Abstract
Compassion is a key motivator of altruistic behavior, but little is known about individuals’ capacity to cultivate compassion through training. We examined whether compassion may be systematically trained by testing whether (i) short-term compassion training increases altruistic behavior, and (ii) individual differences in altruism are associated with training-induced changes in neural responses to suffering. In healthy young adults, we found that compassion training increased altruistic redistribution of funds to a victim encountered outside of the training context. Furthermore, greater altruistic behavior after compassion training was associated with altered activation in regions implicated in social cognition and emotion regulation, including the inferior parietal cortex, dorsolateral prefrontal cortex (DLPFC), and DLPFC connectivity with the nucleus accumbens. These results suggest that compassion can be cultivated with training, where greater altruistic behavior may emerge from increased engagement in neural systems implicated in understanding the suffering of others, executive and emotional control, and reward processing.
Keywords: compassion, meditation, altruism, emotion regulation, fMRI
Compassion and altruism are of great interest to philosophical and scientific inquiry because of their central role in successful societies (Darwin, 2004; Fehr & Fischbacher, 2003; Smith, 2010; Sober, Wilson, & Wilson, 1999). Compassion is the emotional response of caring for and wanting to help those who are suffering (Batson, 1991; Eisenberg, Fabes, & Spinrad, 2006; Goetz, Keltner, & Simon-Thomas, 2010), and may have evolved in humans to foster altruistic acts that increase survival of kin as well as non-kin (Darwin, 2004; Goetz et al., 2010; Sober et al., 1999). These include enhancing the welfare of vulnerable offspring, promoting intimate bonds between partners, and facilitating cooperation among genetically unrelated strangers (Batson, 1991; Darwin, 2004; Goetz et al., 2010; Sober et al., 1999). Despite the clear societal benefits of cultivating compassion, little is known about whether compassion and altruism can be trained, and the neural mechanisms that might underlie such effects.
Contemplative traditions claim that compassion can be enhanced with meditation training, and that this results in greater real-world altruistic behavior (Lutz, Brefczynski-Lewis, Johnstone, & Davidson, 2008). In compassion training, compassion is cultivated towards different people, including loved ones, the self, strangers, difficult persons, and ultimately all people (Salzberg, 1997). Studies indicate that compassion training can improve personal well-being, including stress-related immune responses (Pace et al., 2009), positive affect (Fredrickson, Cohn, Coffey, Pek, & Finkel, 2008; Hutcherson, Seppala, & Gross, 2008) and psychological and physical health (Fredrickson et al., 2008). Compassion training also enhances responses towards others. Expert meditation practitioners show greater empathic neural responses when listening to sounds of others’ suffering during compassion meditation practice compared to controls (Lutz, Brefczynski-Lewis, et al., 2008). Recent work suggests that short-term training can increase prosocial behavior (Leiberg, Klimecki, & Singer, 2011) and increases positive emotions towards those who are suffering (Klimecki, Leiberg, Lamm, & Singer, 2012).
The neural mechanisms by which compassion training alters altruistic responses to suffering remain unknown. Here, we investigated whether short-term compassion training would enhance altruistic behavior towards a victim encountered outside of the training context. Altruistic behavior was assessed using the Redistribution Game, a novel economic decision-making task which models both (i) unfair treatment of a victim and (ii) costly redistribution of funds to the victim. Furthermore, we measured brain activation before and after two weeks of training using functional magnetic resonance imaging (fMRI), and investigated whether increased altruism could be explained by training-induced changes in the neural response to human suffering.
To rigorously test these hypotheses, compassion training (COM) was compared to an active control intervention of reappraisal training (REP). COM trainees cultivated compassion for different targets, and REP trainees practiced re-interpreting personally stressful events to decrease negative affect. Both interventions trained emotion regulation strategies that promote well-being, but they differed in that the goal of COM was to increase empathic concern and the desire to relieve suffering (Lutz et al., 2008), whereas the goal of REP was to decrease one’s personal distress (Ochsner & Gross, 2005). REP provided an ideal control for COM because although the combination of decreased distress and increased empathic concern predicts helping behavior (Batson, 1991; Eisenberg et al., 2006), REP only decreases distress without enhancing concern.
