Abstract
Background
The clinical utility of monoamine releasers such as phenmetrazine or d-amphetamine as candidate agonist medications for cocaine dependence is hindered by their high abuse liability. Phendimetrazine is a clinically available schedule III anorectic that functions as a prodrug for phenmetrazine and thus may have lower abuse liability. This study determined the effects of continuous 14-day treatment with phendimetrazine on cocaine- vs.- food choice in rhesus monkeys (N=4).
Methods
Responding was maintained under a concurrent schedule of food delivery (1-g pellets, fixed-ratio 100 schedule) and cocaine injections (0-0.1 mg/kg/injection, fixed-ratio 10 schedule). Cocaine choice dose-effect curves were determined daily before and during 14-day periods of continuous intravenous treatment with saline or (+)-phendimetrazine (0.32 – 1.0 mg/kg/h). Effects of 14-day treatment with (+)-phenmetrazine (0.1 – 0.32 mg/kg/h; N=5) and d-amphetamine (0.032 – 0.1 mg/kg/h; N=6) were also examined for comparison.
Results
During saline treatment, food was primarily chosen during availability of low cocaine doses (0, 0.0032, and 0.01 mg/kg/injection), and cocaine was primarily chosen during availability of higher cocaine doses (0.032 and 0.1 mg/kg/injection). Phendimetrazine initially decreased overall responding without significantly altering cocaine choice. Over the course of 14 days, tolerance developed to rate decreasing effects, and phendimetrazine dose-dependently decreased cocaine choice (significant at 0.032 mg/kg/injection cocaine). Phenmetrazine and d-amphetamine produced qualitatively similar effects.
Conclusions
These results demonstrate that phendimetrazine can produce significant, though modest, reductions in cocaine choice in rhesus monkeys. Phendimetrazine may be especially suitable as a candidate medication for human studies because of its schedule III clinical availability.
Keywords: cocaine, choice, rhesus monkey, phenmetrazine, phendimetrazine, amphetamine
1. Introduction
The goal of an agonist-based pharmacotherapy for the treatment of cocaine dependence is to utilize a medication that has pharmacological effects similar to the abuse drug, a slow onset to reduce abuse liability, and a long duration of action to facilitate medication compliance (Grabowski et al., 2004). For over a decade, the dopamine/norepinephrine vs. serotonin-selective monoamine releaser d-amphetamine has consistently demonstrated efficacy to decrease cocaine self-administration across a broad range of experimental conditions and in a range of species, including rats (Chiodo et al., 2008; Thomsen et al., 2013), nonhuman primates (Czoty et al., 2011; Negus, 2003), and humans (Greenwald et al., 2010; Rush et al., 2010). Moreover, the efficacy of d-amphetamine to decrease cocaine self-administration in these preclinical and human laboratory studies has been consistent with results from clinical trials (Grabowski et al., 2001; Mariani et al., 2012; Schmitz et al., 2012). Despite this extant evidence for efficacy of d-amphetamine as a candidate agonist medication for cocaine dependence, the clinical utility of d-amphetamine is hindered by its own abuse liability and associated schedule II controlled substance status. The acceptability of agonist-based medications might benefit from identification of novel compounds that have lower abuse liability than amphetamine, but that retain amphetamine's efficacy to decrease cocaine self-administration and its long duration of action.
One approach to decrease the abuse liability of a candidate medication is the development of a prodrug to slow onset of drug effects (Balster and Schuster, 1973; Huttunen et al., 2011; Schindler et al., 2009). Phenmetrazine is a dopamine/norepinephrine vs. serotonin-selective monoamine releaser that has cocaine-like discriminative stimulus effects (Banks et al., 2012; Negus et al., 2009) and decreases cocaine self-administration in nonhuman primates (Banks et al., 2011b, 2013; Negus et al., 2009). However, like d-amphetamine, phenmetrazine has a high abuse liability and is no longer clinically available in the United States (Corwin et al., 1987; Griffiths et al., 1979). Phendimetrazine is an N-methyl analog of phenmetrazine that functions as a prodrug for phenmetrazine (Banks et al., 2012; Rothman et al., 2002). Furthermore, phendimetrazine appears to have reduced abuse potential compared to phenmetrazine in certain drug self-administration assays (Corwin et al., 1987), and is available as a schedule III anorectic approved for the short term treatment of obesity. Overall, this body of literature supports further research on phendimetrazine as a candidate pharmacotherapy for cocaine dependence (Banks et al., 2012; Rothman et al., 2002; Stoops and Rush, 2013).
