Abstract
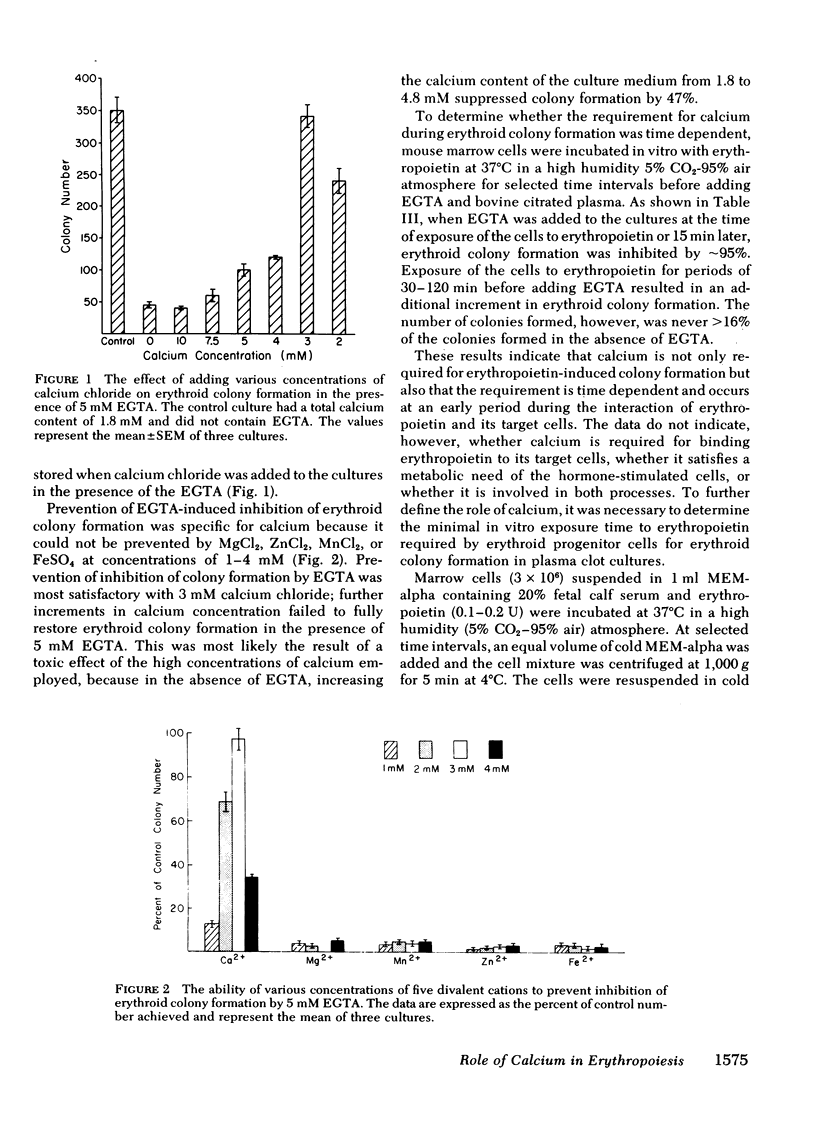
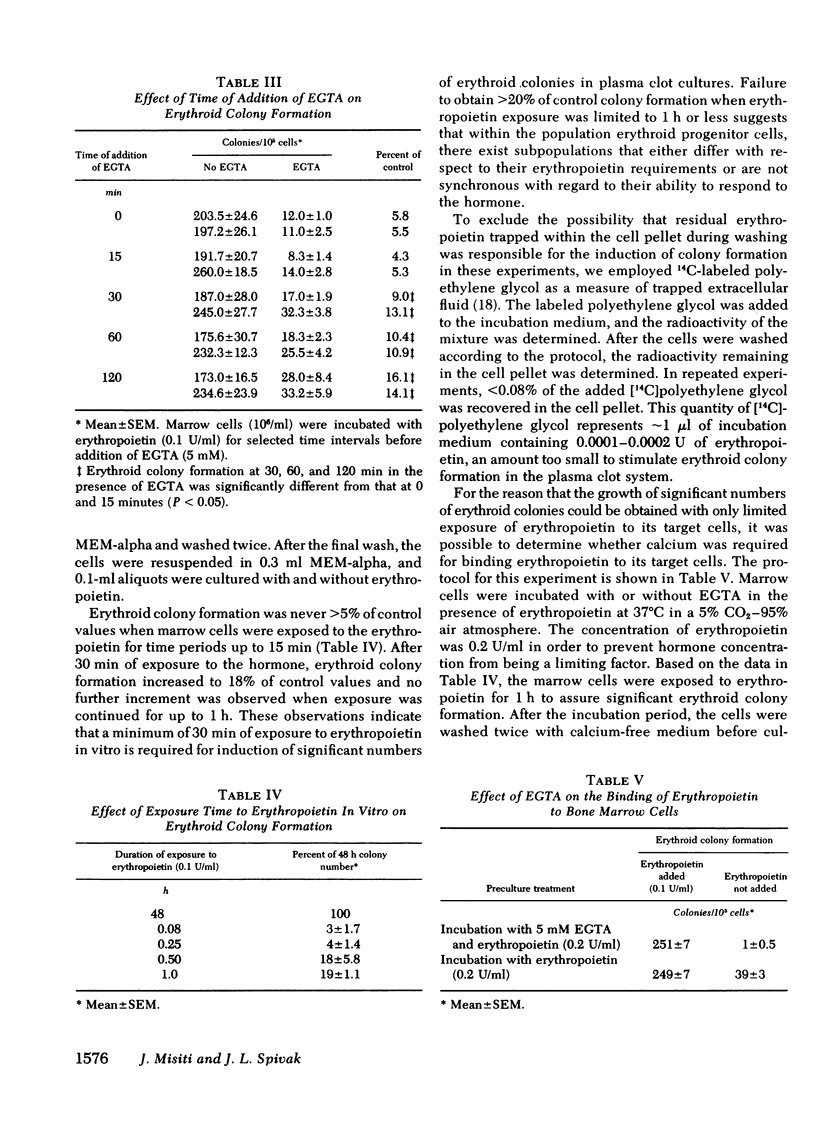
The in vitro plasma clot technique was employed to examine the role of calcium during the interaction of erythropoietin and mouse erythroid progenitor cells. Erythropoietin-induced erythroid colony formation was increased 24% by the carboxylic ionophore A23187 (10 nM), whereas a 35% increase was produced by the carboxylic ionophore Ro 2-2985/1 (1 nM). EGTA (3 mM) inhibited erythropoietin-induced erythroid colony formation. Inhibition of erythroid colony formation by EGTA could be reversed by Ca2+, but not by Mn2+, Mg2+, Zn2+, or Fe2+. At least 30 min exposure of marrow cells to erythropoietin in vitro was required for production of erythroid colonies. EGTA substantially inhibited erythropoietin-induced erythroid colony formation even when the marrow cells were exposed to the hormone for up to 2 h before addition of the chelator. Marrow cells incubated first in calcium-free medium with erythropoietin and then cultured in the presence of calcium but not erythropoietin, failed to form erythroid colonies although colony formation occurred when erythropoietin was provided. Taken together, the data indicate that calcium is required for both extracellular and intracellular events during the interaction of erythropoietin with its target cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. E., Adamson J. W. Modulation of in vitro erythropoiesis. The influence of beta-adrenergic agonists on erythroid colony formation. J Clin Invest. 1977 Jul;60(1):70–77. doi: 10.1172/JCI108771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Adamson J. W. Modulation of in vitro erythropoiesis: enhancement of erythroid colony growth by cyclic nucleotides. Cell Tissue Kinet. 1977 May;10(3):289–298. doi: 10.1111/j.1365-2184.1977.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Goldwasser E. On the mechanism of erythropoietin-induced differentiation. XII. A cytoplasmic protein mediating induced nuclear RNA synthesis. Dev Biol. 1973 Oct;34(2):246–254. doi: 10.1016/0012-1606(73)90353-9. [DOI] [PubMed] [Google Scholar]
- Chang S. C., Sikkema D., Goldwasser E. Evidence for an erythropoietin receptor protein on rat bone marrow cells. Biochem Biophys Res Commun. 1974 Mar 25;57(2):399–405. doi: 10.1016/0006-291x(74)90944-9. [DOI] [PubMed] [Google Scholar]
