Abstract
Context
Persistent pain is common following surgical treatment of breast cancer, but fairly little is known about the changes in sensory processing that accompany such pain syndromes.
Objectives
This study employed quantitative sensory testing (QST) to compare psychophysical responses to standardized noxious stimulation in two groups of women who had previously undergone breast cancer surgery: women with (n=37) and women without (n=34) persistent post-operative pain.
Methods
Participants underwent a single testing session in which responses to a variety of noxious stimuli were assessed.
Results
Findings suggested that women with chronic pain after breast cancer surgery display enhanced temporal summation of mechanical pain, deficits in endogenous pain inhibition, and more intense painful after-sensations compared with those without long-term pain. Some of these group differences were mediated by higher levels of pain catastrophizing in the group of women with persistent pain.
Conclusion
These findings suggest that persistent post-operative pain is associated with alterations in central nervous system pain-modulatory processes. Future treatment studies might benefit from targeting these pain-modulatory systems, and additional studies using functional neuroimaging methods might provide further valuable information about the pathophysiology of long-term post-surgical pain in women treated for breast cancer.
Keywords: hyperalgesia, lumpectomy, quantitative sensory testing, temporal summation, conditioned pain modulation, catastrophizing
Introduction
The development of chronic pain following breast surgery is strikingly common; reviews of the literature on mastectomy and lumpectomy indicate that persistent (i.e., lasting more than three months) post-breast surgery pain occurs in close to 50% of cases, at a variety of sites (1–4). In a recent, large study (over 3,000 women) with long-term (on average, over two years post-surgery) follow-up, a total of 47% of women continued to experience pain after breast cancer treatment (5). Patients report that pain is the most frequent and impairing symptom after breast surgery (6), and persistent pain has a substantial adverse impact on emotional and physical functioning and quality of life among women who have undergone surgical treatment of breast cancer (7–11). The advent of conservative breast surgery (i.e., lumpectomy), although having positive effects on body image (12), has not resulted in a reduction in the rates of persistent post-surgical pain (5,13,14). In fact, several reports even suggest higher rates of post-lumpectomy pain compared with post-mastectomy pain (15, 16). Indeed, some evidence suggests that breast surgery may even increase the experience of pain at distant muscle and joint sites, potentially via alterations in neuroendocrine or inflammatory systems, or direct sensitizing effects on the central nervous system (CNS) (17). The large, and increasing, numbers of annual breast surgeries (18, 19) dictate that millions of women may suffer from treatment-related pain following breast surgery, which historically has been undertreated (20).
Whereas individual differences in long-term, pain-related outcomes following breast cancer treatment are well-documented, it is not entirely clear what differentiates women who develop persistent post-operative pain from those who do not. One factor of interest comprises individual differences in pain sensitivity and pain modulation, evaluated by measuring responses to standardized noxious stimuli under highly controlled conditions (21–23). A number of surgical studies have used Quantitative Sensory Testing (QST) techniques to assess the relationship between basal pain sensitivity and outcomes such as acute post-operative pain. Following limb amputation (24), cholecystectomy (25), anterior cruciate ligament repair (26), gynecologic surgery (27), lower abdominal surgery (28), biopsy (29), and cesarean section (30;31), pre-surgical QST findings were significantly correlated with acute postoperative pain. In each case, individual differences reflecting greater sensitivity to pain (e.g., lower pain thresholds) were associated with more intense acute post-operative pain. Although similar studies of long-term post-operative pain are few, pre-surgical variability in thermal pain responses did predict six-month post-thoracotomy pain outcomes (32), and pre-operative mechanical hyperalgesia was associated with poorer pain-related outcomes at three months after shoulder surgery (33), and at six months post chest surgery (34).
To date, several QST studies have assessed women with post-mastectomy or post-lumpectomy pain (35). Most of these studies perform testing in the affected area of the body, generally testing the operated breast as well as the contralateral breast (36, 37). In general, these studies have noted the presence of hyperalgesia, allodynia, and enhanced temporal summation of pain in the surgical area, and one study (38) has reported lowered pressure pain thresholds at a variety of body sites, suggesting a process akin to central sensitization. Collectively, however, relatively little is known about pain modulation in women with long-lasting pain after breast cancer treatment, and even less is known about the relationship of these factors to psychosocial functioning. Numerous reports have documented the prevalence and impact of negative affective factors in women with persistent pain following breast cancer treatment (7, 13, 39), but these processes have not been studied as potential contributors to maladaptive central pain processing mechanisms. Cognitive and emotional factors such as catastrophizing are strongly related to enhanced pain sensitivity in both healthy adults and patients with chronic pain (40–44), but such psychosocial factors have not been studied as contributors to altered pain modulation in women with chronic pain after breast cancer treatment. Our goal in the present study was to compare matched groups of women with and without persistent pain after lumpectomy on QST measures of pain sensitivity and pain modulation, and to assess the potential contributory role of psychosocial factors to any observed group differences.
Methods
Study Design and Participants
This was a cross-sectional cohort study; 71 women were recruited who had been diagnosed with early-stage breast cancer and had undergone unilateral breast cancer surgery and radiation therapy at least one year prior to participation. All patients were cancer-free at the time of testing (i.e., T0N0M0) and were not undergoing current treatment. Patients were excluded from the study if they had undergone re-operation, had a diagnosis of peripheral neuropathy, a history of myocardial infarction, a chronic pain syndrome (e.g., low back pain, migraine) in which the pain was rated as moderate to severe in intensity, or cognitive limitations that precluded providing self-report data. Recruitment was conducted via local posting of electronic and print advertisements. Flyers were posted around the offices of oncologists and primary care physicians at Brigham & Women’s Hospital. Interested participants called in, and underwent a telephone-based screening before coming in for an initial study visit. Participants received $50 as compensation for their participation.
Questionnaires
Standard demographic information and medical and surgical history information was collected by self-report. Questionnaires administered included:
The Brief Pain Inventory (BPI) (45) is frequently recommended as a measure of pain severity and pain interference for patients with chronic malignant or nonmalignant pain (46).
The Beck Depression Inventory (BDI) is a well-validated, commonly-used, general measure of depressive symptomatology (47).
The Pain Catastrophizing Scale (PCS) (48) is a well-validated, widely-used, self-report measure of catastrophic thinking associated with pain (49). The PCS has good psychometric properties in pain patients and controls (50).
