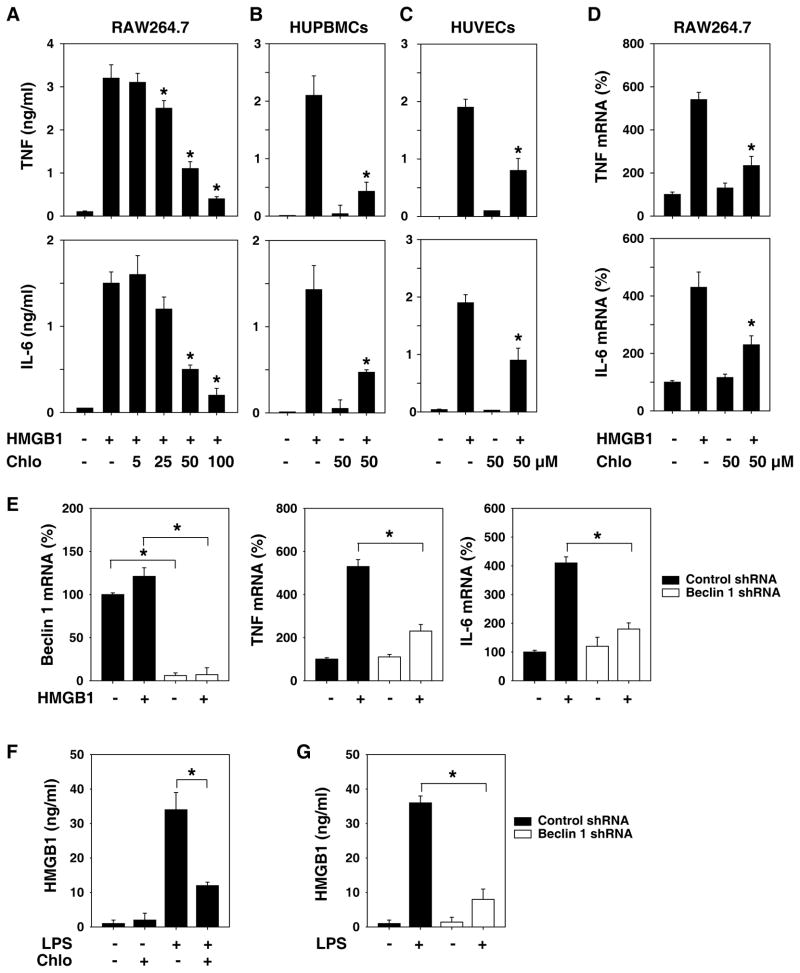
Recombinant HMGB1 has potent cytokine-like stimulating activity in macrophages, monocytes, and endothelial cells [5, 24, 29]. To explore whether chloroquine inhibits the cytokine activity of HMGB1, we treated the murine macrophage cell line RAW264.7 with different doses of chloroquine after rHMGB1 treatment. Indeed, HMGB1-induced TNF and IL-6 release was dose-dependently inhibited by chloroquine in these cells (Fig. 4A). We further confirmed its HMGB1-inhibiting activities using primary HUPBMCs and HUVECs. In HUPBMCs (Fig. 4B) and HUVECs (Fig. 4C), chloroquine abrogated HMGB1-induced TNF and IL-6 release. Furthermore, chloroquine inhibited HMGB1-induced TNF and IL-6 mRNA upregulation in RAW264.7 cells (Fig. 4D) and HUVECs (data not shown). To explore whether the inhibition of autophagy is required for HMGB1-induced TNF and IL-6 mRNA upregulation, we knocked down the essential autophagy gene beclin 1 (the mammalian homolog of yeast ATG6) [30] in RAW264.7 cells. Knockdown of beclin 1 inhibited HMGB1-induced TNF and IL-6 mRNA upregulation (Fig. 4E). We and others recently demonstrated that autophagy regulates starvation- and nigericin (inflammasome agonist)- induced HMGB1 release in fibroblasts and macrophages [31, 32]. Similarly, chloroquine or knockdown of beclin 1 inhibited LPS-induced HMGB1 release in RAW264.7 cells (Fig. 4F and 4G). Taken together, these data suggest that inhibition of autophagy by chloroquine or genetic approaches is capable of effectively inhibiting the cytokine activity and release of HMGB1 in immunity cells.