- Dainiak N., Hoffman R., Maffei L. A., Forget B. G. Potentiation of human erythropoiesis in vitro by thyroid hormone. Nature. 1978 Mar 16;272(5650):260–262. doi: 10.1038/272260a0. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Chopra I. J., Cline M. J. Thyroid hormones stimulate erythropoiesis in vitro. Br J Haematol. 1977 Oct;37(2):173–177. doi: 10.1111/j.1365-2141.1977.tb06833.x. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Cline M. J. Potentiation of erythropoiesis in vitro by dexamethasone. J Clin Invest. 1976 Jan;57(1):57–62. doi: 10.1172/JCI108269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Li C. H. Growth hormone: species-specific stimulation of erythropoiesis in vitro. Science. 1977 Jun 3;196(4294):1112–1113. doi: 10.1126/science.870971. [DOI] [PubMed] [Google Scholar]
- Graber S. E., Bomboy J. D., Jr, Salmon W. D., Jr, Krantz S. B. Effect of erythropoietin preparations on cyclic AMP and cyclic GMP levels in rat fetal liver cell cultures. J Lab Clin Med. 1977 Jul;90(1):162–170. [PubMed] [Google Scholar]
- Graber S. E., Carrillo M., Krantz S. B. Lack of effect of erythropoietin on cyclic adenosine-3',5'-monophosphate levels in rat fetal liver cells. J Lab Clin Med. 1974 Feb;83(2):288–295. [PubMed] [Google Scholar]
- Guilbert L. J., Iscove N. N. Partial replacement of serum by selenite, transferrin, albumin and lecithin in haemopoietic cell cultures. Nature. 1976 Oct 14;263(5578):594–595. doi: 10.1038/263594a0. [DOI] [PubMed] [Google Scholar]
- Kaplan J. G. Membrane cation transport and the control of proliferation of mammalian cells. Annu Rev Physiol. 1978;40:19–41. doi: 10.1146/annurev.ph.40.030178.000315. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Amphotericin inhibition of hematopoiesis in vitro. Am J Hematol. 1977;3:57–62. doi: 10.1002/ajh.2830030107. [DOI] [PubMed] [Google Scholar]
- Ledbetter M. L., Lubin M. Control of protein synthesis in human fibroblasts by intracellular potassium. Exp Cell Res. 1977 Mar 15;105(2):223–236. doi: 10.1016/0014-4827(77)90120-3. [DOI] [PubMed] [Google Scholar]
- Lee T. P., Reed C. E. Effects of steroids on the regulation of the levels of cyclic AMP in human lymphocytes. Biochem Biophys Res Commun. 1977 Oct 10;78(3):998–1004. doi: 10.1016/0006-291x(77)90520-4. [DOI] [PubMed] [Google Scholar]