The 20-item version of the Pain Anxiety Symptoms Scale (PASS) (51) is a measure of fear and anxiety responses that are specific to pain. It is a well-validated and widely used instrument in studies of patients with and without chronic pain.
In addition to these questionnaire measures, participants rated fatigue on a 0–100 visual analogue scale, and rated their current pain intensity on a 0–10 numeric rating scale. Participants also responded to questions about their experience of post-surgical pain. These included “At its most intense, how would you rate the pain following surgery,” and “Are you still experiencing pain from the surgery”?
Session Protocol
Study subjects provided informed consent, and all procedures were approved by the Partners Institutional Review Board at Brigham & Women’s Hospital. Many of these procedures have been described in our previous studies (44, 52, 53). Ratings of current psychological stress (i.e., “How much stress are you feeling right now?”) and current resting clinical pain intensity ratings (on a 0–10 scale) were obtained prior to the testing session, and current verbal ratings of anxiety (on a 0–10 scale, with “no anxiety” and “severe anxiety” as the respective anchors) were obtained during the testing session (44). During the session, subjects were seated comfortably in a reclining chair. First, resting blood pressure and heart rate were assessed, after which participants underwent the psychophysical testing procedures described below:
Psychophysical Pain Testing
First, contact heat stimuli were delivered using a contact thermode (Medoc Advanced Medical Systems, Ramat Yishai, Israel). We used a 9 cm2 thermode applied to the volar forearm, and followed an ascending method of limits paradigm with a rate of rise of 0.5°C/second. Thermal assessment included sampling of warmth and cool thresholds, followed by heat pain thresholds (HPTh) and cold pain thresholds (CPTh), followed by heat pain tolerance (HPTo), all tested on the ventral forearm (44, 54).
Mechanical pain thresholds were assessed using a digital pressure algometer (Somedic, Sollentuna, Sweden). Pressure pain thresholds (PPThs) were determined twice, bilaterally at the trapezius muscle and the metacarpophalangeal joint of the thumb. At each site, mechanical force was applied using a 0.5 cm2 probe covered with polypropylene pressure-transducing material; pressure was increased at a steady rate of 30 kPA/second until the subject indicated that the pressure was “first perceived as painful.” Participants then underwent an assessment of mechanical temporal summation using weighted pinprick stimulators, as in a previous study (44). The lowest-force stimulator that produced a sensation of discomfort (128 or 256 mN for most subjects) was used to apply a train of 10 stimuli to the skin on the dorsum of the hand at the rate of one per second. Participants rated the painfulness of the first, fifth, and tenth stimulus, and also rated any ongoing pain after-sensations 15 seconds following the final stimulus.
Reaction to prolonged pressure pain was ascertained via cuff pressure algometry (CPA). In brief, tonic, deep-tissue, mechanical stimulation is applied using a pneumatic tourniquet cuff, which is inflated to and maintained at a particular pressure (55). The present protocol utilized a Hokanson rapid cuff inflator (D.E. Hokanson, Inc., Bellevue, WA); a standard blood pressure cuff was wrapped comfortably around the lower leg, over the gastrocnemius muscle. A computer-controlled air compressor maintained the pressure at a level that was individually tailored for each subject, to produce a pain intensity rating of 40/100. Cuff inflation was maintained for two minutes, and subjects rated pain intensity at 30-second intervals.
Finally, responses to noxious cold were evaluated using a repeated cold pressor task (CPT), involving immersion of the right hand in a circulating cold water bath maintained at 4°C. In the present protocol, participants underwent a series of three cold pressor tasks, with the first two comprising serial immersions of the right hand for 30 seconds, with two minutes between immersions. The third and final CPT involved an immersion of the right hand lasting until a participant reached pain tolerance (or a three-minute maximum). Participants rated the intensity of the cold pain on a 0–100 scale (“no pain” to “most intense pain imaginable”) at the midpoint of each CPT.
During the first two cold pressor tasks, we also assessed conditioned pain modulation (CPM), a non-invasive test of endogenous pain-inhibitory systems using a heterotopic noxious conditioning stimulation paradigm (56, 57). CPM depends on opioid-mediated supraspinal mechanisms (58), is a sensitive measure of deficits in pain modulation in fibromyalgia and related disorders (59), and has previously been shown to predict the development of long-term clinical pain (32). In the present protocol, during each CPT, PPTh was assessed on the contralateral trapezius. CPM was quantified as percent change in PPTh during the cold pressor tasks relative to baseline PPTh, with an increase in PPTh being expected. Cold pain intensity ratings (0–100) also were obtained at 30-second intervals following each of the cold pressor tests.
Data Analysis
Participants were classified as having (n=37) or not having (n=34) persistent postoperative pain on the basis of their responses to the question “Are you still experiencing pain from the surgery?” as well as their BPI responses. Simple between-group comparisons were made using Chi-square tests (in the case of categorical variables) or t-tests (in the case of continuous variables). The majority of QST variables were found to deviate significantly (P< 0.05) from normality using the Kolmogorov-Smirnov test; consequently, group comparisons on QST variables were conducted using Mann-Whitney U tests. Mediational analyses were conducted using Sobel’s test, a widely-used and recommended method for testing mediation (60, 61). Statistical mediation refers to a case in which one variable (the mediator) “accounts for” the association between an independent/predictor variable and a criterion variable (62, 63). The Sobel test determines whether, when the mediator is included in a regression analysis model with the independent variable, the effect of the independent variable is significantly reduced while the effect of the mediator remains significant. In the present study, we examined whether psychosocial variables that differed between groups statistically “explained” a significant portion of observed group differences in QST responses. Such tests of mediation are commonly performed in cross-sectional studies such as this one, although the design precludes actual causal interpretations. All analyses were performed using SPSS v. 19 (SPSS Inc., Chicago, IL). The sample sizes in prior QST studies of pain responses in women with and without post-lumpectomy pain have ranged from n= 26 (36) to n= 82 (38); based on the moderate to large effect sizes observed in those studies, we estimated that a sample size of n= 72 would provide power of at least 0.80 to detect group differences in pain responses. Alpha was set to α= 0.05 for the study. We elected not to adjust for multiple comparisons, following the convention in other QST studies (44, 64, 65), even those with very large sample sizes (64). While noting that Type II errors can be just as important as Type I errors (66), other researchers have highlighted the overly-conservative nature of correction procedures such as Bonferroni when testing correlated outcomes (67). It is clear from a number of prior studies that there are strong inter-relationships among QST variables (64, 68, 69), and we have, therefore, not applied such (over-conservative) correction methods here.