Figure 4. Chloroquine effectively abrogated HMGB1-induced expression and release of cytokines in immunity cells.
(A–C) RAW 264.7 cells (A), primary human peripheral blood mononuclear cells (HUPBMCs) (B), and human umbilical vein endothelial cells (HUVECs) (C) were stimulated with HMGB1 (1 μg/ml) in the absence or presence of chloroquine at indicated concentrations. At 16 hours after HMGB1 stimulation, levels of TNF and IL-6 in the culture medium were determined by ELISA. Values are mean ± SEM of three independent experiments. * p < 0.05 versus HMGB1 group. (D) In parallel, the mRNA expression level of TNF-α and IL-6 in RAW264.7 cells was detected by real time PCR analysis. Values are mean ± SEM of two independent experiments. * p < 0.05 versus HMGB1 group. (E) RAW 264.7 cells were transfected with beclin 1 shRNA and control shRNA for 48 hours, and then treated with HMGB1 (1 μg/ml) for 16 hours. The mRNA expression levels of beclin 1, TNF-α, and IL-6 in RAW264.7 cells were detected by real time PCR analysis. Values are mean ± SEM of two independent experiments. * p < 0.05. (F) RAW 264.7 cells were stimulated with LPS (200 ng/ml) in the absence or presence of chloroquine at 100 μM. At 24 hours after LPS stimulation, levels of HMGB1 in the culture medium were determined by ELISA. Values are mean ± SEM of three independent experiments. * p < 0.05. (G) RAW 264.7 cells were transfected with beclin 1 shRNA and control shRNA for 48 hours and then treated with LPS (200 ng/ml) for 24 hours. Levels of HMGB1 in the culture medium were determined by ELISA. Values are mean ± SEM of three independent experiments. * p < 0.05.
3.5 Chloroquine inhibits HMGB1-mediated NF-κB activation
HMGB1-induced NF-κB activation has been recognized as a key contributor to the proinflammatory response [11, 12, 33]. To study cholorquine’s effects on this pathway, we next analyzed the influence of chloroquine on HMGB1-mediated NF-κB activation. Chloroquine significantly inhibited HMGB1-induced NF-κB activity as measured by luciferase reporter assay and NF-κB p65 nuclear translocation by Western blot assay (Fig. 5A and B). In unstimulated cells, NF-κB is sequestered in the cytoplasm by IκB inhibitory proteins. NF-κB-activating agents can induce IκB degradation and release NF-κB to enter the nucleus, where it regulates pro-inflammatory gene expression and cytokine release. Chloroquine inhibited HMGB1-induced IκB degradation in RAW264.7 and HUVECs by Western blot analysis (Fig. 5C), suggesting that autophagy is involved in regulation of IκB degradation. To explore whether the inhibition of autophagy is required for IκB degradation, we knocked down the essential autophagy gene beclin 1 and ULK1 (the mammalian homolog of yeast ATG1) in RAW264.7 cells (Fig. 5D). Inhibition of these genes significantly blocked HMGB1-induced microtubule-associated protein 1 light chain 3 (LC3)-II expression (Fig. 5D), an indicator of autophagosome formation [34]. Notably, knockdown of beclin 1 and ULK1 decreased HMGB1-induced IκB degradation (Fig. 5D), NF- κB activity (Fig. 5E), and TNF release (Fig. 5F). Taken together, these data suggest that autophagy-mediated IκB degradation is required for HMGB1-mediated NF-κB activation.
Figure 5. Chloroquine inhibits HMGB1-mediated NF-κB activation.
(A–B) Indicated cells were stimulated with HMGB1 (1 μg/ml, 16 hours) in the absence or presence of chloroquine (“Chlo”, 50 μM), and the activity of NF-κB was assayed by luciferase reporter analysis (A) and p65 level in cytoplasm (“Cyt”)/nucleus (“Nuc”) was assayed by Western blot (B). Values are mean ± SEM of three independent experiments in duplicate. * p < 0.05. Fibrillarin and tubulin were used as a loading control. AU=arbitrary unit. (C) In parallel experiments, the level of IκB in whole cell lysate was assayed by Western blot. Actin was used as a loading control. (D) RAW 264.7 cells were transfected with beclin 1 shRNA, ULK1 shRNA, and control shRNA for 48 hours, and then treated with HMGB1 (1 μg/ml) for 16 hours. The indicated proteins were assayed by Western blot. AU=arbitrary unit. (E–F) In parallel, the activity of NF-κB and TNF release were assayed by luciferase reporter analysis and ELISA, respectively. Values are mean ± SEM of two independent experiments. * p < 0.05.
