Abstract
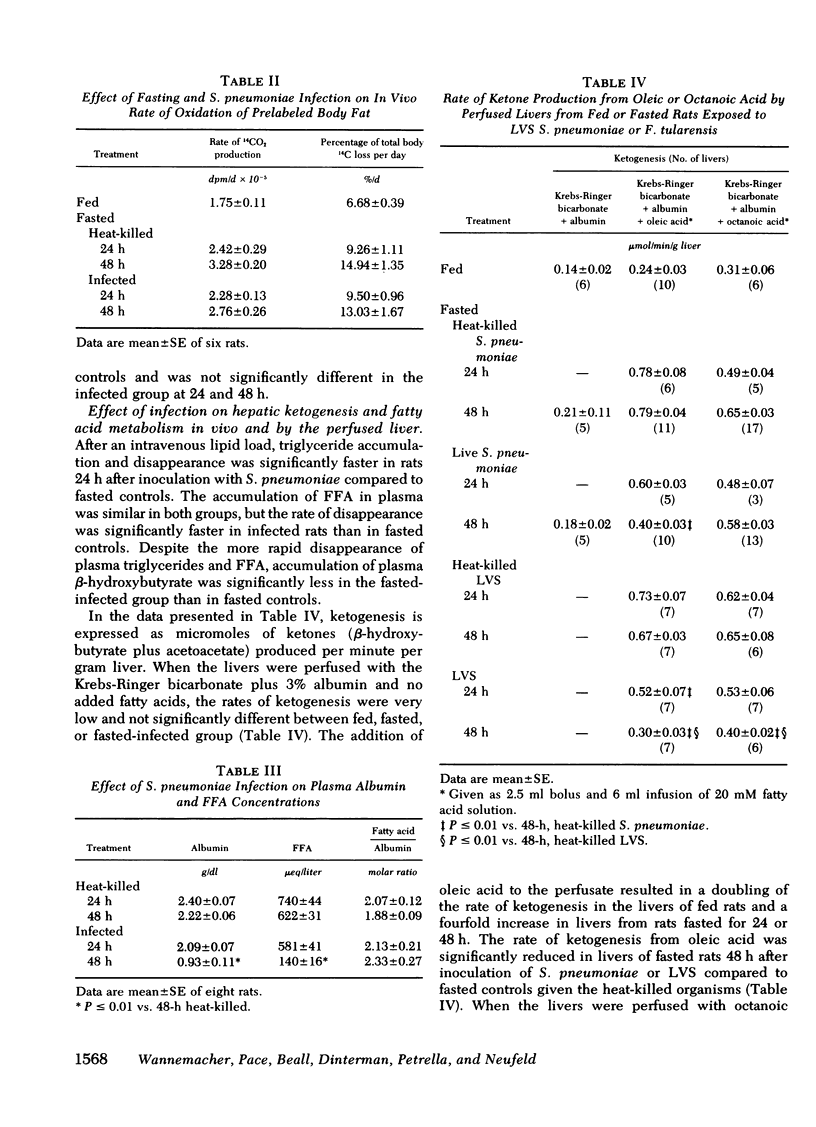
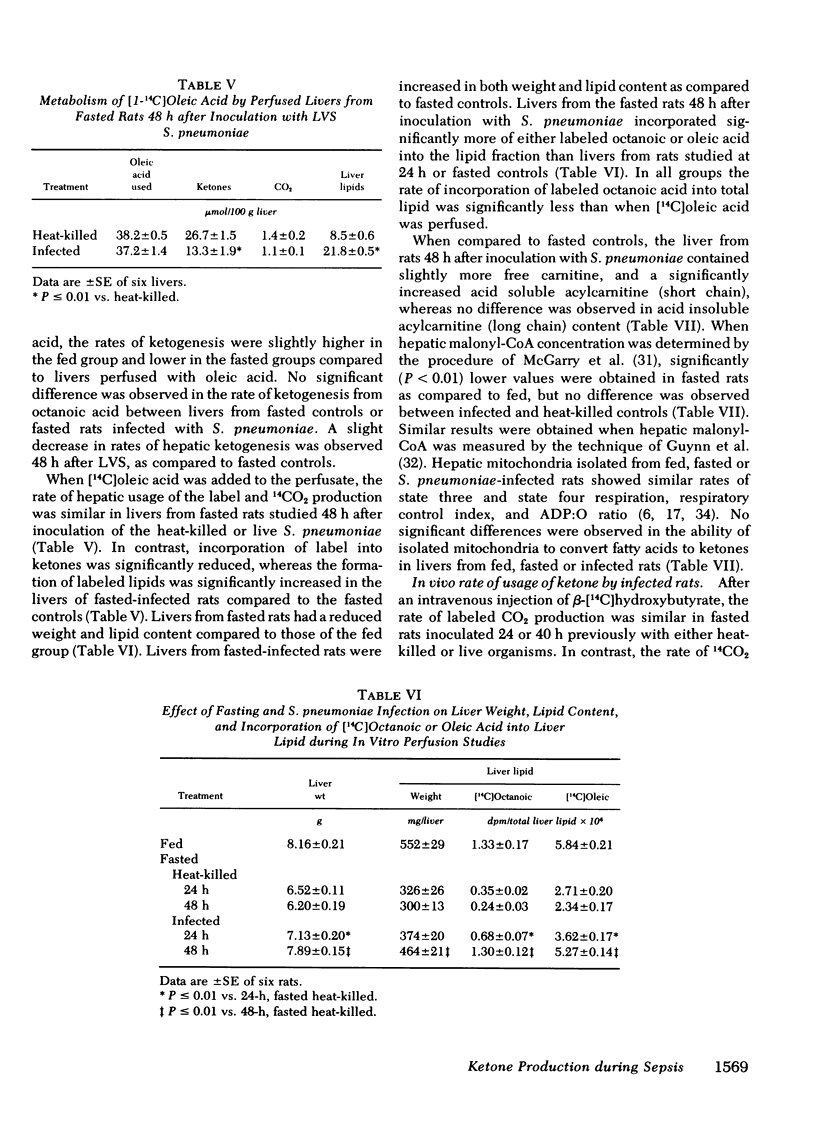
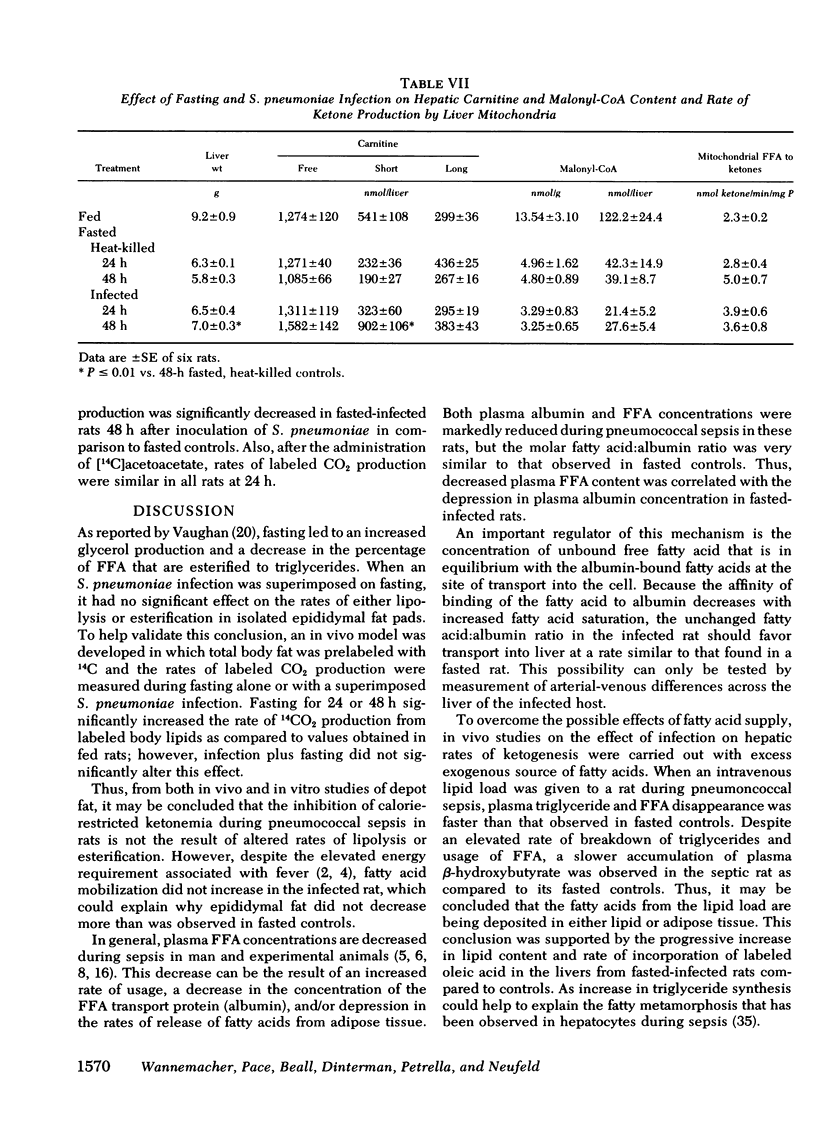
During caloric deprivation, the septic host may fail to develop ketonemia as an adaptation to starvation. Because the plasma ketone body concentration is a function of the ratio of hepatic production and peripheral usage, a pneumococcal sepsis model was used in rats to measure the complex metabolic events that could account for this failure, including the effects of infection on lipolysis and esterification in adipose tissue, fatty acid transport in plasma and the rates of hepatic ketogenesis and whole body oxidation of ketones. Some of the studies were repeated with tularemia as the model infection. From these studies, it was concluded that during pneumococcal sepsis, the failure of rats to become ketonemic during caloric deprivation was the result of reduced ketogenic capacity of the liver and a possibly decreased hepatic supply of fatty acids. The latter appeared to be a secondary consequence of a severe reduction in circulating plasma albumin, the major transport protein for fatty acids, with no effect on the degree of saturation of the albumin with free fatty acids. Also, the infection had no significant effect on the rate of lipolysis or release of fatty acids from adipose tissue. Ketone body usage (oxidation) was either unaffected or reduced during pneumococcal sepsis in rats. Thus, a reduced rate of ketone production in the infected host was primarily responsible for the failure to develop starvation ketonemia under these conditions. The liver of the infected rat host appears to shuttle the fatty acids away from β-oxidation and ketogenesis and toward triglyceride production, with resulting hepatocellular fatty metamorphosis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON J. B., WANNEMACHER R. W., Jr, BANKS W. L., Jr, WUNNER W. H. THE MAGNITUDE AND SIGNIFICANCE OF THE PROTEIN RESERVES IN RATS FED AT VARIOUS LEVELS OF NITROGEN. J Nutr. 1964 Dec;84:383–388. doi: 10.1093/jn/84.4.383. [DOI] [PubMed] [Google Scholar]
- Bates M. W., Krebs H. A., Williamson D. H. Turnover rates of ketone bodies in normal, starved and alloxan-diabetic rats. Biochem J. 1968 Dec;110(4):655–661. doi: 10.1042/bj1100655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel W. R., Fiser R. H., Jr Lipid metabolism during infectious illness. Am J Clin Nutr. 1970 Aug;23(8):1069–1079. doi: 10.1093/ajcn/23.8.1069. [DOI] [PubMed] [Google Scholar]