Results
Questionnaire Data
Women with (n=37) and without (n=34) persistent post-operative pain did not differ (all Ps> 0.2) on demographic variables such as age, ethnicity, education, etc. Similarly, the groups did not differ (Ps> 0.3) on surgery-related variables such as the length of time since surgery, or the operative side. No group differences were evident in the proportion of women who reported having received hormonal treatment (e.g., tamoxifen) (Table 1). All participants in both groups were cancer-free at the time of testing, and none were receiving ongoing chemotherapy or radiation. The groups also did not differ in the use of nonsteroidal anti-inflammatory drugs (NSAIDs) (62% in the group of women with long-term pain, 56% in the group without), and few patients were taking opioid medications (8% in the group with long-term pain, a single patient, representing 3%, in the group without). Group differences were observed on measures of participants’ recall of the maximum intensity of acute post-operative pain, as well as on measures of current pain, pain-related interference, and fatigue, with elevated reports of pain, interference, and fatigue in the group of women with chronic post-lumpectomy pain (Ps< 0.05; Table 1). On affective measures, no significant group differences were observed for depressive symptoms or pain-related anxiety (Ps > 0.2); however, the group of women with chronic post-surgical pain did report higher levels of catastrophizing (P< 0.01).
Table 1.
Comparison of Women With and Without Chronic Post-Surgical Pain
| Chronic Pain (n=37) | No Chronic Pain (n=34) | |
|---|---|---|
| Demographics | ||
| Age | 57.1 ± 8.4 | 59.0 ± 11.1 |
| % Married | 54.1% | 58.8% |
| % White | 73.0% | 85.3% |
| % Employed | 51.4% | 58.9% |
| % Post-Secondary Degree | 78.4% | 85.3% |
| Surgery- and Pain-Related Variables | ||
| Years Since Surgery | 7.4 ± 6.4 | 7.8 ± 6.4 |
| % Right Side Operated | 45.9% | 47.1% |
| % Hormone Therapy | 59.5% | 61.8% |
| Post-op Pain Recall (0–10) | 6.6 ± 3.2 a | 5.0 ± 3.0 |
| BPI Pain Severity | 3.6 ± 2.6 b | 1.1 ± 1.9 |
| BPI Pain Interference | 3.1 ± 2.7 b | 1.0 ± 1.9 |
| Past Week Fatigue (0–100) | 49.8 ± 32.9 b | 26.4 ± 25.5 |
| Psychosocial Factors | ||
| BDI | 11.2 ± 8.1 | 8.7 ± 8.5 |
| PASS | 27.2 ± 19.2 | 20.4 ± 19.6 |
| PCS | 18.4 ± 11.7 b | 8.6 ± 8.8 |
Data are presented as mean ± SD or as percentages.
BPI= Brief Pain Inventory; BDI= Beck Depression Inventory; PASS= Pain Anxiety Symptoms Scale; PCS= Pain Catastrophizing Scale.
P<0.05 for the group comparison.
P<0.01 for the group comparison.
In-Session Data
As expected, verbal reports of current clinical pain (0–10 pain intensity ratings) at the beginning of the QST session were higher in women with persistent post-operative pain (P< 0.001; Table 2). However, blood pressure, heart rate, 0–10 verbal ratings of current stress, and 0–10 verbal ratings of current anxiety were similar between the groups (Ps> 0.2).
Table 2.
Comparison of Psychophysical Responses Among Women With and Without Chronic Post-Surgical Pain
| Chronic Pain (n=37) Mean±SD |
No Chronic Pain (n=34) Mean±SD |
|
|---|---|---|
| Variables of Interest Assessed During the QST Session | ||
| MAP | 96.2 ± 16.1 | 94.5 ± 13.4 |
| HR | 73.0 ± 10.5 | 75.2 ± 13.9 |
| Current Stress (0–10) | 1.8 ± 2.5 | 1.3 ± 2.0 |
| Clinical Pain (0–10) | 3.3 ± 2.7 a | 0.6 ± 1.5 |
| Anxiety during QST (0–10) | 0.7 ± 1.1 | 0.9 ± 1.4 |
| Mechanical and Cold Pressor Responses | ||
| PPTh (KPa)- Trapezius | 312.1 ± 94.4 | 320.8 ± 122.8 |
| PPTh (KPa)- Thumb | 331.8 ± 116.6 | 316.2 ± 111.5 |
| Cuff Pressure (mmHg) | 138.3 ± 44.6 | 145.5 ± 60.4 |
| Cuff Pain (0–100) at 2 Min | 44.7 ± 25.1 | 38.3 ± 23.3 |
| Cold Pain Tolerance (sec) | 47.3 ± 47.0 | 53.0 ± 47.5 |
| CPM Index | 116.0 ± 32.1 b | 138.4 ± 30.1 |
| Other Mechanical and Cold Pressor Data Appear in Figures 1 & 2 | ||
| Thermal Data (in °C) | ||
| Chronic Pain (n=29) | No Chronic Pain (n=27) | |
| Warmth Threshold | 33.8 ± 1.2 | 33.8 ± 0.9 |
| Cool Threshold | 29.3 ± 1.6 | 29.7 ± 1.0 |
| HPTh | 40.5 ± 3.8 | 41.3 ± 3.2 |
| CPTh | 13.2 ± 9.2 | 14.8 ± 9.1 |
| HPTo | 45.1 ± 2.9 | 46.1 ± 2.2 |
QST= Quantitative Sensory Testing; MAP=mean arterial pressure; HR= heart rate; PPTh= Pressure Pain Threshold; KPa= kilopascales; CPM= Conditioned Pain Modulation; HPTh= Heat Pain Threshold; CPTh= Cold Pain Threshold; HPTo= Heat Pain Tolerance.
P<0.01 for the group comparison.
P<0.05 for the group comparison.