4. Discussion
These studies indicate that the anti-malarial drug chloroquine significantly inhibits the systemic release of both early (TNF) and late (HMGB1) cytokines, which are known to mediate the lethality of sepsis and systemic inflammation in human patients. The molecular mechanism of chloroquine’s protective effects occurs in part by inhibition of HMGB1-induced IκB degradation and NF- κB activation.
HMGB1 functions as a DNA-binding protein and regulator of intracellular transcription within the nucleus as a universal cytosolic sensor of nucleic acids and as an extracellular cytokine/inflammatory mediator [12, 35, 36]. HMGB1 can be released into the extracellular milieu under two conditions: 1) HMGB1 is actively secreted from activated immune cells [4]; and 2) HMGB1 is passively released by dying or injured cells [37]. When released into the extracellular compartment, HMGB1 can cause pathological inflammatory responses, including acute lung injury and epithelial barrier dysfunction [29, 38, 39]. Compared to TNF, HMGB1 has a more delayed kinetic profile and provides a wider therapeutic window for clinical use. Therefore, agents capable of selectively attenuating systemic HMGB1 accumulation may prove beneficial in the treatment of lethal sepsis. In this study, we demonstrated that chloroquine is a potential inhibitor of HMGB1 release and activity. More importantly, delayed and repeated administration of chloroquine, beginning at 24 hours after onset of sepsis, rescued mice from lethal sepsis, supporting a therapeutic role for chloroquine in the clinical management of human sepsis.
Activation of the NF-κB plays a central role in inflammation through its ability to induce production and release of multiple proinflammatory cytokines [40]. HMGB1 is an inducer of classical NF-κB (p50/p65) pathway activation [11, 12, 33]. In the nucleus, the NF- κB p65 subunit is a strong activator for a wide variety of genes. NF-κB is kept in an inactive form by the inhibitory subunit Iκ-B in most cell types [41]. The ubiquitin-proteasome system (UPS) and autophagy are two functionally linked major degradation pathways. Previous studies have indicated that UPS is important for LPS-induced degradation of Iκ-B in inflammatory cells [42]. In this study, we demonstrated that autophagy is required for HMGB1-induced Iκ-B degradation. Inhibition of autophagy by chloroquine or likewise, knockdown of critical autophagy genes (e.g. beclin 1 and ULK1) reduced HMGB1-mediated Iκ-B degradation and resulted in the inhibition of NF-κB-mediated cytokine (e.g. TNF and IL-6) release. A study recently demonstrated that autophagy is also required for TNF-induced Iκ-B degradation in the long term [43]. The precise means by which autophagy mediates Iκ-B degradation is an area of ongoing investigation.
In eukaryotic cells, autophagy is constitutively active at low levels, whereas significant upregulation occurs in response to a multitude of stresses [15]. The complex relationship between autophagy and inflammation is dependent on cell type and context [17, 18, 44, 45]. For example, genetic ablation of the autophagy regulator Atg16L1 enables LPS-dependent inflammasome activation [46]. In contrast, autophagy facilitates IFN-γ-induced Signal Transducers and Activators of Transcription (STAT)-1 activation and cellular inflammation [47]. In the tumor microenvironment, autophagy promotes the production of inflammatory mediators (e.g. IL-6, IL-8, and GMCSF) [48]. In addition, the interplay between autophagy and NF-κB signaling pathways is very complex [49–51]. Our findings indicate that HMGB1-mediated autophagy is required for cytokine release in inflammatory cells, partly via regulation of Iκ-B degradation. Our recent studies indicate that HMGB1 is a positive regulator of autophagy in fibroblasts and cancer cells by both transcription-dependent and independent pathways [31, 52–54], but it is unknown whether HMGB1-mediated autophagy was involved in the regulation of inflammation and immunity. In addition, one recent study indicates that autophagy regulates tanshinone IIA sodium sulfonate-mediated HMGB1 degradation in macrophages [55].