The aim of the present study was to determine the effects of 14-day continuous treatment with phendimetrazine on cocaine self-administration by rhesus monkeys responding under a concurrent cocaine-vs.-food choice procedure that has been used previously to examine effects of other candidate medications (Banks et al., 2011b, 2013; Negus, 2003). Effects of 14-day treatment with phenmetrazine and d-amphetamine on cocaine choice were also examined for comparison. We hypothesized that all three compounds would produce similar decreases in cocaine choice and reciprocal increases in food choice. These expected results would support further consideration and development of phendimetrazine as a candidate pharmacotherapy for cocaine dependence.
2. Methods
2.1 Animals
Studies were conducted in up to 6 adult male rhesus monkeys (Macaca mulatta) that had been surgically implanted with double-lumen catheters (Reiss Manufacturing, Blackstone, VA) inserted into a major vein (femoral or jugular) under aseptic procedures as described previously (Banks et al., 2011a). Monkeys weighed 8-12 kg and were maintained on a diet of fresh fruit and food biscuits (Lab Diet High Protein Monkey Biscuits #5045, PMI Nutrition, Inc., St. Louis, MO) provided in the afternoon after the behavioral session. In addition, monkeys could receive up to 50 1-gm banana-flavored pellets (Grain-based Precision Primate Pellets, Test Diets, Richmond, IL) during daily operant sessions (see below). Water was continuously available. A 12 hr light-dark cycle was in effect (lights on from 6 a.m. to 6 p.m.). Four monkeys had prior cocaine self-administration histories with exposure to primarily monoaminergic compounds (Banks et al., 2011a, 2011b, 2013), and two monkeys had previous histories with exposure to primarily opioids in studies of unconditioned behavior and food-maintained responding. These latter two monkeys had not received opioids or other experimental drugs for at least 6 months prior to participating in the studies described in this manuscript.
Animal maintenance and research were conducted in accordance with the eighth edition of the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. The facility was licensed by both the United States Department of Agriculture and the Association for Assessment and Accreditation of Laboratory Animal Care, and protocols were approved by the Institutional Animal Care and Use Committee. Monkeys had visual, auditory and olfactory contact with other monkeys throughout the study. Operant procedures and foraging toys were provided for environmental manipulation and enrichment. Music or videos were also played daily in animal housing rooms to provide additional environmental enrichment.
2.2 Apparatus and Catheter Maintenance
Daily experimental sessions were conducted in each monkey's home cage as previously described (Negus, 2003). Briefly, the front wall was equipped with an operant response panel that included three square response keys arranged horizontally. Only the right and left keys were used in these studies. Each cage was also equipped with a pellet dispenser (Med Associates, Model ENV-203-1000, St. Albans, VT) and two syringe pumps (Model PHM-108, Med Associates), one for each lumen of the double lumen catheter. One syringe pump (the “cocaine” pump) was used to deliver contingent cocaine injections through one lumen of the double-lumen catheter. The second syringe pump (the “treatment” pump) was used to deliver saline or test compounds through the second lumen of the catheter. This “treatment” pump was programmed to deliver 0.1 mL infusions every 20 min from 12:00 noon each day until 11:00 a.m. the next morning. Syringes were checked and refilled or replaced as necessary between 11:00 a.m. and noon. Operation of the operant response panels and data collection were accomplished with computers and software purchased from Med Associates. The intravenous (IV) catheter was protected by a tether system consisting of a custom-fitted nylon vest connected to a flexible stainless steel tether and fluid swivel (Lomir Biomedical, Malone, NY) that permitted monkeys to move freely in the cage. Catheter patency was periodically evaluated by IV administration of ketamine (5 mg/kg) through the catheter lumen and after each test drug treatment that produced a rightward shift in the cocaine choice dose-effect function. The catheter was considered to be patent if IV administration of ketamine produced a loss of muscle tone within 10 s.
2.3 Baseline Procedures
Daily behavioral sessions consisting of five 20-min components separated by 5-min timeout periods were conducted from 9 a.m. to 11 a.m. as described previously (Banks et al., 2011b). During each component, the left, food-associated key was transilluminated red, and completion of the fixed-ratio (FR) requirement, FR100, resulted in the delivery of a 1-g food pellet. The right, cocaine-associated key was transilluminated green, and completion of the FR requirement, FR10, resulted in the delivery of a cocaine dose. The response requirements were set at FR 100 on the food-associated key and FR 10 on the cocaine-associated key for all monkeys, because our previous studies indicated that, under these response requirements, monkeys usually switched from the food-associated key to the drug-associated key during the fourth response period, when an intermediate unit dose of 0.032 mg/kg/inj cocaine was available (Negus, 2003). Consequently, with the variables used in this study, we could detect both leftward and rightward shifts in the cocaine choice dose-effect curves that might result from an experimental manipulation. A different cocaine dose was available during each of the five successive components (0, 0.0032, 0.01, 0.032 and 0.1 mg/kg/injection during components 1-5, respectively), and the cocaine dose was varied by manipulating the duration of the “cocaine” pump activation and the resulting volume of each injection. Stimulus conditions on the cocaine-associated key were also varied by flashing the stimulus lights on and off in 3 s cycles, and longer flashes (and shorter inter-flash intervals) were associated with the availability of higher cocaine doses.