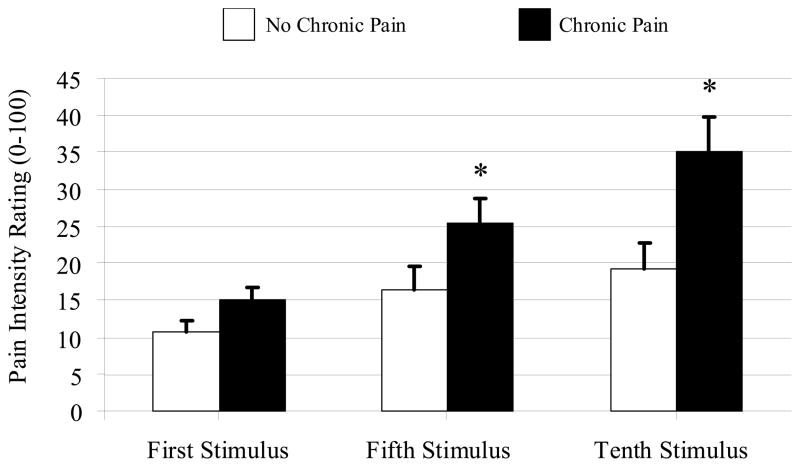
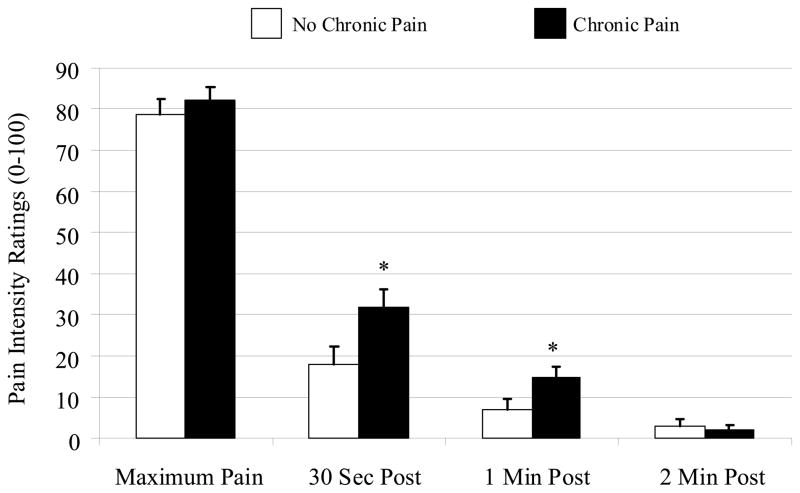
Thermal data were not available for 15 participants because of equipment failure. The two groups did not differ in the proportion of subjects for whom data were not available, and analysis of the 56 subjects with thermal data revealed no group differences in warm or cool threshold, and heat or cold pain threshold (Ps> 0.1). The Mann-Whitney U test for heat pain tolerance approached significance (P= 0.06). In addition, no group differences were found for pressure pain thresholds, for cuff pressure, or for cuff pain ratings (Ps> 0.4), but the chronic pain group did demonstrate increased temporal summation of mechanical pain (P< 0.05), with group differences in pain ratings increasing from the first to the fifth to the tenth punctate stimulus (Fig. 1). Mann-Whitney U tests revealed that the groups did not differ in their rating of the first stimulus (P> 0.05), but that the chronic pain group had higher pain ratings for the fifth (P< 0.05) and tenth (P= 0.01) stimuli. When the groups were compared on ratings of cold pressor pain, no differences emerged for maximum pain ratings, or for cold pain tolerance, but the chronic pain group did rate higher levels of after-sensation pain at 30 and 60 seconds following the termination of the cold pressor water immersion (Ps≤ 0.01). Moreover, the CPM Index (i.e., the ratio of PPTh during cold pressor to baseline PPTh, multiplied by 100) was higher (i.e., a greater increase in PPTh during the cold pressor test, representing a larger CPM effect) in women without long-term post-surgical pain (P= 0.009).
Fig. 1.
Pain ratings (0–100) for repetitive punctuate mechanical stimuli (data presented as means ± SEM). * Groups differ significantly at P< 0.05.
Mediation
Finally, using Sobel’s test, we evaluated whether group differences in catastrophizing mediated group differences in CPM, mechanical temporal summation, or cold pain after-sensations. No mediation was observed for CPM or temporal summation effects, but for cold after-sensations, partial mediation was observed (Sobel’s Z= 2.0, P< 0.05); higher catastrophizing was associated with elevated cold pain after-sensations, and group differences in painful after-sensations were rendered nonsignificant when controlling for catastrophizing.
Discussion
The present findings indicate differences in central pain modulation between women experiencing chronic pain after breast cancer surgery and women who underwent similar surgeries but did not develop chronic pain. Enhanced temporal summation and painful after-sensations in women reporting chronic pain, along with decrements in CPM, may indicate a relative predominance of facilitation over inhibition in central pain processing. The specificity of these effects (e.g., the groups did not differ in various pain threshold measures) makes it unlikely that explanations such as response bias might underlie the observed group differences. Higher levels of catastrophizing among the women with chronic pain did account for group differences in painful after-sensations, but not for the reduction of CPM and amplification of temporal summation observed in the group with chronic pain. Whether such differences in pain-modulatory processes represent pre-existing risk factors that contribute to the development and maintenance of chronic pain after surgery, or whether they are the consequence of chronic pain, is currently unknown, although at least one study has suggested that pre-operative CPM strongly predicts risk for chronic post-surgical pain (32). Longitudinal studies will be necessary to answer this question definitely, and to determine whether interventions that impact pain-modulatory processes can alter the risk or impact of persistent post-operative pain.
It is certainly possible that, for many patients, the surgical injury itself may have induced changes in pain processing. Indeed, the prevailing theory is that the development of persistent pain after an injury such as surgery is the result of plastic events/sensitization of the central and peripheral nervous systems (14,70,71). There is extensive evidence from animal models of persistent pain that an inciting stimulus (chemical, inflammatory, or mechanical injury) can alter expression of proteins at the cellular level, thus changing the phenotype and function of both peripheral and central nociceptive neurons and glial cells (72,73). Human studies also have revealed that tissue injury and surgery can produce widespread sensitization and hyperalgesia (74). Thus, the observed group differences in QST-assessed pain responses may be attributable to post-operative, maladaptive plastic changes in CNS pain processing in women with persisting pain following surgery. Then again, well over a dozen studies have now documented associations between pre-operative individual differences in pain sensitivity/modulation and post-operative pain outcomes (71,75,76). Indeed, such inter-individual variability may reflect genetic variation that contributes to both laboratory-assessed responses to standardized noxious stimuli and to risk for the development of chronic pain (77). Of course, these interpretations are not mutually exclusive, and it seems probable to us that both preoperative characteristics and post-operative changes would likely have contributed to shaping the group differences we observed in the present sample.
