Abstract
Recent studies of children suggest that exposure to elevated manganese (Mn) levels disrupt aspects of motor, cognitive and behavioral functions that are dependent on dopamine brain systems. Although basal ganglia motor functions are well-known targets of adult occupational Mn exposure, the extent of motor function deficits in adults as a result of early life Mn exposure is unknown. Here we used a rodent model early life versus lifelong oral Mn exposure and the Montoya staircase test to determine whether developmental Mn exposure produces long-lasting deficits in sensorimotor performance in adulthood. Long-Evans male neonate rats (n=11/treatment) were exposed daily to oral Mn at levels of 0, 25, or 50 mg Mn/kg/d from postnatal day (PND) 1-21 (early life only), or from PND 1 - throughout life. Staircase testing began at age PND 120 and lasted 1 month to objectively quantify measures of skilled forelimb use in reaching and pellet grasping/retrieval performance. Behavioral reactivity also was rated on each trial. Results revealed that (1) behavioral reactivity scores were significantly greater in the Mn-exposed groups, compared to controls, during the staircase acclimation/training stage, but not the latter testing stages, (2) early life Mn exposure alone caused long-lasting impairments in fine motor control of reaching skills at the higher, but not lower Mn dose, (3) lifelong Mn exposure from drinking water led to widespread impairment in reaching and grasping/retrieval performance in adult rats, with the lower Mn dose group showing the greatest impairment, and (4) lifelong Mn exposure produced similar (higher Mn group) or more severe (lower Mn group) impairments compared to their early life-only Mn exposed counterparts. Collectively, these results substantiate the emerging clinical evidence in children showing associations between environmental Mn exposure and deficits in fine sensorimotor function. They also show that the objective quantification of skilled motor performance using the staircase test can serve as a sensitive measure of early life insults from environmental agents. Supported by NIEHS R01ES018990.
Keywords: adult rats; Manganese; water intake; Montoya staircase test; skilled motor behavior; animal model, persistent effect, developmental exposure
Introduction
Adult manganese (Mn) neurotoxicity, known as ‘manganism’, is identified clinically by motor disturbances that result from the progressive and irreversible damage of the basal ganglia. There also is compelling evidence that neurobehavioral and cognitive functions in children are susceptible to the adverse impacts of elevated Mn exposure [1, 2, 6, 10, 11, 20, 25]. However, relatively little is known about the impacts of early life Mn exposure specifically on neuromotor function in children, and results from epidemiologic studies of psychomotor impairment in Mn-exposed children are inconsistent. Takser et al. [23] reported that elevated Mn exposure was associated with poorer developmental motor outcomes in 3 year old male but not female children, while a recent infant study found no association between Mn exposure and psychomotor development at the first year and second year assessments [6]. He et al. [5] reported poorer manual dexterity was linked to elevated Mn exposure in Chinese adolescent children. More recently, Lucchini et al. [13] reported an association between environmental Mn levels and impairment in the Luria-Nebraska motor coordination test and the Aiming Pursuit hand steadiness test, as well as associations between hair and blood Mn levels and tremor intensity in Italian adolescent children.
However, Hernandez-Bonilla et al. [7] performed extensive motor assessment of Mexican children age 7-11 years old and reported only a subtle negative association between Mn exposure and manual coordination deficits, while Parvez et al. [19] reported poorer psychomotor outcomes associated with arsenic exposure but not Mn exposure among adolescent children in rural Bangladesh. The factors that contribute to the differences in these pediatric epidemiological study outcomes are not known. Participant sample sizes, the developmental period of exposure and different age of the participants, the range of exposure conditions examined and the extent that exposures were known, and statistical control of confounding are all important factors to consider when drawing conclusions from human research (e.g., see ref. 14). Thus, the extent that early life Mn exposure leads to neuromotor function deficits is unclear.
Animal studies are an important counterpart to human studies of environmental toxicant exposure. They allow one to evaluate the long-lasting neurobehavioral and neurochemical consequences of well-defined exposures, while avoiding or controlling for other biological and environmental factors that may not be formally considered in studies with children. In doing so, they can determine whether elevated Mn exposure during early neurodevelopment causes long-lasting changes in cognitive, affective, and motor functions, and thus help inform the causal relationship and biological mechanism(s) underlying the findings from observational studies in children.
However, animal model studies may also possess limitations that impact extrapolation of results to the human condition. For example, the vast majority of animal model studies of early life Mn exposure and neuromotor function assessment have relied almost exclusively on general measures of whole body locomotion and coordination [3, 9, 17]. Results from these studies have provided evidence that even brief period of early life Mn exposure alters locomotor behavior in young animals (e.g., greater distance travelled), although they did not provide information about aspects of fine motor control that may more closely reflect the types of psychomotor outcomes investigated in Mn-exposed children. Thus the extent that the gross motor outcome measures in the animal model studies inform the types of neuromotor function deficits reported in Mn-exposed children is not clear. In light of this, there is a need to conduct assessments of motor function in animal models that are pertinent to the types of psychomotor skills studied in children.
The staircase test was introduced by Montoya et al. [16] to evaluate skilled forelimb use in rodents by means of objective and quantitative measurements in reaching, grasping, and retrieving movements for food pellets located on the descending steps of a left-and-right sided staircase (e.g., 16, 28). The test has been used extensively to assess sensorimotor control of the distal limbs in animal models of Parkinson’s and Huntington’s disease that involved selective neonatal or adult dopaminergic depletions of striatal regions by 6-hydroxydopamine administration [12, 16, 27, 28]. However, the staircase test has received little use in animal studies of chemical exposure. In one such study, Samsam et al. [21] found that the test provided no further evidence of the neuromotor toxicity of harmaline, scopolamine and methyl-scopolamine, and 2,4-dithiobiuret beyond that obtained through direct observation of the behavior of adult rats, leading the authors to conclude that the staircase test was not useful for detecting the fine motor function effects of the studied compounds in adult animals. However, recent modifications of the test using a color-coded pellet counting method have increased the sensitivity of the procedure to detect subtle dysfunction in skilled forelimb performance in dopamine-depleted adult animals [12].
In order to address important public health issues that have recently emerged from epidemiologic studies of Mn-exposed children, we used the Montoya staircase test, modified with a color-coded pellet counting method [12], to determine 1) if a brief period of Mn exposure restricted to the first 21 days of postnatal life produces long-term fine motor function deficits into adulthood, and 2) whether continuous lifelong Mn exposure results in more severe motor impairments than early life exposure alone. We used a neonatal model of Mn exposure and oral dosing regimens that have been shown to produce short- and long-term neurobehavioral and neurochemical toxicity in young and adult rats [8, 9]. We also developed a 5-category scale to rate the behavioral reactivity of the animals during staircase testing. To the best of our knowledge, this is the first study to evaluate fine sensorimotor dysfunction in a neonatal model of Mn exposure using objective measurements that are directly relevant to the types of motor outcomes studied in pediatric Mn research.
2. Methods
2.1. Subjects
Fifty-five adult male Long-Evans rats (Rattus Norvegicus) were used for neurobehavioral assessments (n=11/group), while an additional 60 male littermates (n=7-8/group) were sacrificed at PND 24 and PND 66 to determine blood and brain Mn levels. Subjects were born into the study over a 2 day period from 27 primiparous pregnant Long-Evans rats (gestational day 18, Charles River, Hollister, CA, USA). Twelve – 24 hrs after parturition (designated PND 1); litters were sexed, weighed, and culled to eight pups per litter containing five to six males and the remainder females. Litters were balanced by treatment so that only one male/litter was assigned to a particular treatment × outcome condition (i.e., behavioral outcome or tissue Mn designation). Animals (dams and weaned pups) were fed Harlan Teklad rodent chow #2018 which is reported by the manufacturer to contain 118 mg Mn/kg, and housed in polycarbonate cages at a constant temperature of 21 ± 2 °C. Animals were maintained on a reversed 10:14 light/dark cycle with lights off at 6:00 AM and on at 8:00 PM. Post-weaning starting on PND 22 animals were pair-housed by treatment group assignment. Animals were weighed daily throughout the study. All aspects of testing and feeding were carried during the active (dark) phase of the cycle. All animal care and treatments were approved by the institutional IACUC, and adhered to NIH guidelines set forth in the Guide for the Care and Use of Laboratory Animals [18].