We hypothesize that compassion training should increase altruistic behavior by enhancing neural systems involved in (1) the recognition and understanding of another’s suffering and (2) emotion regulation of responses to suffering that support affiliation and helping behavior. The neuroscience of empathy highlights two systems for understanding the states of others: experience sharing, which involves vicariously sharing the states of others, and mentalizing, which involves explicitly considering and understanding others’ mental states through social inferences as well as self-referential processes (Zaki & Ochsner, 2012; Lamm, Decety, & Singer, 2011). If the neural representation of suffering is increased due to compassion training, then regulatory systems are needed to respond to this suffering with an approach rather than an avoidance response.
Prior theoretical and empirical work suggests that altruistic responses towards another’s suffering can be strengthened through either of two regulatory pathways (Decety & Jackson, 2006): (i) decreasing personal distress, which reduces negative arousal and avoidance behavior, or (ii) increasing empathic concern, which strengthens the motivation to approach and relieve another’s suffering (Batson, 1991). In response to suffering, we predicted that greater altruism in COM would be associated with increased activation in prefrontal cortex (PFC), given its role in controlled processing (Miller & Cohen, 2001), emotion regulation (Ochsner & Gross, 2005; Urry et al., 2006; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008), and fronto-parietal control networks (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008), and decreased amygdala activation, given the amygdala’s role in responding to negative stimuli and distress (Zald, 2003). We also hypothesized that greater prosocial behavior after COM would be associated with increased activation in anterior insula (AI), which has been implicated in studies of empathy and compassion (Immordino-Yang, McColl, Damasio, & Damasio, 2009; Lamm et al., 2011; Lutz, Brefczynski-Lewis, et al., 2008; Singer et al., 2006) and predicts helping (Hein, Silani, Preuschoff, Batson, & Singer, 2010; Masten, Morelli, & Eisenberger, 2011), and would also be correlated with increased activation in nucleus accumbens (NAcc), which has been linked to charitable giving (Harbaugh, Mayr, & Burghart, 2007; Moll et al., 2006) and positive appraisals of aversive stimuli (Wager et al., 2008a).
We will specifically test these hypotheses against the reappraisal group, where the psychological goal is self-focused (to decrease one’s own suffering) rather than other-focused (to decrease others’ suffering in compassion). Although many of the same regions are implicated in reappraisal (Wager et al., 2008), we expect that hypothesized changes (e.g., increases in PFC) will not be associated with altruistic behavior because the behavior is not congruent with reappraisal’s goals.
Method
Participants
56 participants completed the entire protocol, and the final population consisted of 41 participants who believed they were interacting with real players in the Redistribution Game (see Table S1–S2 and Supplementary Material for population and training statistics). These participants were randomized to COM (n = 20, 12 female, mean age = 21.9 years) or REP (n = 21, 13 female, mean age = 22.5), completed two weeks of training (11/14 practice days were required), and attended both fMRI sessions. The groups did not differ in age, gender, baseline trait compassion, or practice time. Participants were healthy adults (18–45 years of age), MRI-compatible, right-handed, and had no previous experience in meditation or cognitive-behavioral therapy. The experiment was approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board, and all subjects gave informed consent and were paid for participation.
Procedure Overview
Participants came to the laboratory on three occasions. At Visit 1, they were randomized to COM or REP, briefly instructed in the assigned strategy, and practiced the fMRI task in a mock MRI scanner. Visit 2 occurred approximately one week later, where they completed the pre-training fMRI scan, and began training later that day. Visit 3 occurred immediately after the 2 weeks of training was completed, and included the post-training fMRI scan and the altruistic behavior task (outside of the scanner).
Trainings
Training consisted of practicing COM or REP using guided audio instructions (via the Internet or compact disc) for 30 minutes/day for 2 weeks. COM trainees practiced cultivating feelings of compassion for different targets (loved one, self, stranger, and difficult person), and REP trainees practiced re-interpreting personally stressful events to decrease negative affect (see Trainings and Fig. S1 in Supplementary Material). Training audio files and written scripts can be downloaded at http://investigatinghealthyminds.org/cihmProjects.html or through the iTunes store (“Healthy Minds at the UW” album).