During each component, monkeys could complete up to a total of 10 ratio requirements on the food- and cocaine-associated keys. Responding on either key, before completing a ratio requirement, reset the ratio requirement on the other key. Completion of each ratio requirement initiated a 3 s timeout, during which all stimulus lights were turned off, and responding had no scheduled consequences. During components when the drug-associated key was not illuminated and a “0” dose of drug was available, responses on this key were still recorded, still reset the FR requirement on the food-associated key, and completion of the FR requirement still counted as one of the 10 allotted ratios and initiated a 3 s timeout. If 10 ratio requirements were completed before the 20-min component had elapsed, all stimulus lights were extinguished, and responding had no scheduled consequences for the remainder of that 20-min component. Choice behavior was considered stable when the lowest unit dose cocaine maintaining at least 80% cocaine choice varied by ≤0.5 log units for three consecutive days.
2.4 Testing Procedures
Once cocaine choice was stable as defined above, test sessions were initiated. Prior to each test, parameters of cocaine choice were assessed for three consecutive days to determine pre-test “baseline” values. During this baseline determination, saline was administered by the “treatment” pump through one lumen of the double-lumen catheter. Subsequently, a 14-day test period was initiated during which a test solution was administered via the “treatment” pump. The test solutions and doses examined in this study were saline, (+)-phendimetrazine (0.32 - 1.0 mg/kg/h), (+)-phenmetrazine (0.1 - 0.32 mg/kg/h) or d-amphetamine (0.032 - 0.1 mg/kg/h). The infusion rate for all test drugs was identical to that used in our previous studies (Banks et al., 2011b, 2013; Negus, 2003). At the conclusion of each 14-day test period (excluding the 14-day saline test), a saline control treatment period was reinstated for at least 5 days and until cocaine choice returned to pretest levels. This interval between successive treatments was implemented to minimize the possibility of any carryover effects from one treatment condition to the next and to provide adequate time for clearance of test drugs before initiation of the next treatment condition. The sequence of saline, phendimetrazine, phenmetrazine and amphetamine testing as well as drug dose were mixed across monkeys. However, all doses of one test drug were administered before testing another drug. Phenmetrazine was tested in five monkeys and d-amphetamine was tested in six monkeys. One monkey died of causes unrelated to the experiment before testing with phendimetrazine, and another monkey exhausted all available catheter sites after testing with phendimetrazine, but before testing with saline. As result, phendimetrazine was tested in five monkeys, and saline was tested in three monkeys. Supplemental Table 11 provides details regarding which monkeys received which test drugs and doses.
2.5 Data Analysis
The primary dependent variables for each component were (1) percent cocaine choice, defined as (number ratios completed on the cocaine-associated key ÷ number of ratios completed)*100, and (2) the number of ratios completed for days 5-7 (7 days) and days 12-14 (14 days). These variables were then plotted as a function of cocaine dose. For each treatment, cocaine choice dose-effect curves were analyzed using a univariate mixed model analysis with cocaine dose and treatment condition as the main factors. A significant main effect of treatment and/or significant interaction was followed by the LSD multiple comparisons post-hoc test to compare test conditions with baseline conditions. Additional dependent variables collected during each session included total choices, total food choices, and total cocaine choices and these variables were analyzed using a one-way RM ANOVA with treatment condition as the main factor. A significant main effect of treatment and/or significant interaction was followed by the Dunnett multiple comparisons post-hoc test to compare test conditions with baseline conditions. The criterion for significance was set a priori at the 95% level of confidence (p < 0.05). All analyses were conducted using JMP Pro 10 for Mac (SAS Institute, Cary, NC).