Animals were food restricted starting on PND 35 in preparation for behavioral testing. Briefly, animals were placed in individual feeding cages and provided a measured amount of food each day, ranging from 14-17 grams as the animals grew, so that their rate of growth was slightly restricted to 90 – 95% of free-feeding animal weights. Animals were fed after they completed testing in the staircase task and were allowed 2 hrs to consume their food allotment. Throughout the study, the amount of food provided was altered on an individual basis if there was evidence of low motivation or aberrant weight loss or gain.
2.2. Neonatal manganese exposure
Neonate rats were orally exposed to Mn doses of 0, 25, or 50 mg Mn/kg/d over PND 1 – 21 (designated ‘early life’), or PND 1 – lifelong (designated ‘lifelong’). For PND 1 – 21 Mn dosing, a 225 mg Mn/mL stock solution of MnCl2 was prepared by dissolving MnCl2·4H2O with Milli-Q™ water; aliquots of the stock solution were diluted with a solution containing 2.5% (w/v) of the natural sweetener stevia to facilitate oral dosing of the neonate pups. Doses were delivered directly into the mouth of the pups in a volume of ~25 μL/dose via micropipette fitted with 1-200 μL flexible polyethylene gel loading pipet tips (Fisher Scientific, Santa Clara, CA, USA). Control animals received only the stevia vehicle. Oral Mn exposure post-weaning (PND 22 – end of study) was via the animal’s drinking water. For this, a 42 mg Mn/mL stock Mn solution was prepared fresh weekly as above and diluted with tap water to a final concentration of 210 or 420 μg Mn/mL in polycarbonate carboys. Stock solutions were made fresh weekly, and water bottles were refilled with fresh water two to three-times per week. Water bottle weights were recorded at refilling to determine water Mn intake per cage, and daily Mn intake per kg body weight based on daily measured body weights. Drinking water Mn concentrations were adjusted weekly as needed to maintain target daily oral Mn intake levels of 25 or 50 mg/kg/d based on measured water intake rates. Rates of drinking water intake were not measurably different between any of the treatment groups throughout the study. For example, drinking water intake by PND 60 rats averaged 0.10 mL/g/d and was not significantly different between exposure groups (data not shown).
The pre-weaning Mn exposure regimens were designed to approximate the relative increases in Mn exposure experienced by infants and young children exposed to Mn-contaminated water or soy-based formulas (or both), compared to Mn ingestion from human breast milk. The post-weaning exposure regimens via drinking water were designed to maintain those daily pre-weaning exposure levels over the animal’s lifetime. A more detailed rationale for these Mn exposure levels is provided elsewhere [8, 9].
2.3. Determination of blood and brain Mn concentrations
Blood and brain Mn concentrations were determined in littermates of the study animals at PND 24 and PND 66 (7 – 8/treatment group and time point), and in the study animals at the completion of all behavioral testing (~PND 400). Animals were euthanized via sodium pentobarbital overdose (75 mg/kg ip) and exsanguination, and whole blood (2 – 3 mL) was collected from the left ventricle of the surgically-exposed heart and stored in EDTA Vacutainers at −20 °C for analyses. Whole brain was immediately removed, bisected into hemispheres, and the hind-brain regions of each hemisphere collected and stored at −80 °C for Mn concentration determinations (forebrain was dedicated to other outcome measures to be reported elsewhere). Tissues were processed for Mn concentrations using trace metal clean techniques, as previously described [9, 22]. Briefly, aliquots of whole blood were digested overnight at room temperature with 16N HNO3 (Optima grade, Fisher Scientific), followed by addition of H2O2 and Milli-Q™ water. Digestates were centrifuged (15,000 × g for 15 min.) and the supernatant collected for Mn analysis. For brain, aliquots of homogenized hind brain tissue (~200 mg wet weight) were dried then digested with hot 16N HNO3, evaporated and redissolved in 1N HNO3 for analyses. Rhodium was added to sample aliquots as an internal standard. Manganese levels were determined using a Thermo Element XR inductively coupled plasma – mass spectrometer, measuring masses 55Mn and 103Rh (the latter for internal standardization). External standardization for Mn used certified SPEX standards (Spex Industries, Inc., Edison, NJ). National Institutes of Standards and Technology SRM 1577b (bovine liver) was used to evaluate procedural accuracy. The analytical detection limit for Mn in blood and brain was 0.04 and 0.015 ng/mL, respectively.
2.4. Staircase apparatus and pellet sizes
In the Staircase test, the animal is confined to the apparatus for a pre-determined test period to retrieve food pellets on the different step levels, using only its right-side forepaw for food pellets on the right, and left-side forepaw for pellets on the left staircase steps. Testing took place in four identical Plexiglas staircase devices modeled after the original design of Montoya et al. [16]. The exact dimensions of the devices have been reported previously [21]. Briefly, the staircase itself was composed of seven descending steps on each side with a bottom floor portion in between where fallen, irretrievable pellets could accumulate. Each step measured 15 × 18 mm and was 6 mm lower than the previous step. A 3 mm deep × 10 mm diameter well was machined into each step to hold the pellets at the beginning of each trial. The highest step was 12 mm below the platform supporting the subject, while the lowest step was 42 mm below the platform.
Two food pellet sizes, 45 mg and 20 mg (BioServ, Holton Industries, Frenchtown, NJ, USA), were used to bait individual steps of the staircase. Pellets of both sizes were colored-coded with six different food dyes (McCormick & Co. MD, USA) following the protocol of Kloth et al. [12], in order to increase the sensitivity of the Staircase test. Steps 1 – 6 were baited with native (white), yellow, orange, green, blue, and purple pellets, respectively. To evaluate the palatability and any preference/aversion of the dye-colored pellets, all animals were given several colored pellets prior to staircase testing for 10 minutes each day for 2 consecutive days; animals readily consumed all colored pellets, and no evidence of preference or aversion was detected.
2.4.1 Testing procedure
The study was comprised of three stages and spanned 1 month, corresponding to subject age ~PND 120 – 150 at testing. The first 8 days of testing constituted habituation and training to reach and grasp the 45 mg pellets from the descending steps of the staircases. The next 12 days (days 9 - 20) corresponded to testing proper with the 45 mg pellet size, and the last 5 days of the study consisted of testing with the 20 mg pellet size. Each subject was given one 10 minute trial per day, 6 days per week for ~1 month, totaling 25 trials per animal.
Prior to each trial, steps 1 – 6 were baited with three 45 mg pellets or five 20 mg pellets per step on each side depending on the stage of testing. The lowest step (7) was not baited with pellets because that location was found to be too difficult to reach for adult male Long-Evans rats [21]. Therefore, in our study the total number of available pellets from the left and right staircases was 36 or 60 for the 45 mg and 20 mg pellets, respectively. After the rat had entered the box and the guillotine-type door had been inserted, the staircases were introduced into the box, and the 10 minute trial was initiated. The 10 minute trial duration was based on evidence that Long-Evans rats obtained the maximum number of pellets in the Staircase test within this time [21]. The order and time of testing were the same each day for each rat. Over that period animals were tested in the same box and handled as much as possible by the same person each day. Experimenters were blinded to the treatment conditions of the animals.
After the 10 minute trial, the staircases and the rat were removed and the following categories of pellet outcomes were determined per step and side: (1) the number of remaining pellets, (2) the number of pellets eaten, (3) the number of pellets misplaced, and (4) the number of pellets lost. The number of ‘pellets remaining’ described those pellets left on the step where they had originally been placed, whereas ‘pellets eaten’ described those pellets that were grasped and consumed. ‘Pellets misplaced’ described all the pellets that were grasped but dropped elsewhere on one of the steps. ‘Pellets lost’ described the category of pellets that ended up on the floor of the apparatus out of reach of the subject. The ‘misplaced pellets’ were counted based on their final step location, whereas the ‘lost pellets’ were counted based on their step of origin at the start of a trial. Finally, the ‘total number of pellets eaten’ was calculated for each rat as the overall sum of pellets eaten across steps and sides of the staircase.
In addition, the level of behavioral reactivity of individual rats during staircase testing and the number of fecal boli left in the box were determined for each trial. Behavioral reactivity was scored according to a 5-category scale at 1, 5, and 10 minutes into the trial; the average of these scores was analyzed per trial. On that scale, a score of 1 (best, lowest reactivity) denoted that the animal was calm, lying flat on top of platform, and readily reaching for pellets on both sides of its body. An intermediate score of 3 denoted less calm behavior with fewer reaching attempts and sporadic attempts to escape the box (e.g., turning in the box). A score of 5 denoted a state of “overreactivity” with frequent turning attempts, biting of the box, and vocalization.