Although we do not have data that bear directly on the neurophysiological mechanisms that may underpin group differences in pain responses, prior studies have hinted at the neurochemical systems that may be involved in mediating CPM and temporal summation. CPM, a sensitive measure of deficits in pain modulation in fibromyalgia and related disorders (59), appears to depend on endogenous opioid-mediated supraspinal mechanisms (58,78). Temporal summation, an analogue of central sensitization, represents an important pathophysiological process that contributes to the development and maintenance of pain states in a number of clinical contexts (79–82). Whereas the temporal summation of pain involves processes at the spinal level, recent functional neuroimaging studies have highlighted the clear contribution of supraspinal processes as well (83–85). Collectively, the modulation of temporal summation appears to involve the activity of descending pain-inhibitory systems, which are known to play crucial roles in pain processing (86). In prior studies, medications such as N-methyl-D-aspartic acid antagonists, gamma-aminobutyric acid agonists, and opioids have been shown to reduce temporal summation of pain (87–91). Interestingly, recent investigations of effective multimodal treatments for pain after lumpectomy or mastectomy have included combination therapies that impact these neurotransmitter systems (92, 93). Future work in this area may benefit from evaluating whether these psychophysical indices of pain modulation could serve as early markers of treatment response.
The finding that catastrophizing mediated some of the group differences in pain modulation is perhaps not surprising. Prior investigations of variability in post-operative pain had also identified psychological variables that contributed significantly to the prediction of at-risk patients (94–97). We found that elevated reports of painful cold after-sensations in the group of women with persistent pain were explained by higher levels of catastrophizing. Numerous studies have indentified painful after-sensations (i.e., prolongation of pain ratings following the cessation of a noxious stimulus) as indices of central sensitization (73) in patients with orofacial pain (64,98), fibromyalgia (99,100), and even in patients without chronic pain (101), and the present findings suggest that affective factors such as catastrophizing may contribute directly to these effects. Our group has previously summarized an expanding literature documenting the breadth of catastrophizing’s maladaptive effects (49). Indeed, catastrophizing has been linked with enhanced temporal summation and impaired pain inhibition in prior studies (41,42,59,102), although in the present sample, catastrophizing only mediated group differences in painful after-sensations. Catastrophizing has been associated with greater post-operative use of opioid analgesics after a painful surgery (103), as well as with reduced acute analgesic benefit of opioids in a laboratory setting (104). Functional neuroimaging studies have suggested that elevated catastrophizing is related to enhanced activation of pain-processing brain areas such as anterior cingulate cortex and amygdala during the administration of calibrated noxious stimuli (105–107), potentially reflecting a deficit in endogenous opioid-mediated pain inhibition.
A number of limitations to the current study should be noted. We do not have pre-operative data available, having evaluated participants many years after their surgeries. It would have been preferable to collect prospective data, including a pre-surgical application of our QST battery, and detailed treatment-related information (for example, we do not have precise data on past exposure to chemotherapeutic agents as part of subjects’ previous breast cancer treatment). Because of the cross-sectional design of this study, we are unable to determine whether the observed group differences pre-dated the surgery, or were created by the surgery and subsequent experience of persisting post-operative pain. Because the average time since the surgery was over seven years, recall of pre- and peri-surgical experiences is unlikely to be highly reliable. It is also possible that, because recruitment for the study involved self-selection, differential motivations for responding to the advertisement may have been present for the two groups, although such factors did not manifest in any detectable demographic difference between groups. In addition, it is important to note that medication usage did not appear substantially different between the groups In particular, NSAID use was similar in the chronic pain and pain-free groups, and only a few patients were taking opioids, highlighting the relatively mild nature of the clinical pain in this study. Since chronic opioid administration has been associated with decrements in CPM (108), this is an important consideration. The lack of complete thermal data is a complicating factor in the interpretation of this study’s findings. Although we did not find significant group differences in thermal pain threshold or tolerance, reductions in power because of a smaller sample size likely contributed to this negative finding. Finally, the present study was restricted to lumpectomy patients; the inclusion of mastectomy patients, although potentially adding variability to the sample, would have permitted examination of differences between surgical approaches, as in some other recent (non-QST) reports (5). Despite these limitations, the current report adds to a small body of literature documenting alterations in pain responses among women with post-mastectomy or post-lumpectomy pain (35–38). The present findings are among the first to document alterations in pain-modulatory processes rather than pain threshold measures, and, to our knowledge, this is the first study to indicate that some of the observed changes in pain responses may be attributable to psychosocial characteristics such as catastrophizing.
Fig. 2.
Cold pain ratings during and after cold pressor testing (data presented as means ± SEM). * Groups differ significantly at P≤ 0.01.
Acknowledgments
This work was supported by National Institutes of Health grant R21 CA120500 (RRE).