- Blackburn G. L. Lipid metabolism in infection. Am J Clin Nutr. 1977 Aug;30(8):1321–1332. doi: 10.1093/ajcn/30.8.1321. [DOI] [PubMed] [Google Scholar]
- Border J. R., Chenier R., McManamy R. H., La Duca J., Seibel R., Birkhahn R., Yu L. Multiple systems organ failure: muscle fuel deficit with visceral protein malnutrition. Surg Clin North Am. 1976 Oct;56(5):1147–1167. doi: 10.1016/s0039-6109(16)41035-2. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Blackburn B. S., Wannemacher R. W., Jr, McGann V. G., Beisel W. R., Dupont H. L. Sequential changes in the concentration of specific serum proteins during typhoid fever infection in man. J Lab Clin Med. 1976 Apr;87(4):577–585. [PubMed] [Google Scholar]
- Crespin S. R., Greenough W. B., 3rd, Steinberg D. Stimulation of insulin secretion by infusion of free fatty acids. J Clin Invest. 1969 Oct;48(10):1934–1943. doi: 10.1172/JCI106160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton C., Kowalski C. Automated colorimetric determination of free fatty acids in biologic fluids. Clin Chem. 1967 Sep;13(9):744–751. [PubMed] [Google Scholar]
- DiMarco J. P., Hoppel C. Hepatic mitochondrial function in ketogenic states. Diabetes, starvation, and after growth hormone administration. J Clin Invest. 1975 Jun;55(6):1237–1244. doi: 10.1172/JCI108042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRITZ I. B. Factors influencing the rates of long-chain fatty acid oxidation and synthesis in mammalian systems. Physiol Rev. 1961 Jan;41:52–129. doi: 10.1152/physrev.1961.41.1.52. [DOI] [PubMed] [Google Scholar]
- Flatt J. P., Blackburn G. L. The matabolic fuel regulatory system: implications for protein-sparing therapies during caloric deprivation and disease. Am J Clin Nutr. 1974 Feb;27(2):175–187. doi: 10.1093/ajcn/27.2.175. [DOI] [PubMed] [Google Scholar]
- Foster D. W. Studies in the ketosis of fasting. J Clin Invest. 1967 Aug;46(8):1283–1296. doi: 10.1172/JCI105621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guynn R. W., Veloso D., Veech R. L. The concentration of malonyl-coenzyme A and the control of fatty acid synthesis in vivo. J Biol Chem. 1972 Nov 25;247(22):7325–7331. [PubMed] [Google Scholar]