2.4.2 Dependent variables
Skilled forelimb performance in reaching and grasping movements was evaluated for each step using the following parameters: the number of pellets taken, the number of pellets eaten, and the corresponding percent of success. Pellets taken per step were calculated as: number of initial pellets − number of pellets remaining. The number of pellets eaten was calculated using the formula: number of initial pellets − (number of pellets remaining + number of pellets misplaced + number of pellets lost). The percent of success was calculated as: (pellets eaten/pellets taken) × 100. For these calculations, pellets misplaced described all the pellets that remained somewhere on any step other than the step of origin, while the pellets lost described all the pellets that were lost to the floor of the apparatus, out of reach of the animal. From these primary parameters, the percent of pellets misplaced and the percent of pellets lost were computed as the [(pellets misplaced/pellets taken) × 100] and [(pellets lost/pellets taken) × 100]. The maximum forelimb extension was also determined by the stringent criteria of the lowest step where zero pellets remained, out of a starting total of 6 (45 mg pellets) or 10 (20 mg pellets) pellets per step for both sides.
2.5 Statistical methods
The behavior data were analyzed by means of a mixed model analysis of variance (ANOVA), which handled the repeated measurements on each animal [29]. The tissue Mn concentration data were analyzed using a one-way ANOVA and Tukey’s post hoc test for pairwise comparisons; data were log transformed before analyses if necessary to achieve normal distribution and variance equality.
The daily averaged behavioral reactivity scores and the number of fecal boli were analyzed temporally over the three stages of the study, corresponding to (I) training (days 1 - 8); (II) testing with 45 mg pellet size (days 9 - 20); and (III) testing with 20 pellet size (days 21 - 25). The total number of pellets eaten per trial was analyzed over the first 20 days of the experiment (stages I and II) to assess motor learning in experimental groups. Finally, analyses of skilled forelimb performance were conducted step-by-step for the pooled data from stage II (days 9 – 20 of testing with the 45 mg pellets), and the pooled data from stage III (days 21 – 25 of testing with the 20 mg pellets). For this, the staircase data were averaged across the left and right sides based on results showing that skilled forelimb performance (i.e., the number of pellets taken and eaten) was not significantly different between the left and the right staircases (data not shown). Treatment group, corresponding to the five Mn exposure conditions, was included in all models as the between-subject variable. In addition, the models also included study day, stage of the study, or step level of the staircase depending on the data analyzed. Relevant interactions were also included in all statistical models.
For each outcome analyzed, a mean was calculated for each rat and factor (e.g., day or step) included in the statistical model. Skewed data were subjected to a square root transformation prior to analysis. The significance level was set at p<0.05. In addition, effects of 0.10> p >0.05 are reported if they helped form a coherent pattern of Mn-related effects across end points analyzed. Significant main effects of treatment group were followed by single-degree of freedom contrasts, whereas significant interactions involving this factor were followed by tests of simple main effects at each level of the interacting factor (e.g., step). The Satterhwaite method was used to adjust the denominator degrees of freedom for each simple test. Statistical analyses were conducted using SAS 9.3 for Windows (SAS Institute, Cary, NC, USA) on a mainframe computer, or JMP 10.0 (SAS Institute).
3. Results
3.1. Adult body weights
All animals gained weight during the period of staircase evaluation (ANOVA, main effect of day, F (26, 1298) = 81.38, p<0.0001). Group mean adult body weights ranged from ~420 – 460 g and were not significantly different among the treatment groups over all days of the study (F (4, 50) =0.92, p=0.46).
3.2. Mn exposure and resultant blood and brain Mn levels in littermate groups
Blood and brain Mn levels were measured in littermates to the study animals on PND 24 and 66, and in the study animals following completion of all behavioral testing (~PND 400) as an indication of body Mn levels during development preceding or after staircase testing, which started ~PND 120. Blood and brain Mn levels in PND 24 littermates exposed to Mn over PND 1 – 21 (i.e., the ‘early life Mn’ groups) were increased ~6-fold and 2.4-fold, respectively, in the 25 mg/kg/d group compared to control, and by ~10-fold and 3-fold, respectively, in the early life 50 mg/kg/d treatment group compared to control (ANOVA blood F(2,18) = 59.4, p<0.0001; brain F(2,19) = 19.5, p<0.0001) (Table 1). In PND 66 animals, blood and brain Mn levels remained slightly though significantly elevated in the lifelong Mn groups, but not in the early life Mn groups, compared to PND 66 controls (ANOVA blood F(4, 29) = 16.1, p<0.0001; brain F(4, 29) = 7.26, p=0.0004) (Table1). Similarly, in the ~PND 400 study subject animals, blood and brain Mn levels remained slightly though significantly elevated in the lifelong Mn groups, but not in the early life Mn groups, compared to ~PND 400 controls (ANOVA blood F(4, 43) = 21.6, p<0.0001; brain F(4, 28) = 10.3, p<0.0001) (Table1). Notably, blood and brain Mn levels dropped substantially in all PND 66 and ~PND 400 groups compared to levels in their PND 24 counterparts. Average blood Mn levels in PND 66 animals ranged from ~10 – 20 ng/mL across all treatment groups, and in PND400 animals from ~6 – 14 ng/mL, all well within the range of blood Mn levels in environmentally exposed children [13].
Table 1.
Blood and brain Mn concentrations in male littermates of the study animals (PND 24, 66), and in the study subjects at the completion of behavioral testing (~PND 400).
| Treatment | PND 24 | PND 66 | PND ~400 | |||
|---|---|---|---|---|---|---|
| Blood Mn* | Brain Mn | Blood Mn | Brain Mn | Blood Mn | Brain Mn | |
| Control | 23.6 ± 1.04a# |
4.28 ± 0.47a |
9.71 ± 0.447a |
2.14 ± 0.019a |
5.81± 0.342a |
2.06 ± 0.052a,b |
| Early 25 mg/kg/d |
186 ± 36.8b |
11.5 ± 1.59b |
11.3 ± 0.823a,b |
2.30 ± 0.072a |
7.26 ± 0.614a |
2.00 ± 0.063a |
| Early 50 mg/kg/d |
267 ± 30.0c |
13.4 ± 1.98b |
15.4 ± 1.12b |
2.28 ± 0.058a |
6.65 0.487a |
1.91 ± 0.022a |
| Lifelong 25 mg/kg/d |
NA | NA | 14.6 ± 1.05b |
2.36 ± 0.039a,b |
9.69 ± 0.442b |
2.28 ± 0.082b,c |
| Lifelong 50 mg/kg/d |
NA | NA | 20.4 ± 1.53c |
2.58 ± 0.077b |
13.7 ± 1.25c |
2.54 ± 0.120c |
Blood Mn in ng/mL, brain Mn in μg/g dry weight.
Data are expressed as means ± SE (n=6 – 11/group). Within a developmental period and type of tissue, treatment groups with different superscripts are statistically different from one another, based on Tukey’s posthoc test on log transformed data.
3.3. Manganese exposure impacts behavioral reactivity during staircase training
Two rats (one control rat and one lifelong 50 Mn mg/kg/d rat) constantly failed to obtain pellets from the steps of the staircases even after 8 days of training. Because these animals gave incomplete data, they were excluded from all subsequent analyses. As a result, the staircase data analyzed and reported here included observations from a total of 53 subjects.