Altruistic Behavior Task: Redistribution Game
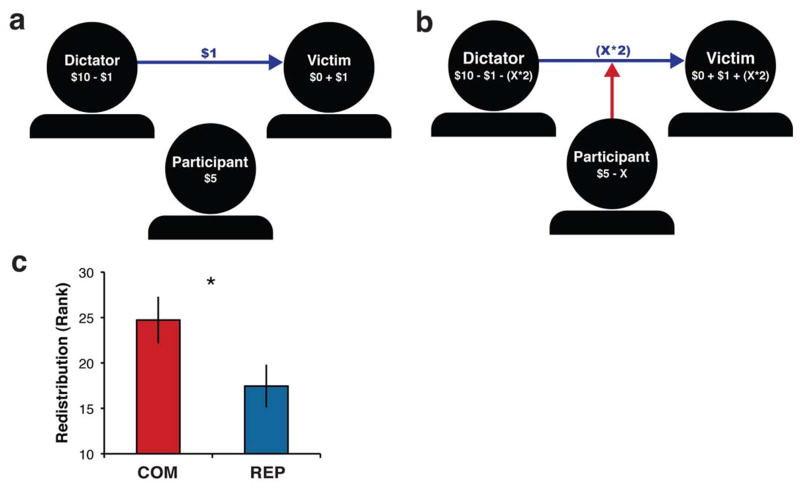
We tested whether COM could impact altruistic behavior outside of the training context using the Redistribution Game (Fig. 1). This economic decision-making task models both (i) unfair treatment of a victim and (ii) costly redistribution of funds to the victim. Using anonymous online interactions, participants first observed a dictator (endowed with $10) transfer an unfair amount of money ($1) to a victim (endowed with no money) (Fig. 1a). After witnessing this violation of the fairness norm (Fehr & Fischbacher, 2003), participants could choose to spend any amount of their own endowment ($5) to redistribute twice the amount of funds from the dictator to the victim (Fig. 1b). Participants were paid the amount that was left in the endowment after making the decision.
Fig 1.
The Redistribution Game. (a) The dictator transfers an unfair amount of money ($1/$10) to the victim, while the participant observes. (b) The participant can choose to spend any X amount of $5 in order to compel redistribution of funds from the dictator to the victim. (c) After two weeks of training, COM trainees (n = 20) redistributed more funds than REP trainees (n = 21; * independent sample t(39) = 2.09, p < .05, d = 0.65; COM mean rank = 24.725 or $1.14, REP mean rank = 17.45 or $0.62) and participants with no training in an independent validation sample (Fig. S2). Redistribution of $2 (rank = 35.5/41) results in an equal distribution between the Dictator and Victim ($5 each). The $1.14 spent by COM was 1.84 times more money than the $0.62 spent by REP and increased the distribution between the dictator and the victim by 57% (inequality of $3.44 instead of $8). In contrast, REP increased the distribution by only 31% (inequality of $5.52 instead of $8). Redistribution responses were rank-transformed based on 41 participants. Error bars denote standard error of the mean rank.
Participants were told that they were playing the game with live players over the Internet. Effects of demand characteristics on behavior were minimized by presenting the game as a unique study, describing it in purely economic terms, never instructing participants to use training, removing the physical presence of players and experimenters during game play, and enforcing real monetary consequences of participants’ behavior. Because compassionate behavior is specifically evoked by unfairness, all participants observed the same pre-programmed unfair dictator offer ($1/$10). At the end of the entire protocol, participants were debriefed and asked whether they believed they were playing against real people in the game. Data were only analyzed for participants who believed the paradigm (Table S2 in Supplementary Material).
fMRI Paradigm
To determine whether altruistic behavior was predicted by changes in neural responses to human suffering, participants were scanned using fMRI before and after training while employing their assigned emotion regulation strategy. When presented with images of human suffering (SUFFERING) or non-suffering (NEUTRAL), COM trainees were instructed to evoke feelings of compassion while silently repeating compassion-generating phrases. In contrast, REP trainees were instructed to decrease negative emotions by silently re-interpreting the emotional meaning of the images. See Supplementary Material for fMRI data acquisition parameters.
SUFFERING images depicted emotional distress, physical pain, or acts of violence (e.g., burn victim, crying child). NEUTRAL images depicted people in non-emotional situations, such as working or walking on a street. Two parallel sets of images (20 SUFFERING and 16 NEUTRAL) were created to ensure that participants viewed different images before and after training. Set order was counterbalanced and randomized. Images were pseudorandomized so that 3 or more images from either condition were not presented in a row. Image randomization was performed once for each set and then fixed. Images were balanced across sets for published normative ratings of valence and arousal, as well as stimulus properties of hue, luminance, and saturation (all P’s > .1).