In summary, we demonstrate here that chloroquine, as well as its derivative hydroxychloroquine, may have therapeutic potential for lethal sepsis and systemic inflammation. The molecular target of chloroquine action is enigmatic, but it is an effective inhibitor of HMGB1 release and pro-inflammatory function in macrophage/monocyte/endothelial cells in vitro and in animal models of lethal endotoxemia and sepsis in vivo. Moreover, autophagy-dependent Iκ-B degradation pathways were reported. It will be of interest to assess the pharmacological activity of chloroquine as an inhibitor of HMGB1 signaling in other animal models of local and systemic inflammation. Given the inflammatory genomic response in mice is significantly different to that observed in humans [56], it is of great interest to explore whether chloroquine also inhibits HMGB1 inflammatory signaling and protects lethal sepsis in humans.
Acknowledgments
We thank Christine Heiner (Departments of Surgery and Anesthesiology, University of Pittsburgh) for her critical reading of the manuscript. This work was supported by grants from The National Natural Sciences Foundation of China (30973234 and 31171328 to L.C.; 81270616 to Y.Y.; 81100359 to M.Y.) and a grant from the National Institutes of Health (R01CA160417 to D.T.).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest or financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–7. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–4. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 3.Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–33. [PubMed] [Google Scholar]
- 4.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 5.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae JS, Rezaie AR. Activated protein C inhibits high mobility group box 1 signaling in endothelial cells. Blood. 2011 doi: 10.1182/blood-2011-06-360701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99:12351–6. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson S, Pettila V, Tenhunen J, Laru-Sompa R, Hynninen M, Ruokonen E. HMGB1 as a predictor of organ dysfunction and outcome in patients with severe sepsis. Intensive Care Med. 2008;34:1046–53. doi: 10.1007/s00134-008-1032-9. [DOI] [PubMed] [Google Scholar]
- 11.Andersson U, Tracey KJ. HMGB1 Is a Therapeutic Target for Sterile Inflammation and Infection. Annu Rev Immunol. 2011;29:139–62. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 13.Olatunde IA. The present status of chloroquine in the drug treatment of malaria. Afr J Med Sci. 1972;3:77–91. [PubMed] [Google Scholar]
- 14.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–12. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 15.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–93. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–22. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–35. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158–75. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Noriega A, Grubb JH, Talkad V, Sly WS. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980;85:839–52. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meinao IM, Sato EI, Andrade LE, Ferraz MB, Atra E. Controlled trial with chloroquine diphosphate in systemic lupus erythematosus. Lupus. 1996;5:237–41. doi: 10.1177/096120339600500313. [DOI] [PubMed] [Google Scholar]
- 21.Miller HG, Foster JB, Newell DJ, Barwick DD, Brewis RA. Multiple Sclerosis: Therapeutic Trials of Chloroquine, Soluble Aspirin, and Gammaglobulin. British medical journal. 1963;2:1436–9. doi: 10.1136/bmj.2.5370.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haydu GG. Rheumatoid arthritis therapy; a rationale and the use of chloroquine diphosphate. Am J Med Sci. 1953;225:71–5. [PubMed] [Google Scholar]
- 23.Yasuda H, Leelahavanichkul A, Tsunoda S, Dear JW, Takahashi Y, Ito S, et al. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am J Physiol Renal Physiol. 2008;294:F1050–8. doi: 10.1152/ajprenal.00461.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Wang H, Mason JM, Levine J, Yu M, Ulloa L, et al. Recombinant HMGB1 with cytokine-stimulating activity. J Immunol Methods. 2004;289:211–23. doi: 10.1016/j.jim.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, et al. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol. 2007;81:741–7. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang D, Kang R, Xiao W, Wang H, Calderwood SK, Xiao X. The Anti-inflammatory Effects of Heat Shock Protein 72 Involve Inhibition of High-Mobility-Group Box 1 Release and Proinflammatory Function in Macrophages. J Immunol. 2007;179:1236–44. doi: 10.4049/jimmunol.179.2.