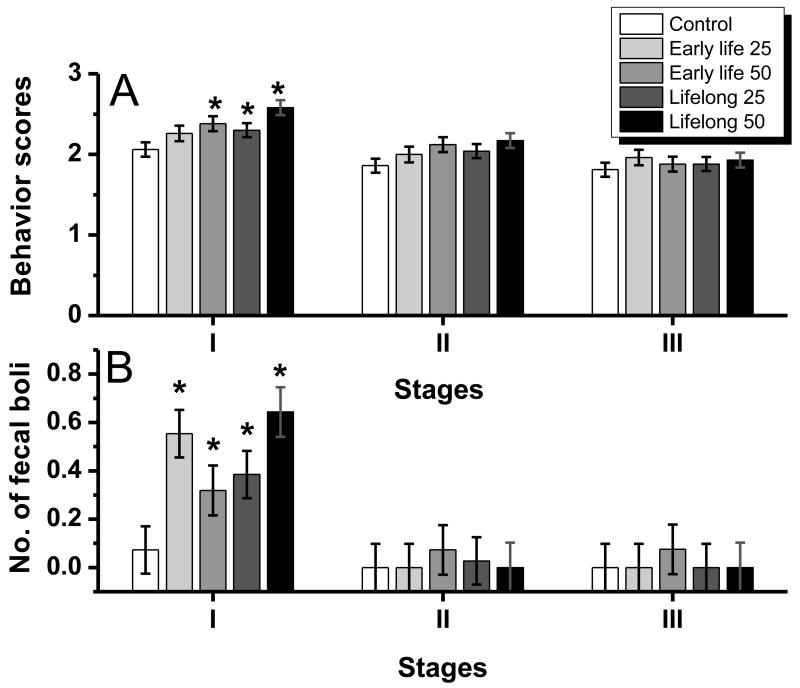
The behavioral reactivity of the rats improved with successive trials on the task; the behavioral reactivity scores and the number of fecal boli decreased significantly over the three stages of the study (ANOVA, respectively, F(2, 92)=67.25, p<0.001 and F(2, 144)=38.7, p<0.0001) (Fig. 1A, B). Over all stages combined, the Mn-treated groups tended to exhibit greater behavioral reactivity (i.e., increased scores; F (4, 46) =2.22, p=0.08), but no difference in the number of fecal boli (not shown). However, the interaction of treatment group by testing stage approached significance for the behavioral reactivity scores (F (8, 92) =1.98, p=0.06) and the rate of defecation (F (8, 144) =1.72, p=0.09), suggesting that improvement on the task was different between exposure groups.
Figure 1.
(A) Behavioral reactivity scores and (B) number of fecal boli per day of the five Mn exposure groups across the three stages of the staircase study (stage I, II, and III, corresponded to day 1-8, 9-20, and 21-25 of the study, respectively). Daily scores were averaged across days per rat; bars/symbols represent the mean ±S.E. (n=11-12/group). *p<0.05 relative to the control group. Manganese doses in mg Mn/kg/d (see text).
Subsequent analysis using tests of the simple main effect at each stage revealed that for both behavioral/stress measurements the interactions arose from significant group differences that emerged during stage I but not during stage II or stage III (for behavioral reactivity, F(4, 82)=4.35, p=0.003, and for defecation, F(4, 144)= 4.84, p=0.001). Contrasts further indicated that during stage I, all Mn-treated groups, except the early life 25 Mn mg/kg/d group, exhibited significantly greater behavioral reactivity (i.e., higher scores) compared to controls (all p’s <0.05). Similarly, during stage I only the number of fecal boli was significantly higher in all of the Mn exposure groups compared to the control group (all p’s < 0.05).
3.4. Skilled forelimb performance
3.4.1 Manganese exposure did not impact forelimb motor skill learning
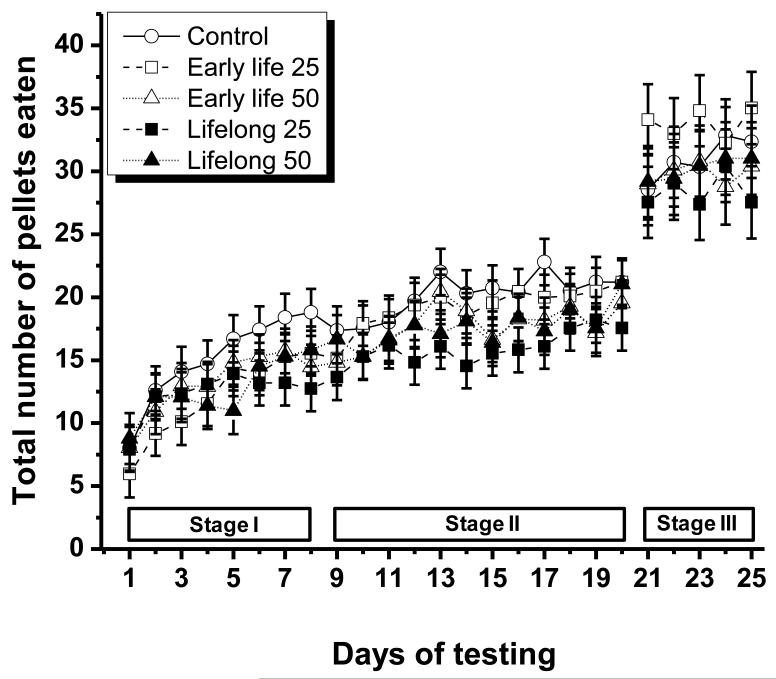
All the treatment groups ate an increasing number of 45 mg pellets per trial over the first 20 days of testing, reflecting motor skill learning (ANOVA, main effect of day, F(19, 878)=35.4, p<.0001) (Fig. 2). This improvement was most pronounced during the first 8 days of testing (stage I) when the number of total pellets eaten increased by ~100% compared to an increase of ~30% for the remaining testing days (9-20 or stage II). There was no significant effect of treatment group on the daily total number of 45 mg pellets eaten, nor was there a significant treatment group × day interaction (F(4, 48)=0.84, p=0.51, and F(76, 878)=1.10, p=0.27, respectively).
Figure 2.
Number of total pellets eaten for the five Mn exposure groups over the three stages of the study (training, testing with 45 mg pellets, testing with 20 mg pellets). Testing with the 45 mg pellets occurred over days 1 – 20, while testing with the 20 mg pellets occurred over days 21 – 25 of the study. Data represent the mean ± S.E. (n=11-12/group). Manganese doses in mg Mn/kg/d (see text).
Similarly, there was no significant treatment group effect on the daily total number of 20 mg pellets eaten during stage III of the experiment (day 21-25; F(4, 184)=0.80, p=0.53). There also was no significant effect of test day during that stage (F (4, 184) =0.80, p=0.53), indicating that by then the animals had achieved their maximum performance ability on the staircase task.
3.4.2. Early life and lifelong Mn exposure reduced forelimb maximum reaching distance
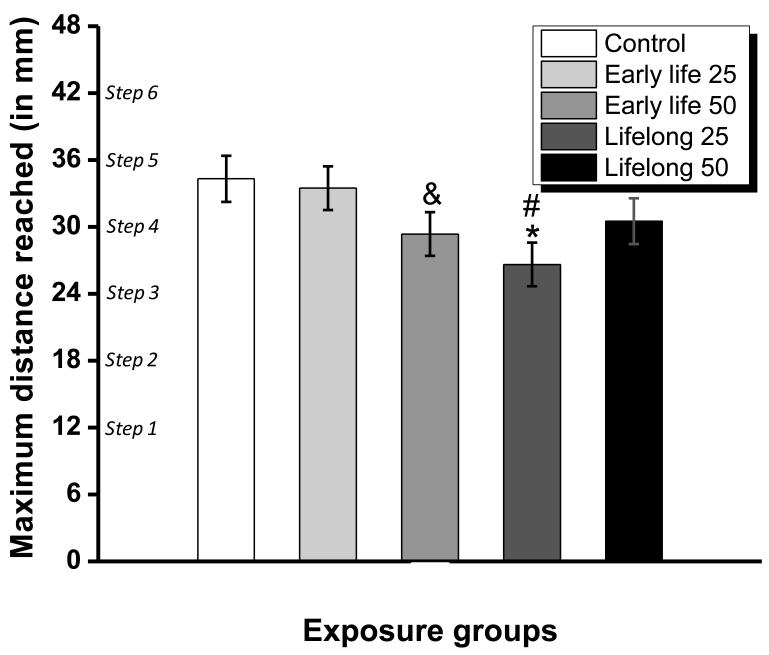
The maximum forelimb reaching distance on the task, defined as the lowest step distance where zero pellets remained, was 30 mm across all groups, corresponding to step 4 of the staircase. The analysis revealed a trending significant main effect of treatment group on maximum forelimb reaching distance (ANOVA, F (4, 48) =2.46, p=0.06, see Fig. 3). Contrasts further identified that the early life 50 mg Mn/kg/d group (p=0.09), but not the early life 25 mg Mn/kg/d group (p=0.77), tended to reach over significantly shorter distances than the control group. In addition, the maximum forelimb reaching distance was significantly reduced in the lifelong 25 mg Mn/kg/d group compared with controls (p=0.009) or the early life 25 mg Mn/kg/d group (p=0.02), but was no different in the lifelong 50 mg Mn/kg/d or the early life 25 mg Mn/kg/d group when compared with the controls (p’s=0.20 and 0.70, respectively).
Figure 3.