- MILLER L. L. Some direct actions of insulin, glucagon, and hydrocortisone on the isolated perfused rat liver. Recent Prog Horm Res. 1961;17:539–568. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. The regulation of ketogenesis from octanoic acid. The role of the tricarboxylic acid cycle and fatty acid synthesis. J Biol Chem. 1971 Feb 25;246(4):1149–1159. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. The regulation of ketogenesis from oleic acid and the influence of antiketogenic agents. J Biol Chem. 1971 Oct 25;246(20):6247–6253. [PubMed] [Google Scholar]
- McGarry J. D., Guest M. J., Foster D. W. Ketone body metabolism in the ketosis of starvation and alloxan diabetes. J Biol Chem. 1970 Sep 10;245(17):4382–4390. [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Robles-Valdes C., Foster D. W. Role of carnitine in hepatic ketogenesis. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4385–4388. doi: 10.1073/pnas.72.11.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Stark M. J., Foster D. W. Hepatic malonyl-CoA levels of fed, fasted and diabetic rats as measured using a simple radioisotopic assay. J Biol Chem. 1978 Nov 25;253(22):8291–8293. [PubMed] [Google Scholar]
- Neufeld H. A., Pace J. A., White F. E. The effect of bacterial infections on ketone concentrations in rat liver and blood and on free fatty acid concentrations in rat blood. Metabolism. 1976 Aug;25(8):877–884. doi: 10.1016/0026-0495(76)90120-7. [DOI] [PubMed] [Google Scholar]
- Pace J. A., Wannemacher R. W., Jr, Neufeld H. A. Improved radiochemical assay for carnitine and its derivatives in plasma and tissue extracts. Clin Chem. 1978 Jan;24(1):32–35. [PubMed] [Google Scholar]
- Powanda M. C., Dinterman R. E., Wannemacher R. W., Jr, Herbrandson G. D. Distribution and metabolism of phenylalanine and tyrosine during tualraemia in the rat. Biochem J. 1974 Oct;144(1):173–176. doi: 10.1042/bj1440173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan N. T., Blackburn G. L., Clowes H. A., Jr Differential tissue sensitivity to elevated endogenous insulin levels during experimental peritonitis in rats. Metabolism. 1974 Nov;23(11):1081–1089. doi: 10.1016/0026-0495(74)90075-4. [DOI] [PubMed] [Google Scholar]
- Seyffert W. A., Jr, Madison L. L. Physiologic effects of metabolic fuels on carbohydrate metabolism. I. Acute effect of elevation of plasma free fatty acids on hepatic glucose output, peripheral glucose utilization, serum insulin, and plasma glucagon levels. Diabetes. 1967 Nov;16(11):765–776. doi: 10.2337/diab.16.11.765. [DOI] [PubMed] [Google Scholar]