Footnotes
Disclosures
None of the authors have any financial or other conflicts of interest with regard to this study or its findings.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93:1123–1133. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 2.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12:725–746. doi: 10.1016/j.jpain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Vadivelu N, Schreck M, Lopez J, Kodumudi G, Narayan D. Pain after mastectomy and breast reconstruction. Am Surg. 2008;74:285–296. [PubMed] [Google Scholar]
- 4.Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005;119:16–25. doi: 10.1016/j.pain.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Gartner R, Jensen MB, Nielsen J, et al. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302:1985–1992. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 6.Lauridsen MC, Overgaard M, Overgaard J, Hessov IB, Cristiansen P. Shoulder disability and late symptoms following surgery for early breast cancer. Acta Oncol. 2008;47:569–575. doi: 10.1080/02841860801986627. [DOI] [PubMed] [Google Scholar]
- 7.Kudel I, Edwards RR, Kozachik S, et al. Predictors and consequences of multiple persistent postmastectomy pains. J Pain Symptom Manage. 2007;34:619–627. doi: 10.1016/j.jpainsymman.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Karki A, Simonen R, Malkia E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37:180–188. doi: 10.1080/16501970410024181. [DOI] [PubMed] [Google Scholar]
- 9.Green CR, Hart-Johnson T, Loeffler DR. Cancer-related chronic pain: examining quality of life in diverse cancer survivors. Cancer. 2011;117:1994–2003. doi: 10.1002/cncr.25761. [DOI] [PubMed] [Google Scholar]
- 10.Peuckmann V, Ekholm O, Rasmussen NK, et al. Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. Eur J Pain. 2009;13:478–485. doi: 10.1016/j.ejpain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Peuckmann V, Ekholm O, Rasmussen NK, et al. Health-related quality of life in long-term breast cancer survivors: nationwide survey in Denmark. Breast Cancer Res Treat. 2007;104:39–46. doi: 10.1007/s10549-006-9386-6. [DOI] [PubMed] [Google Scholar]
- 12.Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Holzel D. Quality of life following breast-conserving therapy or mastectomy: results of a 5-year prospective study. Breast J. 2004;10:223–231. doi: 10.1111/j.1075-122X.2004.21323.x. [DOI] [PubMed] [Google Scholar]
- 13.Rief W, Bardwell WA, Dimsdale JE, et al. Long-term course of pain in breast cancer survivors: a 4-year longitudinal study. Breast Cancer Res Treat. 2011;130:579–586. doi: 10.1007/s10549-011-1614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 15.Caffo O, Amichetti M, Ferro A, et al. Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat. 2003;80:39–48. doi: 10.1023/A:1024435101619. [DOI] [PubMed] [Google Scholar]
- 16.Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6:453–459. doi: 10.1093/oxfordjournals.annonc.a059215. [DOI] [PubMed] [Google Scholar]
- 17.Andrykowski MA, Curran SL, Carpenter JS, et al. Rheumatoid symptoms following breast cancer treatment: a controlled comparison. J Pain Symptom Manage. 1999;18:85–94. doi: 10.1016/s0885-3924(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 18.Golub RM, Bennett CL, Stinson T, Venta L, Morrow M. Cost minimization study of image-guided core biopsy versus surgical excisional biopsy for women with abnormal mammograms. J Clin Oncol. 2004;22:2430–2437. doi: 10.1200/JCO.2004.06.154. [DOI] [PubMed] [Google Scholar]
- 19.Tatrow K, Montgomery GH, Avellino M, Bovbjerg DH. Activity and sleep contribute to levels of anticipatory distress in breast surgery patients. Behav Med. 2004;30:85–91. doi: 10.3200/BMED.30.2.85-94. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter JS, Andrykowski MA, Sloan P, et al. Postmastectomy/postlumpectomy pain in breast cancer survivors. J Clin Epidemiol. 1998;51:1285–1292. doi: 10.1016/s0895-4356(98)00121-8. [DOI] [PubMed] [Google Scholar]
- 21.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10:556–572. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Pavlakovic G, Petzke F. The role of quantitative sensory testing in the evaluation of musculoskeletal pain conditions. Curr Rheumatol Rep. 2010;12:455–461. doi: 10.1007/s11926-010-0131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Backonja MM, Walk D, Edwards RR, et al. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009;25:641–647. doi: 10.1097/AJP.0b013e3181a68c7e. [DOI] [PubMed] [Google Scholar]
- 24.Nikolajsen L, Ilkjaer S, Jensen TS. Relationship between mechanical sensitivity and postamputation pain: a prospective study. Eur J Pain. 2000;4:327–334. doi: 10.1053/eujp.2000.0194. [DOI] [PubMed] [Google Scholar]
- 25.Bisgaard T, Klarskov B, Rosenberg J, Kehlet H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain. 2001;90:261–269. doi: 10.1016/S0304-3959(00)00406-1. [DOI] [PubMed] [Google Scholar]
- 26.Werner MU, Duun P, Kehlet H. Prediction of postoperative pain by preoperative nociceptive responses to heat stimulation. Anesthesiology. 2004;100:115–119. doi: 10.1097/00000542-200401000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Granot M, Zimmer EZ, Friedman M, Lowenstein L, Yarnitsky D. Association between quantitative sensory testing, treatment choice, and subsequent pain reduction in vulvar vestibulitis syndrome. J Pain. 2004;5:226–232. doi: 10.1016/j.jpain.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Hsu YW, Somma J, Hung YC, et al. Predicting postoperative pain by preoperative pressure pain assessment. Anesthesiology. 2005;103:613–618. doi: 10.1097/00000542-200509000-00026. [DOI] [PubMed] [Google Scholar]
- 29.Soyupek S, Bozlu M, Armagan A, Ozorak A, Perk H. Does experimental pain assessment before biopsy predict for pain during transrectal ultrasound-guided prostate biopsy? Urology. 2007;70:681–684. doi: 10.1016/j.urology.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Granot M, Lowenstein L, Yarnitsky D, Tamir A, Zimmer EZ. Postcesarean section pain prediction by preoperative experimental pain assessment. Anesthesiology. 2003;98:1422–1426. doi: 10.1097/00000542-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen PR, Norgaard L, Rasmussen LS, Kehlet H. Prediction of post-operative pain by an electrical pain stimulus. Acta Anaesthesiol Scand. 2007;51:582–586. doi: 10.1111/j.1399-6576.2007.01271.x. [DOI] [PubMed] [Google Scholar]
- 32.Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 33.Gwilym SE, Oag HC, Tracey I, Carr AJ. Evidence that central sensitisation is present in patients with shoulder impingement syndrome and influences the outcome after surgery. J Bone Joint Surg Br. 2011;93:498–502. doi: 10.1302/0301-620X.93B4.25054. [DOI] [PubMed] [Google Scholar]