Maximum forelimb step distance reached for each treatment group, as defined by the lowest step where all food pellets had been taken. Bars represent the mean ± S.E. (n=11-12/group). * indicates p<0.05 versus control; ‘&’ indicates p>0.05 and p<0.10 versus control; ‘+’ indicates p<0.05 versus the early life 25 mg Mn/kg/d group. Manganese doses in mg Mn/kg/d (see text).
3.4.3 Early life and lifelong Mn exposure produced deficits in forelimb sensorimotor performance
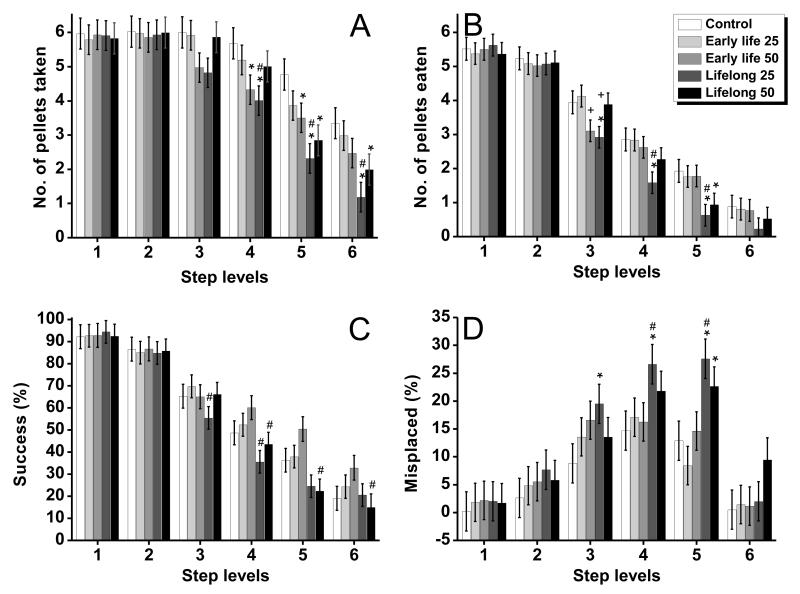
For both pellet sizes, the number of pellets taken, pellets eaten, and the percent success [i.e., (pellets eaten/pellets taken) × 100] were all significantly decreased on the lower, more difficult to reach steps (significant main effect of step level, F(5, 240) = 96.7, p<0.0001; F(5, 240) = 384, p <0.0001; F(5, 240)=271, p<0.0001,for pellets taken, eaten, and percent success, respectively). Consistent with these results, a significantly greater percent of pellets were misplaced and lost from the difficult to reach steps of the staircase (F (5, 234) =40.8, p<0.0001 and F (5, 237) =189.3, p<0.0001, respectively).
Importantly, Mn-treated rats performed significantly worse on the more difficult to reach steps compared to controls across several performance outcomes for both pellet sizes (e.g., Fig. 4A-D for the 45 mg pellet size trials; ANOVAs treatment group × step interaction for the outcomes number of pellets taken, F(20, 240)=1.88, p=0.01; pellets eaten, F(20, 240)=2.10, p=0.005; percent of success, F(20, 234)=2.13, p=0.004; and percent of pellets misplaced, F(20, 229) =1.73, p=0.03)). The interaction of treatment group by step level was not significant for the percent of pellets lost for either pellet size (45 mg pellets, F (20, 237) = 1.04, p = 0.41; and 20 mg pellets, F (20, 230) =1.04, p=0.42).
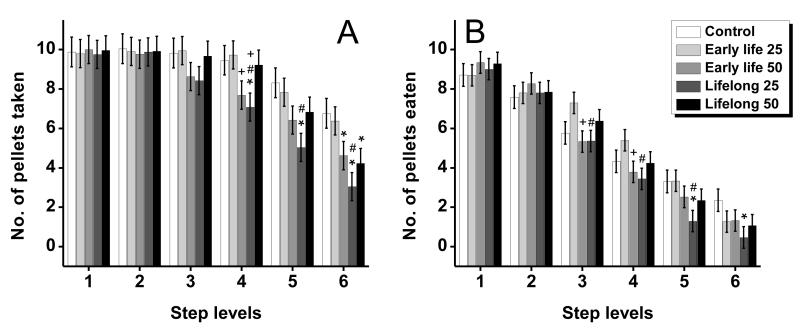
Figure 4.
(A) Number of 45 mg pellets taken, (B) number of pellets eaten, (C) the percent of success, and (D) the percent of misplaced pellets of control and Mn-exposed groups as a function of step level (reaching difficulty) on the staircase. Bars represent the mean ± S.E. (n=11-12/group). * indicates p<0.05 versus control; ‘#’ indicates p<0.05 versus the early life Mn exposure group within each Mn dose level; ‘+’ indicates p<0.05 for the 25 mg Mn/kg/d group versus the 50 mg Mn/kg/d group within each level of exposure duration. Manganese doses in mg Mn/kg/d (see text).
Follow up tests of the simple main effect at each step level further revealed that across outcomes the significant group by step interactions were driven by significant differences among Mn exposure conditions specifically at steps that required forelimb function to retrieve a pellet (i.e., steps 3 – 6) versus the higher steps 1 and 2 where food pellets could also be obtained directly with the mouth (e.g., simple effect for pellets taken from steps 3 – 6, respectively, F(4, 138)=1.71, p=0.15, F(4, 138)=2.38, p=0.05, F(4, 138)=4.54, p=0.002, and F(4, 138)=3.76, p=0.006; and for pellets eaten from steps 3 – 6, respectively, F(4, 114)=2.89, p=0.02, F(4, 114)=2.74, p=0.03, F(4, 114)=3.25, p=0.01, and F(4, 114)=0.69, p=0.60)). Notably, the effect of Mn treatment on skilled forelimb performance became more pronounced as the level of step difficulty increased on the task. This was reflected in the effect size of the impairment, indicated by the magnitude of the single-degrees of freedom contrasts from controls (Table 2), which achieved statistical significance starting at step 3 of the staircase, and then typically became larger with increasing step level (i.e., difficulty).
Table 2.
Summary of treatment group differences and t probabilities in the number of pellets taken, pellets eaten, the percent of success, and the percent of misplaced pellets, as a function of difficult to reach step levels (3-6) for trials with 45 mg and 20 mg food pellets. Group contrasts at the first two highest step levels were not significant for any outcomes and pellet size (data not shown).
| 45 mg pellet size | 20 mg pellet size | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Step levels |
Pellets taken |
Pellets eaten |
Success % |
Mispl. % |
Pellets taken |
Pellets eaten |
Success % |
Mispl. % |
|
|
Early life 50 vs.
Control |
3 | 0.10 | 0.07 | 0.61 | 0.10 | 0.25 | 0.58 | 0.59 | 0.28 |
| 4 | 0.03 | 0.61 | 0.28 | 0.74 | 0.09 | 0.49 | 0.54 | 0.27 | |
| 5 | 0.04 | 0.74 | 0.07 | 0.72 | 0.07 | 0.32 | 0.48 | 0.65 | |
| 6 | 0.16 | 0.81 | 0.08 | 0.90 | 0.04 | 0.20 | 0.71 | 0.91 | |
|
| |||||||||
|
Lifelong 25 vs.
Control |
3 | 0.06 | 0.03 | 0.19 | 0.03 | 0.18 | 0.60 | 0.82 | 0.02 |
| 4 | 0.01 | 0.01 | 0.08 | 0.02 | 0.02 | 0.26 | 0.69 | 0.01 | |
| 5 | 0.00 | 0.01 | 0.12 | 0.00 | 0.00 | 0.01 | 0.10 | 0.00 | |
| 6 | 0.00 | 0.16 | 0.84 | 0.76 | 0.00 | 0.02 | 0.06 | 0.38 | |
|
| |||||||||
|
Lifelong 50 vs.
Control |
3 | 0.83 | 0.90 | 0.92 | 0.35 | 0.89 | 0.45 | 0.45 | 0.57 |
| 4 | 0.29 | 0.23 | 0.50 | 0.16 | 0.82 | 0.92 | 0.91 | 0.03 | |
| 5 | 0.00 | 0.04 | 0.07 | 0.05 | 0.17 | 0.24 | 0.32 | 0.03 | |
| 6 | 0.04 | 0.46 | 0.63 | 0.09 | 0.02 | 0.12 | 0.79 | 0.86 | |
|
| |||||||||
|
Early life 25 vs.