Participants were instructed to regulate their emotional responses to the images over 3 blocks. Each block began with a 20-second (s) fixation baseline period. Participants then received both an auditory and visual instruction (3s) of either “Compassion” or “Reappraisal” (depending on group assignment), which was followed by a fixation cross (5–7s). They applied the assigned regulation strategy to a series of 12 images. Each image was presented for 12s and separated by a fixation inter-trial interval trial (5–11s, randomized). Blocks ended with a final fixation baseline (17–38s). After each block, participants saw each image again for 2s and rated the arousal (1 = Least Arousing to 7 = Most Arousing) of each image.
Behavioral analysis
Across all participants, the redistribution response was positively skewed (skewness = 1.5, SE = 0.37), and two participants qualified as outliers (> 3 SDs from the population mean). Because of these violations of normality, we rank-ordered the behavioral response across both groups so that strong assumptions were not made about the scaling or normality of the residuals. Parametric statistics were then performed on the ranked data. To test the mean difference between groups, an independent samples t-test was performed on the ranks. For in-depth analyses and discussion of redistribution values and ranks, see Supplementary Material.
fMRI analysis
A series of tests were conducted to identify regions in which changes due to training predicted greater redistribution in COM vs. REP. A whole-brain interaction contrast (Group × Redistribution) was tested on neural change scores (see Interaction analysis). Follow-up tests were conducted using both across- and within-subject analyses to identify regions that were functionally connected to clusters identified in the interaction analysis (see IPC conjunction analysis) and networks involved in emotion regulation (see PPI analysis). Finally, we investigated whether reported arousal was associated with either redistribution or neural changes (see Correlational analyses). See Supplementary Material for full details.
Interaction analysis
Individual functional and structural MRI brain data were pre-processed and normalized to Montreal Neurological Institute (MNI) space. Each participant’s neural response to suffering during regulation was estimated with the SUFFERING-NEUTRAL contrast at each fMRI scan time point (PRE, POST) using standard first-level analyses (see Supplementary Material), and beta coefficients were converted to percent signal change (PSC). Training-induced changes were calculated by subtracting POST-PRE PSC values. To identify regions where training-related changes specifically predicted greater redistribution in COM, a second-level Group (COM, REP) × Redistribution voxelwise interaction was performed, controlling for main effects of Group and Redistribution. First, whole-brain analyses were conducted and corrected for multiple comparisons (p < 0.01 after voxelwise thresholding at p < 0.01) using Monte Carlo simulation. This analysis yielded the right inferior parietal cortex (IPC). In order to decompose the interactions, mean PSC change scores were extracted from the clusters for each participant and analyzed to yield parameter estimates and determine the directionality of the relationship for each group. These values were used for descriptive and diagnostic purposes only (Vul, Harris, Winkielman, & Pashler, 2009). In region of interest (ROI) analyses, data from the Group × Redistribution interaction were corrected for multiple comparisons (p < .01 after voxelwise thresholding at p < 0.01) using Monte Carlo simulation within bilateral a priori ROIs of the amygdala, insula, and NAcc.
IPC conjunction analysis
To identify regions that may be functionally connected to the IPC in order to increase redistribution in COM vs. REP, a conjunction analysis was performed requiring voxels to be (1) correlated with POST-PRE changes in IPC activation across-subject in both groups (voxelwise p < 0.01) and (2) identified in the original Group × Redistribution interaction (voxelwise p < 0.01). This analysis identified a cluster in the dorsolateral PFC (DLPFC; whole-brain corrected at p < 0.01 after conjunction voxelwise thresholding at p < 0.001).
Psychophysiological interactions (PPI) analysis
To determine regions in which altered PFC connectivity predicted redistribution in COM, a PPI analysis were performed using the DLPFC seed region identified by the IPC conjunction analysis. The PPI regressor consisted of comparing DLPFC connectivity in the SUFFERING vs. NEUTRAL condition. POST-PRE interaction betas were regressed with redistribution scores, and voxelwise regression maps were corrected for multiple comparisons (p < .01 after voxelwise thresholding at p < 0.01) using Monte Carlo simulation within each bilateral ROI (amygdala, insula, and NAcc). For descriptive purposes, mean PPI change betas were extracted from the clusters for each participant and analyzed to yield parameter estimates and determine the directionality of the relationship for each group.
Correlational analyses
COM may increase altruistic behavior by decreasing personal distress evoked by suffering (Batson, 1991; Eisenberg et al., 2006). To test this, arousal change scores were computed (POST[SUFFERING-NEUTRAL] – PRE[SUFFERING-NEUTRAL]) and correlated with altruistic redistribution in each group. To examine whether changes in arousal were associated with changes in neural responses to suffering, correlations were computed between arousal change scores and neural change scores identified in the previous fMRI analyses in each group.