- Luckasen J. R., White J. G., Kersey J. H. Mitogenic properties of a calcium ionophore, A23187. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5088–5090. doi: 10.1073/pnas.71.12.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager D., Bernstein A. Early transport changes during erythroid differentiation of Friend leukemic cells. J Cell Physiol. 1978 Mar;94(3):275–285. doi: 10.1002/jcp.1040940305. [DOI] [PubMed] [Google Scholar]
- Maino V. C., Green N. M., Crumpton M. J. The role of calcium ions in initiating transformation of lymphocytes. Nature. 1974 Sep 27;251(5473):324–327. doi: 10.1038/251324b0. [DOI] [PubMed] [Google Scholar]
- McLeod D. L., Shreeve M. M., Axelrad A. A. Improved plasma culture system for production of erythrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974 Oct;44(4):517–534. [PubMed] [Google Scholar]
- Negendank W. G., Collier C. R. Ion contents of human lymphocytes. The effects of concanavalin A and ouabain. Exp Cell Res. 1976 Aug;101(1):31–40. doi: 10.1016/0014-4827(76)90408-0. [DOI] [PubMed] [Google Scholar]
- Popovic W. J., Brown J. E., Adamson J. W. The influence of thyroid hormones on in vitro erythropoiesis. Mediation by a receptor with beta adrenergic properties. J Clin Invest. 1977 Oct;60(4):907–913. doi: 10.1172/JCI108845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- Singer J. W., Samuels A. I., Adamson J. W. Steroids and hematopoiesis. I. The effect of steroids on in vitro erythroid colony growth: structure/activity relationships. J Cell Physiol. 1976 Jun;88(2):127–134. doi: 10.1002/jcp.1040880202. [DOI] [PubMed] [Google Scholar]
- Whitney R. B., Sutherland R. M. Requirement for calcium ions in lymphocyte transformation stimulated by phytohemagglutinin. J Cell Physiol. 1972 Dec;80(3):329–337. doi: 10.1002/jcp.1040800303. [DOI] [PubMed] [Google Scholar]
- Zalman F., Maloney M. A., Patt H. M. Differential response of early erythropoietic and granulopoietic progenitors to dexamethasone and cortisone. J Exp Med. 1979 Jan 1;149(1):67–72. doi: 10.1084/jem.149.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijff B., Gerritsen W. J., Oerlemans A., Demel R. A., van Deenen L. L. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. I. Specificity of the membrane permeability changes induced by the polyene antibiotics. Biochim Biophys Acta. 1974 Feb 26;339(1):30–43. doi: 10.1016/0005-2736(74)90330-7. [DOI] [PubMed] [Google Scholar]