Lifelong 25 |
3 | 0.07 | 0.01 | 0.05 | 0.22 | 0.13 | 0.01 | 0.04 | 0.01 |
| 4 | 0.05 | 0.01 | 0.02 | 0.05 | 0.01 | 0.01 | 0.36 | 0.06 | |
| 5 | 0.01 | 0.01 | 0.07 | 0.00 | 0.01 | 0.01 | 0.04 | 0.00 | |
| 6 | 0.00 | 0.20 | 0.61 | 0.91 | 0.00 | 0.29 | 0.69 | 0.85 | |
|
| |||||||||
|
Early life 50 vs.
Lifelong 50 |
3 | 0.15 | 0.09 | 0.88 | 0.53 | 0.32 | 0.19 | 0.20 | 0.62 |
| 4 | 0.28 | 0.45 | 0.03 | 0.25 | 0.14 | 0.56 | 0.62 | 0.28 | |
| 5 | 0.29 | 0.07 | 0.00 | 0.11 | 0.69 | 0.82 | 0.09 | 0.07 | |
| 6 | 0.44 | 0.59 | 0.03 | 0.12 | 0.71 | 0.74 | 0.51 | 0.95 | |
|
| |||||||||
|
Early life 25 vs.
Early life 50 |
3 | 0.12 | 0.02 | 0.52 | 0.53 | 0.19 | 0.01 | 0.02 | 0.13 |
| 4 | 0.16 | 0.63 | 0.30 | 0.86 | 0.05 | 0.04 | 0.50 | 0.74 | |
| 5 | 0.55 | 0.97 | 0.10 | 0.19 | 0.16 | 0.29 | 0.76 | 0.92 | |
| 6 | 0.40 | 0.93 | 0.27 | 0.95 | 0.09 | 0.94 | 0.06 | 0.32 | |
|
| |||||||||
|
Lifelong 25 vs.
Lifelong 50 |
3 | 0.10 | 0.04 | 0.16 | 0.23 | 0.23 | 0.20 | 0.31 | 0.09 |
| 4 | 0.10 | 0.14 | 0.29 | 0.34 | 0.04 | 0.31 | 0.78 | 0.67 | |
| 5 | 0.40 | 0.51 | 0.77 | 0.32 | 0.08 | 0.19 | 0.52 | 0.20 | |
| 6 | 0.20 | 0.53 | 0.48 | 0.16 | 0.26 | 0.45 | 0.12 | 0.49 | |
3.4.4. Early life versus lifelong Mn exposure produced different patterns of deficits in forelimb grasping and reaching skills
Since quantitatively similar results were obtained with both the 45 mg and 20 mg pellet size trials, only the findings from analysis of the 45 mg pellet size trials are reported below in order to streamline the presentation of the results across endpoints and step levels. First, we describe the treatment group contrasts involving the early life Mn exposure groups and the control group to show that this duration of exposure resulted in selective long-lasting sensorimotor performance deficits even 3 months after the cessation of Mn exposure. Secondly, we report the contrasts involving the lifelong Mn exposure groups and the controls to shown that this duration of treatment produced a type of global impairment in skilled forelimb functions. Finally, we report comparisons specifically between the early life vs. lifelong Mn groups to show that lifelong Mn exposure was more detrimental to sensorimotor performance than the early life only exposure.
Lasting impact of early life only Mn exposure
Early life exposure to 25 mg Mn/kg/d (pre-weaning, PND 1 – 21) did not produce any detectable long-term impairment in skilled forelimb performance. These animals took and ate as many pellets as the controls from the more difficult steps of the staircase, indicating that reaching and grasping movements were not impaired in this group (Fig. 4A-D, Table 2). In contrast, the early life 50 mg Mn/kg/d group displayed selective deficits compared to the control group; they took fewer pellets than controls from the difficult to reach steps (e.g., steps 3, 4, and 5, respectively, Table 2), but were not impaired in the number of pellets eaten from the same difficult step levels (Fig. 4A, B). As a result, the early life 50 mg Mn/kg/d group reached a higher percent of success than the control from these steps of the staircase, since percent success was defined as pellets eaten/pellets taken × 100 (Fig. 4C). In line with these results, the percent of pellets misplaced and pellets lost were not different in the early life 50 mg Mn/kg/d and control groups (Fig. 4D), further supporting that their ability to manipulate and eat pellets was not measurably affected.
Impact of lifelong Mn exposure
The lifelong 25 mg Mn/kg/d group was the most consistently impaired at taking and eating pellets, while also misplacing a greater percent of pellets (Fig. 4A-D, Table 2). Specifically, this group took and ate significantly fewer pellets than the control from steps 3 - 5 of the staircase, with the treatment effect size (i.e., significance level) generally increasing with step level/difficulty (Table 2). Consistent with this, the percent of pellets misplaced was significantly greater in the lifelong 25 mg Mn/kg/d group compared to the control group at these same step levels (Fig. 4D, Table 2). The lifelong 50 mg Mn/kg/d group was also impaired compared to controls, but to a lesser extent than the lifelong 25 mg Mn/kg/d group. The lifelong 50 mg Mn/kg/d group took significantly fewer pellets than the control group but only from the two lowest steps of the staircase (5 and 6), while eating significantly fewer pellets than controls only from step 5 (Fig. 4A-D, Table 2). However, there were no differences between the lifelong 50 mg Mn/kg/d group and controls in the percent of pellets misplaced for any of the steps of the staircase.
Impact of lifelong versus early life manganese exposure
The early life only versus lifelong duration of Mn exposure produced different patterns of deficits that were dependent on the Mn dose administered. For example, the lifelong 25 mg Mn/kg/d group performed significantly worse than their early life 25 mg Mn/kg/d counterparts in the number of pellets taken and pellets eaten from all lower steps of the staircase (Fig. 4A, B, Table 2). And correspondingly, their percent of success was lower than that of the early life 25 mg Mn/kg/d group from steps 3, 4, and 5 (Fig. 4C, Table 2), while their percent of pellets misplaced was significantly higher on steps 4 and 5 of the staircase (Fig. 4D, Table 2).
In contrast, lifelong exposure to 50 mg Mn/kg/d did not appear to significantly exacerbate the effects produced by early life only exposure to 50 mg Mn/kg/d. The specific contrasts between the early life only versus the lifelong 50 mg Mn/kg/d groups showed no significant differences in the number of pellets taken or the number of pellets eaten for any of the steps (Table 2). There was, however, some evidence of group differences in the percent of success outcome, with the lifelong Mn group performing worse than their early life only Mn exposure counterpart for steps 5 and 6 (Fig. 4C, Table 2). However, both groups misplaced and lost a similar percent of pellets even from difficult step levels. Collectively, these results suggest that lifelong exposure to the higher 50 mg Mn/kg/d dose produces little additional impairment of skilled forelimb performance over that already produced by exposure restricted to early pre-weaning life.
4. Discussion
We investigated the impacts of early life and lifelong oral Mn exposure on skilled motor performance in adult rats, using the staircase test of fine motor function relevant to the types of motor skill deficits reported in Mn-exposed children (e.g., 13). The staircase test taps essential motor functions required to successfully take and eat small food items from the staircase, including advancing the limb over the food, opening the digits in preparation for grasping, grasping and manipulating the food pellet, and withdrawing the paw to place the food in the mouth. Our results show for the first time that early life Mn exposure restricted to the pre-weaning period produced selective long-lasting impairment in reaching skills in adults, and that lifelong Mn exposure produced wider-spread deficits in both reaching and grasping skills (Figs. 4, 5, Table 2).
Figure 5.
(A) Number of 20 mg pellets taken, and (B) number of pellets eaten by the control and Mn-exposed groups, as a function of step level (reaching difficulty) of the staircase. Bars represent the mean ± S.E. (n=11-12/group). * indicates p<0.05 versus control; ‘#’ indicates p<0.05 versus the early life Mn exposure group within each Mn dose level; ‘+’ indicates p<0.05 for the 25 mg Mn/kg/d group versus the 50 mg Mn/kg/d group within each level of exposure duration. Manganese doses in mg Mn/kg/d (see text).