Results
Altruistic Redistribution
Findings in an independent validation sample (N = 72) confirmed that altruistic redistribution is a behavioral signature of compassion: individuals who endorsed greater levels of trait empathic concern (Davis, 1980) redistributed more money, r(70) = 0.43, p < 0.001 (Fig. S2). After two weeks of training, individuals trained in COM spent more money to redistribute funds to the victim compared to those trained in REP (Fig. 1c; independent sample t(39) = 2.09, p < 0.05, d = 0.65) as well as individuals with no training in the validation sample (Fig. S3). See Supplementary Material for detailed method and analyses. COM participants spent 1.84 times more money than REP ($1.14 vs. $0.62) and increased the distribution between the dictator and the victim by 57%. In contrast, REP increased the distribution by only 31%. This demonstrates that purely mental training in compassion can result in observable altruistic changes towards a victim, even when individuals are not explicitly cued to generate compassion.
Neuroimaging
Group differences in neural change and altruistic redistribution
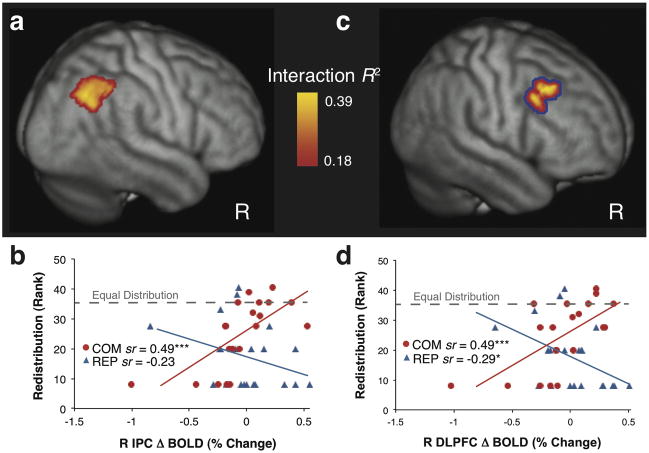
We hypothesized that greater altruism due to COM would be predicted by training-related changes in the neural responses to suffering. The whole-brain Group × Redistribution interaction test revealed that training-induced changes in right inferior parietal cortex (IPC) activation were differentially associated with altruistic redistribution in COM vs. REP (Fig. 2a; p < 0.01 corrected; Tables S3–S4). In COM, greater IPC activation due to training was associated with greater redistribution, and this was not found in REP (Fig. 2b; Table S5). Within the a priori ROIs, no region survived correction at p < 0.01. See Fig. S4 and Supplementary Material for exploratory analyses within the ROIs.
Fig 2.
Greater redistribution after COM is predicted by changes in the neural response to suffering. (a) Training-induced (POST-PRE) BOLD changes in right IPC while regulating emotional responses evoked by images of human suffering were differentially associated with post-training altruistic redistribution in COM vs. REP. The cluster was significant after correction (p < .01) using cluster-extent thresholding based on Monte Carlo simulation within the whole brain (Tables S3–4). Images and coordinates are in MNI space. Interaction R2 indicates the proportion of variance in BOLD change accounted for by the Group (COM, REP) × Redistribution interaction. (b) Trainingrelated increases in right IPC activation were associated with greater subsequent altruistic redistribution in COM (n = 20; *** P < 0.001; Table S5), whereas no significant effect was found in REP (n = 21). (c) The right DLPFC was identified by a conjunction test of (1) the correlation with IPC POST-PRE change scores (voxelwise threshold of p < 0.01) and (2) the original Group × Redistribution Interaction (voxelwise threshold of p < 0.01, shown in red-yellow). The cluster was significant after correction (p < 0.01) using cluster-extent thresholding based on Monte Carlo simulation within the whole brain (Tables S3–4). The blue line indicates that the cluster was identified by the conjunction test. (d) Training-related increases in right DLPFC activation were associated with greater redistribution in COM (*** p < 0.001) and less redistribution in REP (* p < 0.05; Table S5).Δ BOLD (% change) in b and d indicates POST-PRE changes in brain response to human suffering (SUFFERING-NEUTRAL), and sr indicates the semipartial correlation of redistribution and neural change in each group. The dashed line indicates redistribution of $2 (rank = 35.5/41) which results in an equal $5 distribution between the dictator and victim.