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang R, Tang D, Livesey KM, Schapiro NE, Lotze MT, Zeh HJ. The Receptor for Advanced Glycation End-Products (RAGE) Protects Pancreatic Tumor Cells Against Oxidative Injury. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2010.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conner JR, Smirnova, Moseman AP, Poltorak A. IRAK1BP1 inhibits inflammation by promoting nuclear translocation of NF-kappaB p50. Proc Natl Acad Sci U S A. 2010;107:11477–82. doi: 10.1073/pnas.1006894107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–60. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 30.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–80. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, et al. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881–92. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dupont N, Jiang S, Pilli M, Ornatowski W, Bhattacharya D, Deretic V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1beta. Embo J. 2011;30:4701–11. doi: 10.1038/emboj.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang D, Kang R, Xiao W, Zhang H, Lotze MT, Wang H, et al. Quercetin prevents LPS-induced high-mobility group box 1 release and proinflammatory function. Am J Respir Cell Mol Biol. 2009;41:651–60. doi: 10.1165/rcmb.2008-0119OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 36.Tang D, Kang R, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal. 2011;14:1315–35. doi: 10.1089/ars.2010.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 38.Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288:L958–65. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- 39.Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 40.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 41.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 42.Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–62. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 43.Colleran A, Ryan A, O’Gorman A, Mureau C, Liptrot C, Dockery P, et al. Autophagosomal IkappaB alpha degradation plays a role in the long term control of tumor necrosis factor-alpha-induced nuclear factor-kappaB (NF-kappaB) activity. J Biol Chem. 2011;286:22886–93. doi: 10.1074/jbc.M110.199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vernon PJ, Tang D. Eat-Me: Autophagy, Phagocytosis, and Reactive Oxygen Species Signaling. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Q, Kang R, Zeh HJ, Lotze MT, Tang D. DAMPs and autophagy: Cellular adaptation to injury and unscheduled cell death. Autophagy. 2013;9:451–8. doi: 10.4161/auto.23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–8. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 47.Chang YP, Tsai CC, Huang WC, Wang CY, Chen CL, Lin YS, et al. Autophagy facilitates IFN-gamma-induced Jak2-STAT1 activation and cellular inflammation. J Biol Chem. 2010;285:28715–22. doi: 10.1074/jbc.M110.133355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez-Outschoorn UE, Whitaker-Menezes D, Lin Z, Flomenberg N, Howell A, Pestell RG, et al. Cytokine production and inflammation drive autophagy in the tumor microenvironment: role of stromal caveolin-1 as a key regulator. Cell Cycle. 2011;10:1784–93. doi: 10.4161/cc.10.11.15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Criollo A, Senovilla L, Authier H, Maiuri MC, Morselli E, Vitale I, et al. The IKK complex contributes to the induction of autophagy. Embo J. 2010;29:619–31. doi: 10.1038/emboj.2009.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qing G, Yan P, Xiao G. Hsp90 inhibition results in autophagy-mediated proteasome-independent degradation of IkappaB kinase (IKK) Cell Res. 2006;16:895–901. doi: 10.1038/sj.cr.7310109. [DOI] [PubMed] [Google Scholar]
- 51.Djavaheri-Mergny M, Amelotti M, Mathieu J, Besancon F, Bauvy C, Souquere S, et al. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J Biol Chem. 2006;281:30373–82. doi: 10.1074/jbc.M602097200. [DOI] [PubMed] [Google Scholar]
- 52.Tang D, Kang R, Livesey KM, Kroemer G, Billiar TR, Van Houten B, et al. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 2011;13:701–11. doi: 10.1016/j.cmet.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, Yang M, Kang R, Wang Z, Zhao Y, Yu Y, et al. HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia. 2011;25:23–31. doi: 10.1038/leu.2010.225. [DOI] [PubMed] [Google Scholar]
- 54.Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, et al. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299–310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Li W, Zhu S, Jundoria A, Li J, Yang H, et al. Tanshinone IIA sodium sulfonate facilitates endocytic HMGB1 uptake. Biochem Pharmacol. 2012 doi: 10.1016/j.bcp.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]