2.6 Drugs
(−)-Cocaine HCl was provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). (+)-Phendimetrazine fumarate and (+)-phenmetrazine hemi-fumarate was synthesized by BE Blough (Research Triangle Institute, Research Triangle Park, NC). d- Amphetamine hemisulfate was purchased from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in sterile saline, and all solutions were filter-sterilized using a 0.22-μm Millipore filter (Millipore Corp, Billerica, MA). Drug doses were calculated using the salt forms listed above.
3. Results
3.1 Baseline choice between cocaine and food and effects of 14-day saline treatment
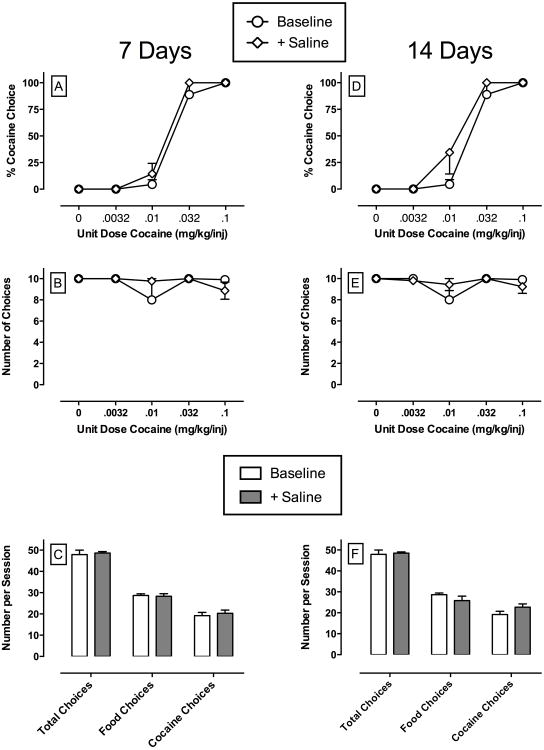
Under baseline conditions, monkeys primarily chose food when the unit cocaine dose was low (0 – 0.01 mg/kg/injection) and almost exclusively reallocated their behavior to the cocaine-associated key during availability of higher unit cocaine doses (0.032 – 0.1 mg/kg/injection) (Figures 1-4 top panels; open circles). Furthermore, monkeys typically completed the maximum number of choices during each component of the behavioral session (Figures 1-4 middle panels; open circles). Figure 1 shows the stability of the cocaine choice dose-effect function over 14 consecutive days when saline was continuously administered as the test solution. The left column shows the effects of saline treatment on A) percent cocaine choice, B) number of choices per component, and C) number of choices per session for 7 days, whereas the right column shows the effects on the same experimental endpoints for 14 days of saline treatment. Mixed model analysis failed to demonstrate a significant main effect or interaction of saline treatment on any experimental endpoint. These results provide evidence of the stability of the cocaine choice dose-effect function over the 14-day treatment period.
Figure 1.
Effects of continuous 14-day treatment with saline on choice between cocaine and food in rhesus monkeys (n=3). The top panels show saline treatment effects on cocaine dose-effect curves. Top and middle abscissae: unit dose of cocaine in milligrams per kilogram per injection (log scale). Top ordinates: percent cocaine choice. Middle ordinates: the number of choices completed per component. The bottom panels show summary data for total choices, food choices and cocaine choices summed across all cocaine doses. Bottom abscissae: experimental endpoint. Bottom ordinates: number of choices per session. All points and bars represent mean data ± SEM obtained during days 5-7 and 12-14 of each 14-day treatment period from three monkeys. Baseline points and bars represent mean data ± SEM obtained during the 3 days preceding each treatment period while saline was infused through the “treatment” lumen of the double lumen catheter.
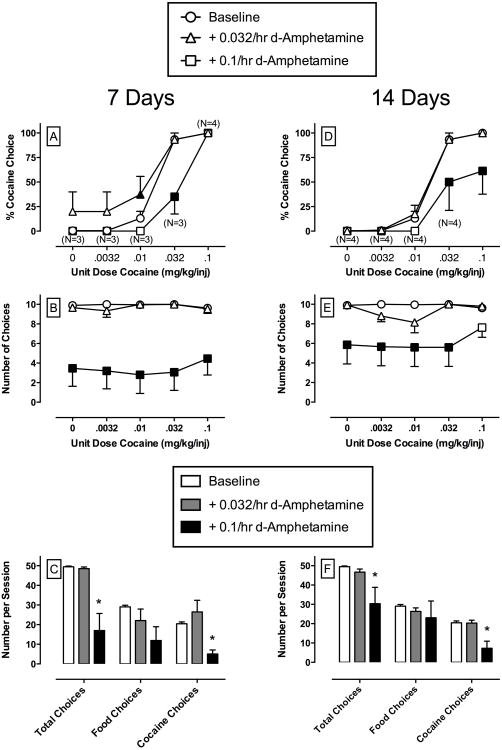
Figure 4.