- 34.Lautenbacher S, Huber C, Schofer D, et al. Attentional and emotional mechanisms related to pain as predictors of chronic postoperative pain: a comparison with other psychological and physiological predictors. Pain. 2010;151:722–731. doi: 10.1016/j.pain.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 35.Werner MU, Mjobo HN, Nielsen PR, Rudin A. Prediction of postoperative pain: a systematic review of predictive experimental pain studies. Anesthesiology. 2010;112:1494–1502. doi: 10.1097/ALN.0b013e3181dcd5a0. [DOI] [PubMed] [Google Scholar]
- 36.Gottrup H, Andersen J, Arendt-Nielsen L, Jensen TS. Psychophysical examination in patients with post-mastectomy pain. Pain. 2000;87:275–284. doi: 10.1016/S0304-3959(00)00291-8. [DOI] [PubMed] [Google Scholar]
- 37.Vilholm OJ, Cold S, Rasmussen L, Sindrup SH. Sensory function and pain in a population of patients treated for breast cancer. Acta Anaesthesiol Scand. 2009;53:800–806. doi: 10.1111/j.1399-6576.2009.01938.x. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-Lao C, Cantarero-Villanueva I, Fernandez-de-Las-Penas C, et al. Widespread mechanical pain hypersensitivity as a sign of central sensitization after breast cancer surgery: comparison between mastectomy and lumpectomy. Pain Med. 2011;12:72–78. doi: 10.1111/j.1526-4637.2010.01027.x. [DOI] [PubMed] [Google Scholar]
- 39.Torer N, Nursal TZ, Caliskan K, et al. The effect of the psychological status of breast cancer patients on the short-term clinical outcome after mastectomy. Acta Chir Belg. 2010;110:467–470. doi: 10.1080/00015458.2010.11680657. [DOI] [PubMed] [Google Scholar]
- 40.Vase L, Nikolajsen L, Christensen B, et al. Cognitive-emotional sensitization contributes to wind-up-like pain in phantom limb pain patients. Pain. 2011;152:157–162. doi: 10.1016/j.pain.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22:730–737. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- 42.Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Exp Brain Res. 2008;186:79–85. doi: 10.1007/s00221-007-1206-7. [DOI] [PubMed] [Google Scholar]
- 43.Geisser ME, Casey KL, Brucksch CB, et al. Perception of noxious and innocuous heat stimulation among healthy women and women with fibromyalgia: association with mood, somatic focus, and catastrophizing. Pain. 2003;102:243–250. doi: 10.1016/S0304-3959(02)00417-7. [DOI] [PubMed] [Google Scholar]
- 44.Edwards RR, Wasan AD, Michna E, et al. Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. J Pain. 2011;12:953–963. doi: 10.1016/j.jpain.2011.02.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- 48.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 49.Edwards RR, Calahan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011;7(4):216–224. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- 50.Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain. 2002;96:319–324. doi: 10.1016/S0304-3959(01)00463-8. [DOI] [PubMed] [Google Scholar]
- 51.McCracken LM, Dhingra L. A short version of the Pain Anxiety Symptoms Scale (PASS-20): preliminary development and validity. Pain Res Manag. 2002;7:45–50. doi: 10.1155/2002/517163. [DOI] [PubMed] [Google Scholar]
- 52.Edwards RR, Kronfli T, Haythornthwaite JA, et al. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140:135–144. doi: 10.1016/j.pain.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edwards RR, Wasan AD, Bingham CO, III, et al. Enhanced reactivity to pain in patients with rheumatoid arthritis. Arthritis Res Ther. 2009;11:R61. doi: 10.1186/ar2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edwards RR, Haythornthwaite JA, Sullivan MJ, Fillingim RB. Catastrophizing as a mediator of sex differences in pain: differential effects for daily pain versus laboratory-induced pain. Pain. 2004;111:335–341. doi: 10.1016/j.pain.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 55.Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol. 2010;6(10):599–606. doi: 10.1038/nrrheum.2010.107. [DOI] [PubMed] [Google Scholar]
- 56.Yarnitsky D, Arendt-Nielsen L, Bouhassira D, et al. Recommendations on terminology and practice of psychophysical DNIC testing [letter] Eur J Pain. 2010;14:339. doi: 10.1016/j.ejpain.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23:611–615. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]
- 58.Sprenger C, Bingel U, Buchel C. Treating pain with pain: supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. Pain. 2011;152:428–439. doi: 10.1016/j.pain.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 59.van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain. 2010;11:408–419. doi: 10.1016/j.jpain.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 60.MacKinnon DP, Fairchild AJ. Current directions in mediation analysis. Curr Dir Psychol Sci. 2009;18:16. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baron R, Kenny D. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Personal Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 63.Judd CM, Kenny DA, McClelland GH. Estimating and testing mediation and moderation in within-subject designs. Psychol Methods. 2001;6:115–134. doi: 10.1037/1082-989x.6.2.115. [DOI] [PubMed] [Google Scholar]
- 64.Greenspan JD, Slade GD, Bair E, et al. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA Case Control Study. J Pain. 2011;12:T61–T74. doi: 10.1016/j.jpain.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rahim-Williams B, Riley JL, III, Williams AK, Fillingim RB. A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Med. 2012;13(4):522–540. doi: 10.1111/j.1526-4637.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blakesley RE, Mazumdar S, Dew MA, et al. Comparisons of methods for multiple hypothesis testing in neuropsychological research. Neuropsychology. 2009;23:255–264. doi: 10.1037/a0012850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhalang K, Sigurdsson A, Slade GD, Maixner W. Associations among four modalities of experimental pain in women. J Pain. 2005;6:604–611. doi: 10.1016/j.jpain.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Hastie BA, Riley JL, III, Robinson ME, et al. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116:227–237. doi: 10.1016/j.pain.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 70.McGreevy K, Bottros MM, Raja SN. Preventing chronic pain following acute pain: risk factors, preventive strategies, and their efficacy. Eur J Pain Suppl. 2011;5:365–372. doi: 10.1016/j.eujps.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilder-Smith OH. Chronic pain and surgery: a review of new insights from sensory testing. J Pain Palliat Care Pharmacother. 2011;25:146–159. doi: 10.3109/15360288.2010.505256. [DOI] [PubMed] [Google Scholar]
- 72.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilder-Smith OH, Schreyer T, Scheffer GJ, Arendt-Nielsen L. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: a pilot study. J Pain Palliat Care Pharmacother. 2010;24:119–128. doi: 10.3109/15360281003706069. [DOI] [PubMed] [Google Scholar]