4.1. Early life Mn exposure causes a selective long-lasting motor disorder in adulthood
Skilled forelimb use was significantly but selectively impaired in adult animals exposed to 50 mg Mn/kg/d during early (pre-weaning) life. For example, this group tended to reach over significantly shorter distances than the controls (Fig. 3), suggesting impaired forelimb extension/reaching. Consistent with a deficit in reaching movements, this group also exhibited significant reductions in the number of food pellets taken from steps 3-5 of the staircase, relative to the controls (Figs. 4A and 5A). In contrast, however, the grasping and pellet retrieval skills of the early life 50 mg Mn/kg/d group were not significantly impaired; these subjects ate as many pellets, and exhibited levels of success (%) and pellets misplaced (%) comparable to controls on all steps of the staircase (Figs. 4B-D, 5B). The nature of the forelimb performance deficits in the early life 50 mg Mn/kg/d group, i.e., impaired forelimb reaching but not grasping/retrieval, point to a selective long-lasting vulnerability of reaching movements in these adult subjects, which is consistent with the abnormal reaching skills reported in adult rats after neonatal DA-depletion [26].
In contrast, animals exposed early in life to the slightly lower 25 mg Mn/kg/d dose were not measurably impaired in any of the performance outcomes compared to controls. This suggests that the early life Mn exposure regimens used here, which differed in Mn concentration only by a factor of two, spanned the threshold of measurable impairment of forelimb sensorimotor function. This negative finding is also consistent with the profile of spared behavioral and learning functions reported previously in animals treated with the same early life 25 mg Mn/kg/d exposure regimen [8, 9].
It is noteworthy that the deficits in forelimb sensorimotor function discussed above were identified in adults approximately 3 months after their last oral Mn dose on PND 21, when blood and brain Mn levels had long since returned to background levels (Table 1, and see ref. 8 for additional time points). These long-lasting deficits suggest permanent or irreversible damage to the basal ganglia systems of the adult rat brain as a result of early life Mn exposure, consistent with evidence from our prior studies showing that adult (PND ~100) rats exposed to the same levels of pre-weaning Mn early in life exhibited increased expression of dopamine D2 receptors and activated astrocytes in frontal – subcortical neuronal circuits [8]. Previous studies in animal models have provided evidence that early life exposure to Mn levels similar to those used here can result in hyperkinetic behavior in young mice, rats, and monkeys [5, 9, 17], but the nature of those previously reported deficits specific to sensorimotor function, and the extent that the deficits were lasting (i.e., permanent), was not clear.
4.2. Lifelong Mn exposure produces a widespread motor disorder in adulthood
Lifelong oral exposure to Mn produced widespread impairment in skilled motor performance that was apparent across multiple staircase test outcomes in adult rats. Additionally, while the general nature of deficits in reaching and pellet grasping/retrieval performance was similar in the lifelong 25 and 50 mg Mn/kg/d groups relative to controls, the deficits appeared most extensive in the animals given the lower Mn dose. For example, the lifelong 25 mg Mn/kg/d group appeared the most impaired in maximum reaching distance (Fig. 3), as well as in the number of pellets taken and eaten from the moderate and more difficult to reach steps of the staircase (Figs. 4A, B, 5A, B). This pattern of impairment indicates that lifelong exposure to Mn at the lower dose not only reduced forelimb extension, but also impaired the execution of reaching and grasping/retrieval movements that were required to successfully take and eat pellets from the staircase. Consistent with this, the lifelong 25 mg Mn/kg/d group also misplaced a higher percent of pellets than the controls on steps 3-5 of the staircase (Fig. 4D).
The group exposed to the higher lifelong 50 mg Mn/kg/d dose also exhibited clear evidence of sensorimotor deficits in grasping/retrieval of pellets, but the deficits were generally restricted to the most difficult to reach steps 5 and 6 of the staircase and the larger (45 mg) pellet size. This group showed no measurable impairment in the maximum distance reached (Fig. 3), suggesting normal forelimb extension. Instead, the lifelong 50 mg Mn/kg/d group was significantly impaired relative to controls in the number of pellets taken and eaten from the most difficult steps of the staircase (Figs. 4A, B, and 5A, B). Consistent with this, the lifelong 50 mg Mn/kg/d group also misplaced a higher percent of pellets than the controls but only on step 5 (Fig. 4D). Notably, however, this group showed no impairment in staircase performance on the moderately difficult steps 3 and 4, in contrast to the performance deficits evident in the lower lifelong 25 mg Mn/kg/d group.
Collectively, these results point to significant disruption of the basal ganglia motor circuit from lifelong Mn exposure, in line with prior studies demonstrating abnormalities of digit flexion after neonatal dopamine depletion [26], neonatal hypoxia-ischemia [24], or unilateral nigrostriatal lesion generated in adulthood [12, 28]. The current results also show that the staircase test is a sensitive measure to detect different degrees of impairment in skilled forelimb use in adulthood following lifelong Mn exposure.
4.3 Early life versus lifelong Mn exposure differentially impairs skilled motor performance
We were particularly interested in determining whether lifelong Mn exposure produced similar or more severe deficits in skilled motor performance compared to early life Mn exposure alone. Specific contrasts between the early life and lifelong exposure groups showed that at the lower 25 mg Mn/kg/d dose, the lifelong Mn group performed significantly more poorly than their early life counterpart across performance outcomes and step levels. For instance, the lifelong 25 mg Mn/kg/d group was significantly more impaired in maximum reaching distance, as well as in the number of pellets taken and eaten across both pellet sizes. Notably too this group misplaced a higher percent of pellets on steps 4 and 5, compared to their early life Mn counterpart (Figs. 4A, B, D, 5A, B). Thus, at this lower Mn dose, lifelong oral Mn exposure produced significant widespread skilled motor deficits in adults, while the same exposure level restricted to the early life pre-weaning period produced no measurable impairment. This, again, appears to indicate that lifelong exposure to the lower 25 mg Mn/kg/d dose contributed to cumulative neurological damage incurred beyond the early life (pre-weaning) developmental period that resulted in the emergence of sensorimotor performance deficits in adulthood.
At the higher 50 mg Mn/kg/d dose, the impact of Mn exposure duration on skilled motor performance was different than at the lower Mn dose discussed above. For the most part, the impairment of the lifelong and the early life 50 mg Mn/kg/d groups was similar across performance measures and step level difficulty on the staircase. Both exposure groups extended their forelimb over similar maximum distances, and both took, ate, and misplaced a comparable number of pellets from the staircase steps (Fig. 3; Figs. 4A, B, D and 5A, B). Notably, there was some evidence that the lifelong 50 mg Mn/kg/d group had a significantly lower percent success (i.e., pellets eaten/taken) compared to their early life counterpart from steps 4-6 of the staircase, but this effect seems to reflect the trending higher percent success of the early life group over all treatment groups. Closer examination of the pellets eaten and taken data that make up the percent success outcome (i.e., % success = pellets eaten/taken × 100) shows that the early life 50 mg Mn/kg/d group was not impaired in the number of pellets eaten, but was impaired in the number of pellets taken from steps 4-6. In contrast, the lifelong Mn group ate proportionately fewer pellets of what they took. Thus, overall the continuous exposure to 50 mg Mn/kg/d in drinking water caused little additional impairment in skilled motor behavior beyond that produced by early life exposure at the same dose.
As noted above, the motor difficulties displayed by the Mn-exposed rats are reminiscent of the forelimb movement disorder described previously in pre-clinical models of mesotelencephalic dopamine dysfunction on rodent skilled motor behavior [12, 15, 16, 28]. In those studies adult rats administered unilateral or bilateral dopamine lesions showed clear deficits in the number of pellets taken and eaten from the staircase, and in reaching for a single pellet on a shelf, compared to sham-operated controls. These results suggest that deficits in skilled limb performance described herein in the Mn-exposed groups may have been mediated at least in part by the toxicity of Mn on dopamine neuromotor systems that regulate these functions in adulthood. Furthermore, the different patterns of impairment seen in the current study suggest that the nature and/or severity of dopamine neuromotor system dysfunction were different between the adult groups, depending upon the dosage and duration of developmental Mn exposure.
4.4. Conclusions
This study provides compelling new evidence that early life Mn exposure alone can cause long-lasting impairment in fine motor control of forelimb reaching and grasping/retrieval movements in adult rats. We demonstrate also that continuous postnatal Mn exposure from drinking water intake can give rise to additional widespread impairment in skilled forelimb function in adulthood. Additionally, because this enduring impact was quantifiable at the higher Mn dose and not at the lower dose tested, there may be a possible threshold for the enduring impact of early life Mn exposure on skilled motor behavior in adulthood. Further studies are needed to evaluate if non-motor functions mediated by dopamine systems (i.e., working memory and attention) also exhibit a similar long-lasting threshold effect in adult animals.