The IPC is implicated in experience sharing as part of the mirror neuron network (Gallese, Keysers, & Rizzolatti, 2004; Lamm et al., 2011), and we investigated whether the IPC was functionally connected to other regions that also differentially predicted redistribution between groups. The IPC conjunction test (see Method) identified the dorsolateral PFC (DLPFC; Fig. 2c and Tables S3–S4; p < 0.01 whole-brain corrected; no other regions were identified), where greater increases in DLPFC activation predicted greater altruistic redistribution in COM, and the opposite relationship was found in REP (Fig. 2d; Table S5). The changes in IPC and DLPFC were highly coupled (COM r(18) = 0.92, p < 0.001; REP r(19) = 0.79, p < 0.001), and both regions differentially predicted redistribution between groups. These findings suggest that fronto-parietal executive control networks (Dosenbach et al., 2008; Vincent et al., 2008) may be recruited by COM in order to regulate emotions and increase altruistic behavior.
DLPFC PPI connectivity changes and altruistic redistribution
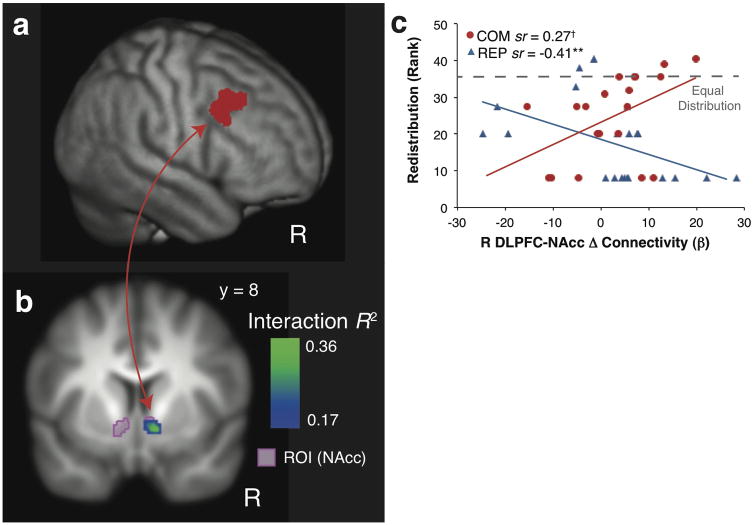
Emotion regulation is thought to involve the influence of the PFC over other regions such as the amygdala, insula, and NAcc (Ochsner & Gross, 2005; Urry et al., 2006; Wager et al., 2008). Using psychophysiological interactions (PPI), we tested whether changes in task-related functional connectivity between DLPFC and amygdala, DLPFC and NAcc, or DLPFC and insula, predicted greater altruistic redistribution in COM vs. REP. Using the DLPFC cluster defined by the IPC conjunction test as a seed (Fig. 3a), we found a significant interaction in the NAcc, demonstrating that DLPFC-NAcc connectivity was differentially associated with redistribution between COM and REP (Fig. 3b; p < 0.01 corrected within the ROI; Tables S3–S4). COM trainees who showed increased DLPFC-NAcc connectivity redistributed more funds after training, where REP trainees who showed increased DLPFC-NAcc connectivity redistributed less money after training (Fig. 3c, Table S5; see Supplementary Material for discussion of the directionality of the connectivity). No relationship was found in the insula or the amygdala.
Fig 3.
Greater training-induced connectivity with the DLPFC predicts redistribution in COM vs. REP. (a) DLPFC cluster identified by the conjunction test was used as the seed region in the PPI analysis. (b) Training-related changes (POST-PRE) in right DLPFCNAcc connectivity were differentially associated with post-training altruistic redistribution in COM vs. REP. The cluster was significant after correction (p < .01) using cluster-extent thresholding based on Monte Carlo simulation within a bilateral NAcc ROI (shown in purple; Tables S3–4). Images and coordinates are in MNI space. Interaction R2 indicates the proportion of variance in BOLD change accounted for by the Group (COM, REP) × Redistribution interaction. (c) Training-related increases in right DLPFC-NAcc connectivity were associated with greater redistribution in COM (n = 20;† p = 0.07) and less redistribution in REP (** p < 0.01; Table S5). Δ Connectivity (®) indicates COM-induced changes (POST-PRE) in PPI connectivity betas (SUFFERING vs. NEUTRAL), and sr indicates the semipartial correlation of redistribution and PPI change in each group. The dashed line indicates redistribution of $2 (rank = 35.5/41), which results in an equal $5 distribution between the dictator and victim.