- VAUGHAN M. The production and release of glycerol by adipose tissue incubated in vitro. J Biol Chem. 1962 Nov;237:3354–3358. [PubMed] [Google Scholar]
- VON BRAND T., MERCADO T. I. Quantitative and histochemical studies on liver lipids of rats infected with Plasmodium berghei. Am J Hyg. 1958 May;67(3):311–320. doi: 10.1093/oxfordjournals.aje.a119937. [DOI] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr, Kaminski M. V., Jr, Neufeld H. A., Dinterman R. E., Bostian K. A., Hadick C. L. Protein-sparing therapy during pneumococcal infection in rhesus monkeys. JPEN J Parenter Enteral Nutr. 1978 Sep;2(4):507–518. doi: 10.1177/014860717800200402. [DOI] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr Key role of various individual amino acids in host response to infection. Am J Clin Nutr. 1977 Aug;30(8):1269–1280. doi: 10.1093/ajcn/30.8.1269. [DOI] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr, Klainer A. S., Dinterman R. E., Beisel W. R. The significance and mechanism of an increased serum phenylalanine-tyrosine ratio during infection. Am J Clin Nutr. 1976 Sep;29(9):997–1006. doi: 10.1093/ajcn/29.9.997. [DOI] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr, Powanda M. C., Pekarek R. S., Beisel W. R. Tissue amino acid flux after exposure of rats to Diplococcus pneumoniae. Infect Immun. 1971 Nov;4(5):556–562. doi: 10.1128/iai.4.5.556-562.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Veloso D., Ellington E. V., Krebs H. A. Changes in the concentrations of hepatic metabolites on administration of dihydroxyacetone or glycerol to starved rats and their relationship to the control of ketogenesis. Biochem J. 1969 Sep;114(3):575–584. doi: 10.1042/bj1140575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenser T. V., DeRubertis F. R., Curnow R. T. Effects of prostaglandins on hepatic adenylate cyclase activity and cyclic adenosine 3',5',-monophosphate content. Endocrinology. 1974 May;94(5):1404–1410. doi: 10.1210/endo-94-5-1404. [DOI] [PubMed] [Google Scholar]
- Zivin J. A., Snarr J. F. An automated colorimetric method for the measurement of 3-hydroxybutyrate concentration. Anal Biochem. 1973 Apr;52(2):456–461. doi: 10.1016/0003-2697(73)90048-1. [DOI] [PubMed] [Google Scholar]