These findings substantiate the emerging clinical evidence in children showing associations between environmental Mn exposure and deficits in fine sensorimotor function. Most notably, Lucchini et al. [13] recently reported associations between environmental Mn exposure and impairment in the Luria-Nebraska motor coordination test and the Aiming Pursuit hand steadiness test, as well as associations between body Mn levels (hair Mn and blood Mn levels) and tremor intensity in the dominant hand in adolescents age 11–14 years. Impairments on these tests reflect deficits in motor coordination, hand dexterity, and difficulties in coordinating movements of the two hands and arms, and in making skillful actions to grasp, move, or assemble small objects (e.g., 5, 13), many of which are aspects of sensorimotor function also tapped by the modified Montoya staircase test used here. It is noteworthy, however, that other studies of children have shown only small and subtle motoric disturbances or normal psychomotor development related to elevated Mn exposure [6, 7, 23]. In light of our staircase results, however, these disparate findings in motor outcomes may be attributed, at least in part, to the limitations of these pediatric studies in characterizing the intensity and the duration of Mn exposure in developing children, since our results provide compelling evidence of a complex Mn exposure – neuromotor disorder relationship that varies across Mn exposure level, duration, and motor function outcome measure.
Highlights.
Early Mn exposure caused persistent impairments in reaching skill at the higher dose.
Lifelong Mn exposure produced widespread deficits in reaching and grasping skills at the lower dose.
Lifelong Mn exposure led to similar or more severe impairments than early exposure.
Acknowledgments
The authors would like to thank Javier Alvarado, Jade Fee, Ramon Garcia, Kyle Goetz, Mikayla Richter, Robin Rogue, and Monica Torres for their valuable assistance in staircase testing. We also thank Tom Jursa for analytical assistance, and Rachel Eastman, Richard Cathey, and Emma Hiolski for assistance in the study, and Barbara Strupp and Myla Strawderman for assistance with study design and data analyses. We also thank Dr. Philip Bushnell for providing the staircase devices that made this study possible. This research was funded by a grant from the National Institutes of Health (R01ES018990) to DRS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect. 2007;115:122–7. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouchard MF, Sauvé S, Barbeau B, Legrand M, Brodeur M-È, Bouffard T, et al. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect. 2011;119:138–43. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordova FM, Aguiar AS, Peres TV, Lopes MW, Gonçalves FM, Remor AP, et al. In vivo manganese exposure modulates Erk, Akt and Darpp-32 in the striatum of developing rats, and impairs their motor function. PloS one. 2012;7:e33057. doi: 10.1371/journal.pone.0033057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, et al. Neurobehavioral evaluation of rhesus monkey infants fed cow’s milk formula, soy formula, or soy formula with added manganese. Neurotoxicol Teratol. 2005;27:615–27. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 5.He P, Liu DH, Zhang GQ. Effects of high-level-manganese sewage irrigation on children’s neurobehavior. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese journal of preventive medicine] 1994;28:216–8. [PubMed] [Google Scholar]
- 6.Henn BC, Ettinger AS, Schwartz J, Téllez-Rojo MM, Lamadrid-Figueroa H, Hernández-Avila M, et al. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology. 2010;21:433–439. doi: 10.1097/ede.0b013e3181df8e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernández-Bonilla D, Schilmann A, Montes S, Rodríguez-Agudelo Y, Rodríguez-Dozal S, Solís-Vivanco R, et al. Environmental exposure to manganese and motor function of children in Mexico. Neurotoxicology. 2011;32:615–21. doi: 10.1016/j.neuro.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Kern CH, Smith DR. Preweaning Mn exposure leads to prolonged astrocyte activation and lasting effects on the dopaminergic system in adult male rats. Synapse. 2011;65:532–44. doi: 10.1002/syn.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kern CH, Stanwood GD, Smith DR. Preweaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse. 2010;64:363–78. doi: 10.1002/syn.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan K, Factor-Litvak P, Wasserman GA, Liu X, Ahmed E, Parvez F, et al. Manganese exposure from drinking water and children’s classroom behavior in Bangladesh. Environ Health Perspect. 2011;1003397:1501–1506. doi: 10.1289/ehp.1003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan K, Wasserman GA, Liu X, Ahmed E, Parvez F, Slavkovich V, et al. Manganese exposure from drinking water and children’s academic achievement. Neurotoxicology. 2012;33:91–7. doi: 10.1016/j.neuro.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kloth V, Klein A, Loettrich D, Nikkhah G. Colour-coded pellets increase the sensitivity of the staircase test to differentiate skilled forelimb performances of control and 6-hydroxydopamine lesioned rats. Brain Res bulletin. 2006;70:68–80. doi: 10.1016/j.brainresbull.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Lucchini RG, Guazzetti S, Zoni S, Donna F, Peter S, Zacco A, et al. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology. 2012;33:687–96. doi: 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menezes-Filho JA, Bouchard M, Sarcinelli P, de N, Moreira JC. Manganese exposure and the neuropsychological effect on children and adolescents: a review. Rev Panam Salud Publica. 2009;26:541–8. doi: 10.1590/s1020-49892009001200010. [DOI] [PubMed] [Google Scholar]
- 15.Miklyaeva EI, Castañeda E, Whishaw IQ. Skilled reaching deficits in unilateral dopamine-depleted rats: impairments in movement and posture and compensatory adjustments. J Neurosci. 1994;14:7148–58. doi: 10.1523/JNEUROSCI.14-11-07148.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991;36:219–28. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- 17.Moreno JA, Yeomans EC, Streifel KM, Brattin BL, Taylor RJ, Tjalkens RB. Age-dependent susceptibility to manganese-induced neurological dysfunction. Toxicol Sci. 2009;112:394–404. doi: 10.1093/toxsci/kfp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Research Council . Guide for the care and use of laboratory animals. 8th ed. National Academy Press; Washington D.C.: 2011. [Google Scholar]
- 19.Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, et al. Arsenic exposure and motor function among children in Bangladesh. Environ Health Perspect. 2011;119:1665–70. doi: 10.1289/ehp.1103548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horacio Riojas-Rodríguez, Rodolfo Solís-Vivanco, Astrid Schilmann, Sergio Montes, Rodríguez S, Camilo Ríos, et al. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ Health Perspect. 2010;118:1465–70. doi: 10.1289/ehp.0901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samsam TE, Gadrinab LG, Bushnell PJ. Toxicological evaluation of the staircase test for assessing fine motor movements. Neurotoxicol Teratol. 2003;26:113–20. doi: 10.1016/S0892-0362(03)00093-X. [DOI] [PubMed] [Google Scholar]
- 22.Smith DR, Osterloh JD, Niemeyer S, Flegal AR. Stable isotope labeling of lead compartments in rats with ultralow lead concentrations. Environ Res. 1992;57:190–207. doi: 10.1016/s0013-9351(05)80079-9. [DOI] [PubMed] [Google Scholar]
- 23.Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology. 2003;24:667–74. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 24.Tomimatsu T, Fukuda H, Endoh M, Mu J, Watanabe N, Kohzuki M, et al. Effects of neonatal hypoxic-ischemic brain injury on skilled motor tasks and brainstem function in adult rats. Brain Res. 2002;926:108–17. doi: 10.1016/s0006-8993(01)03311-x. [DOI] [PubMed] [Google Scholar]
- 25.Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, et al. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2006;114:124–9. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whishaw IQ, Funk DR, Hawryluk SJ, Karbashewski ED. Absence of sparing of spatial navigation, skilled forelimb and tongue use and limb posture in the rat after neonatal dopamine depletion. Physiol Behav. 1987;40:247–53. doi: 10.1016/0031-9384(87)90215-0. [DOI] [PubMed] [Google Scholar]
- 27.Whishaw IQ, Pellis SM, Gorny BP, Pellis VC. The impairments in reaching and the movements of compensation in rats with motor cortex lesions: an endpoint, videorecording, and movement notation analysis. Behav Brain Res. 1991;42:77–91. doi: 10.1016/s0166-4328(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 28.Whishaw IQ, Woodward NC, Miklyaeva E, Pellis SM. Analysis of limb use by control rats and unilateral DA-depleted rats in the Montoya staircase test: movements, impairments and compensatory strategies. Behav Brain Res. 1997;89:167–77. doi: 10.1016/s0166-4328(97)00057-0. [DOI] [PubMed] [Google Scholar]
- 29.Wolfinger R, O’Connell M. Generalized linear mixed models: a pseudolikelihood approach. J Stat Comput Simu. 1993;4:233–44. [Google Scholar]