Arousal correlations with altruistic redistribution and neural change
COM may increase altruistic behavior by decreasing personal distress evoked by suffering (Batson, 1991; Eisenberg et al., 2006). We found that decreases in reported arousal to the images were correlated with greater redistribution in COM (r(18) = −0.45, p < .05) but not REP (r(19) = 0.09, p = 0.70). We further investigated whether decreases in arousal in were associated with neural changes, and found that greater DLPFC-NAcc connectivity was correlated with decreases in arousal in COM (r(18) = −0.64, p < 0.01) but not REP (r(19) = −0.13, p = 0.59). Decreases in arousal were not associated with IPC or DLPFC changes in either group (all p’s ≥ 0.21).
Discussion
Individuals who trained in compassion for two weeks were more altruistic towards a victim after witnessing an unfair social interaction compared to individuals who trained in reappraisal and to a validation control group. Importantly, this demonstrates that a purely mental training can generalize to behavioral domains by impacting social behavior outside of the training context. Furthermore, greater altruistic responses were correlated with training-related changes in the neural response to suffering, providing evidence for functional neuroplasticity in the circuitry underlying compassion and altruism.
The pattern of neural changes in COM suggest that compassion trainees aid individuals who are treated unfairly by enhancing neural mechanisms that support (1) the understanding of others’ states, (2) greater fronto-parietal executive control, and (3) up-regulation of positive emotion systems. Greater IPC activation specifically predicted greater redistribution in COM and not REP, suggesting that IPC recruitment is a unique neural marker for compassion traininginduced altruism. This region has been implicated in the human mirror neuron system (Gallese, Keysers, & Rizzolatti, 2004), and may reflect greater simulation of the suffering of others. If the signal of others’ suffering is indeed increased by COM, this leads to an emotion regulatory challenge which requires trainees to approach rather than avoid suffering in order to engage in prosocial behavior. This transformation of emotional response may have been instantiated by a fronto-parietal executive control network (Dosenbach et al., 2008; Vincent et al., 2008) in order to increase altruistic behavior in COM. The coordinated activation of the IPC and DLPFC in COM may reflect greater sustained attention and goal maintenance (Miller & Cohen, 2001) to help others, as well as integration of information from systems that process both external information (of others’ suffering) and internal information (the goal to help; Vincent et al., 2008).
Regulation of internal information may include increasing positive emotions towards others’ suffering, as reflected by the increased DLPFC-NAcc connectivity that predicts redistribution in COM. This may represent increasing positive appraisals of aversive stimuli (Wager et al., 2008a) by enhancing the reward value of the victim’s well-being (e.g., caring) and increasing the anticipated reward (Knutson & Cooper, 2005) of helping the victim. Furthermore, decreased reported arousal after COM may be due to enhancement of reward-related neural systems. These findings also support research that suggests compassion training enhances positive emotions and neural substrates of affiliation (Klimecki et al., 2012).
Interestingly, the relationship between training-induced neural changes (DLPFC and DLPFC-NAcc connectivity) and altruistic behavior were not unique to COM. In fact, greater changes in these regions predicted less redistribution in REP. This may be due to the differing regulatory goals between COM and REP. In COM, the goal is to increase caring for people who are suffering and to help, whereas the goal in REP is to decrease personal negative emotions. In a social context, the goals of COM and REP are opposing (other vs. self-focused), and this may explain the cross-over interaction effects. In REP, neural changes may have resulted in decreased helping of others in order to serve the primary goal of decreasing personal negative affect.
A clear limitation of this study is that altruistic behavior was not measured at pre-training, although a separate validation sample was used to estimate pre-training levels. Future research may build on this study’s findings by measuring altruism at baseline which can strengthen claims that COM increases altruism (Leiberg et al., 2011). Furthermore, positive and negative emotions may be measured using methodology that is less susceptible to demand characteristics such as facial electromyography and skin conductance response. Future research should employ longitudinal designs to determine the length of COM needed to have sustained behavioral effects.
Finally, these results build on existing evidence that the adult human brain may demonstrate functional and structural changes after mental training (Davidson & McEwen, 2012; Klingberg, 2010; Lutz, Slagter, et al., 2008) and extend these findings to include socio-emotional domains such as compassion and altruism. Our findings support the possibility that compassion and altruism can be viewed as trainable skills rather than stable traits. This lays groundwork for future research to explore whether compassion-related trainings can benefit fields that depend on altruism and cooperation (e.g., medicine) as well as clinical subgroups characterized by deficits in compassion such as psychopathy (Blair, 2007).