- 75.Abrishami A, Chan J, Chung F, Wong J. Preoperative pain sensitivity and its correlation with postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2011;114:445–457. doi: 10.1097/ALN.0b013e3181f85ed2. [DOI] [PubMed] [Google Scholar]
- 76.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12:725–746. doi: 10.1016/j.jpain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 77.Diatchenko L, Slade GD, Nackley AG, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 78.Willer JC, Le Bars D, De Broucker T. Diffuse noxious inhibitory controls in man: involvement of an opioidergic link. Eur J Pharmacol. 1990;182:347–355. doi: 10.1016/0014-2999(90)90293-f. [DOI] [PubMed] [Google Scholar]
- 79.Eide PK. Wind-up and the NMDA receptor complex from a clinical perspective. Eur J Pain. 2000;4:5–15. doi: 10.1053/eujp.1999.0154. [DOI] [PubMed] [Google Scholar]
- 80.Stubhaug A, Breivik H, Eide PK, Kreunen M, Foss A. Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anaesthesiol Scand. 1997;41:1124–1132. doi: 10.1111/j.1399-6576.1997.tb04854.x. [DOI] [PubMed] [Google Scholar]
- 81.Eide PK, Stubhaug A. Relief of trigeminal neuralgia after percutaneous retrogasserian glycerol rhizolysis is dependent on normalization of abnormal temporal summation of pain, without general impairment of sensory perception. Neurosurgery. 1998;43:462–472. doi: 10.1097/00006123-199809000-00036. [DOI] [PubMed] [Google Scholar]
- 82.Bradley LA, McKendree-Smith NL, Alarcon GS, Cianfrini LR. Is fibromyalgia a neurologic disease? Curr Pain Headache Rep. 2002;6:106–114. doi: 10.1007/s11916-002-0006-9. [DOI] [PubMed] [Google Scholar]
- 83.Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008;12:1078–1089. doi: 10.1016/j.ejpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129:130–142. doi: 10.1016/j.pain.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tran TD, Wang H, Tandon A, Hernandez-Garcia L, Casey KL. Temporal summation of heat pain in humans: Evidence supporting thalamocortical modulation. Pain. 2010;150:93–102. doi: 10.1016/j.pain.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 87.Harding LM, Kristensen JD, Baranowski AP. Differential effects of neuropathic analgesics on wind-up-like pain and somatosensory function in healthy volunteers. Clin J Pain. 2005;21:127–132. doi: 10.1097/00002508-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 88.Staud R, Vierck CJ, Robinson ME, Price DD. Effects of the N-methyl-D-aspartate receptor antagonist dextromethorphan on temporal summation of pain are similar in fibromyalgia patients and normal control subjects. J Pain. 2005;6:323–332. doi: 10.1016/j.jpain.2005.01.357. [DOI] [PubMed] [Google Scholar]
- 89.Lomas LM, Picker MJ. Behavioral assessment of temporal summation in the rat: sensitivity to sex, opioids and modulation by NMDA receptor antagonists. Psychopharmacology (Berl) 2005;180:84–94. doi: 10.1007/s00213-005-2153-2. [DOI] [PubMed] [Google Scholar]
- 90.Eichenberger U, Giani C, Petersen-Felix S, et al. Lumbar epidural fentanyl: segmental spread and effect on temporal summation and muscle pain. Br J Anaesth. 2003;90:467–473. doi: 10.1093/bja/aeg100. [DOI] [PubMed] [Google Scholar]
- 91.Enggaard TP, Poulsen L, Arendt-Nielsen L, et al. The analgesic effect of codeine as compared to imipramine in different human experimental pain models. Pain. 2001;92:277–282. doi: 10.1016/s0304-3959(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 92.Gartner R, Kroman N, Callesen T, Kehlet H. Multimodal prevention of pain, nausea and vomiting after breast cancer surgery. Minerva Anestesiol. 2010;76:805–813. [PubMed] [Google Scholar]
- 93.Patarica-Huber E, Boskov N, Pjevic M. Multimodal approach to therapy-related neuropathic pain in breast cancer. J BUON. 2011;16:40–45. [PubMed] [Google Scholar]
- 94.Kalkman CJ, Visser K, Moen J, et al. Preoperative prediction of severe postoperative pain. Pain. 2003;105:415–423. doi: 10.1016/S0304-3959(03)00252-5. [DOI] [PubMed] [Google Scholar]
- 95.Janssen KJ, Moons KG, Kalkman CJ, Grobbee DE, Vergouwe Y. Updating methods improved the performance of a clinical prediction model in new patients. J Clin Epidemiol. 2008;61:76–86. doi: 10.1016/j.jclinepi.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 96.Janssen KJ, Vergouwe Y, Kalkman CJ, Grobbee DE, Moons KG. A simple method to adjust clinical prediction models to local circumstances. Can J Anaesth. 2009;56:194–201. doi: 10.1007/s12630-009-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Janssen KJ, Kalkman CJ, Grobbee DE, et al. The risk of severe postoperative pain: modification and validation of a clinical prediction rule. Anesth Analg. 2008;107:1330–1339. doi: 10.1213/ane.0b013e31818227da. [DOI] [PubMed] [Google Scholar]
- 98.Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Sex differences in temporal summation of pain and after sensations following repetitive noxious mechanical stimulation. Pain. 2004;109:115–123. doi: 10.1016/j.pain.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 99.Staud R, Robinson ME, Vierck CJ, et al. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–222. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 100.Staud R, Spaeth M. Psychophysical and neurochemical abnormalities of pain processing in fibromyalgia. CNS Spectr. 2008;13:12–17. doi: 10.1017/s109285290002678x. [DOI] [PubMed] [Google Scholar]
- 101.Riley JL, III, King CD, Wong F, Fillingim RB, Mauderli AP. Lack of endogenous modulation and reduced decay of prolonged heat pain in older adults. Pain. 2010;150:153–160. doi: 10.1016/j.pain.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Granot M, Weissman-Fogel I, Crispel Y, et al. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter? Pain. 2008;136(1–2):142–149. doi: 10.1016/j.pain.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 103.Jacobsen PB, Butler RW. Relation of cognitive coping and catastrophizing to acute pain and analgesic use following breast cancer surgery. J Behav Med. 1996;19:17–29. doi: 10.1007/BF01858172. [DOI] [PubMed] [Google Scholar]
- 104.Fillingim RB, Hastie BA, Ness TJ, et al. Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol Psychol. 2005;69:97–112. doi: 10.1016/j.biopsycho.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 105.Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 106.Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Increased affective bias revealed using experimental graded heat stimuli in young depressed adults: evidence of “emotional allodynia”. Psychosom Med. 2008;70:338–344. doi: 10.1097/PSY.0b013e3181656a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Berna C, Leknes S, Holmes EA, et al. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry. 2010;67:1083–1090. doi: 10.1016/j.biopsych.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 108.Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain - new perspective of opioid-induced hyperalgesia. Pain. 2008;139:431–438. doi: 10.1016/j.pain.2008.05.015. [DOI] [PubMed] [Google Scholar]