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (P01-AT004952 and T32-MH018931, PI: RJD; P30-HD003352, PI: Seltzer), Department of Psychology (Hertz Award; ASF and HYW); Fetzer Institute, Impact Foundation, J. W. Kluge Foundation, Mental Insight Foundation (RJD); Mind and Life Institute (HYW); and gifts from Bryant Wangard, Ralph Robinson, Keith and Arlene Bronstein (RJD).
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Batson CD. The altruism question. Hillsdale, NJ: Erlbaum; 1991. [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11(9):387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Darwin C. The descent of man and selection in relation to sex. London, England: Penguin; 2004. Original work published in 1871. [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nature Neuroscience. 2012;15(5):689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA. A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology. 1980;10:85. [Google Scholar]
- Decety J, Jackson PL. A social-neuroscience perspective on empathy. Current directions in psychological science. 2006;15(2):54. [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Spinrad TL. Prosocial development. In: Eisenberg N, Damon W, Lerner RM, editors. Handbook of child psychology: Vol. 3. Social, emotional, and personality development. New York: Wiley; 2006. pp. 646–718. [Google Scholar]
- Fehr E, Fischbacher U. The nature of human altruism. Nature. 2003;425(6960):785–791. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Cohn MA, Coffey KA, Pek J, Finkel SM. Open hearts build lives: Positive emotions, induced through loving-kindness meditation, build consequential personal resources. Journal of Personality and Social Psychology. 2008;95(5):1045–1062. doi: 10.1037/a0013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Sciences. 2004;8(9):396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Goetz JL, Keltner D, Simon-Thomas E. Compassion: An evolutionary analysis and empirical review. Psychological bulletin. 2010;136(3):351. doi: 10.1037/a0018807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316(5831):1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- Hein G, Silani G, Preuschoff K, Batson CD, Singer T. Neural responses to ingroup and outgroup members’ suffering predict individual differences in costly helping. Neuron. 2010;68(1):149–160. doi: 10.1016/j.neuron.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Hutcherson CA, Seppala EM, Gross JJ. Loving-kindness meditation increases social connectedness. Emotion. 2008;8(5):720–724. doi: 10.1037/a0013237. [DOI] [PubMed] [Google Scholar]
- Immordino-Yang MH, McColl A, Damasio H, Damasio A. Neural correlates of admiration and compassion. Proceedings of the National Academy of Sciences, USA. 2009;106(19):8021–8026. doi: 10.1073/pnas.0810363106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimecki OM, Leiberg S, Lamm C, Singer T. Functional Neural Plasticity and Associated Changes in Positive Affect After Compassion Training. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs142. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends in Cognitive Sciences. 2010;14(7):317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology. 2005;18(4):411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54(3):2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Leiberg S, Klimecki O, Singer T. Short-Term Compassion Training Increases Prosocial Behavior in a Newly Developed Prosocial Game. PLoS ONE. 2011;6(3):e17798. doi: 10.1371/journal.pone.0017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ. Regulation of the neural circuitry of emotion by compassion meditation: effects of meditative expertise. PloS One. 2008;3(3):e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in Cognitive Sciences. 2008;12(4):163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Morelli SA, Eisenberger NI. An fMRI investigation of empathy for “social pain” and subsequent prosocial behavior. NeuroImage. 2011;55(1):381–388. doi: 10.1016/j.neuroimage.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences, USA. 2006;103(42):15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, Issa MJ, et al. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34(1):87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg S. Lovingkindness: The Revolutionary Art of Happiness. Boston, MA: Shambhala; 1997. [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439(7075):466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. The Theory of Moral Sentiments. London, England: Penguin Classics; 2010. Original work published in 1759. [Google Scholar]
- Sober PE, Wilson PDS, Wilson DS. Unto Others: The Evolution and Psychology of Unselfish Behavior. Harvard University Press; 1999. [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. Journal of Neurophysiology. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly High Correlations in fMRI Studies of Emotion, Personality, and Social Cognition. Perspectives on Psychological Science. 2009;4(3):274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Ochsner K. The neuroscience of empathy: progress, pitfalls and promise. Nature Neuroscience. 2012;15(5):675–680. doi: 10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.