Effects of continuous 14-day treatment with d-amphetamine (0.032 – 0.1 mg/kg/h) on choice between cocaine and food in rhesus monkeys (n=6). Filled symbols indicate significantly different (p < 0.05) from baseline (saline control) within a cocaine dose. Asterisk indicates significantly different (p < 0.05) from baseline (saline control) conditions. Numbers in parentheses indicate the number of subjects contributing to that data point if fewer than the total number of subjects tested during treatment with 0.1 mg/kg/h d-amphetamine. This number indicates d-amphetamine treatment eliminated responding in one or more subjects during that component of the choice procedure. Other details as in Figure 1.
3.2 Effects of (+)-phendimetrazine on cocaine choice
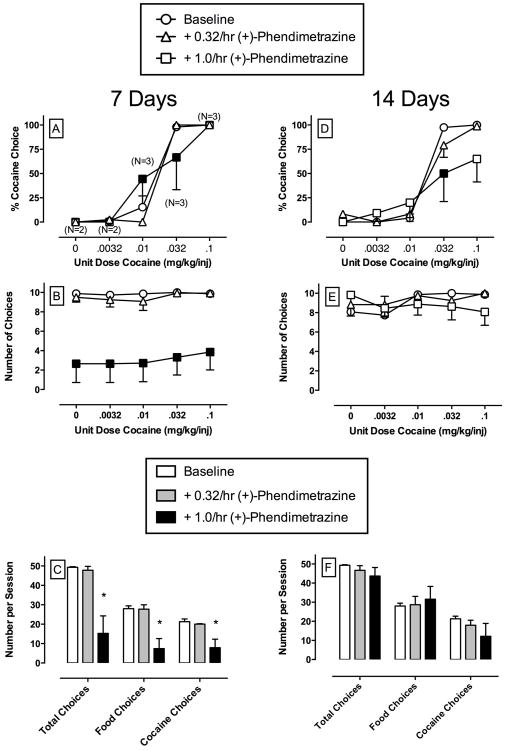
Figure 2 shows the effects of continuous 14-day treatment with (+)-phendimetrazine. As in the previous figure, and in all subsequent figures, the left column shows the mean effects of treatment during days 5-7, whereas the right column shows the effects of treatment during days 12-14. During the first 7 days of phendimetrazine treatment, mixed model analysis demonstrated a significant main effect of cocaine dose (F4,16.5 = 76.6, p<0.05) and a significant cocaine dose × phendimetrazine interaction (F8,24.5 = 3.3, p<0.05) for percent cocaine choice dependent measures (Panel 2A). Post-hoc analysis revealed that cocaine choice significantly increased during 0.01 mg/kg/injection cocaine availability and significantly decrease during 0.032 mg/kg/injection cocaine availability during treatment with 1.0 mg/kg/h phendimetrazine. For the number of choices completed per component, mixed model analysis demonstrated a significant main effect of phendimetrazine treatment (F2,7 = 8.5, p<0.05). Post-hoc analysis revealed that 1.0 mg/kg/h phendimetrazine significant decreased the number of choices completed during all components of the behavioral session (Panel 2B). For the number of choices completed per session (Panel 2C), one-way RM ANOVA demonstrated a significant main effect of phendimetrazine treatment on total choices (F2,11 = 11.7, p<0.05), food choices (F2,11 = 11.5, p<0.05), and cocaine choices (F2,11 = 6.8, p<0.05). Post-hoc analysis revealed that 1.0 mg/kg/h phendimetrazine significantly decreased total, food, and choices.
Figure 2.
Effects of continuous 14-day treatment with (+)-phendimetrazine (0.32 – 1.0 mg/kg/h) on choice between cocaine and food in rhesus monkeys (n=4). Filled symbols indicate significantly different (p < 0.05) from baseline (saline control) within a cocaine dose. Asterisk indicates significantly different (p < 0.05) from baseline (saline control) conditions. Numbers in parentheses indicate the number of subjects contributing to that data point if fewer than the total number of subjects (4) tested during treatment with 1.0 mg/kg/h phendimetrazine. This number indicates phendimetrazine treatment eliminated responding in one or more subjects during that component of the choice procedure. Other details as in Figure 1.
At the end of 14 days of phendimetrazine treatment, mixed model analysis demonstrated a significant main effect of cocaine dose (F4,14.5 = 55.4, p<0.05) and a significant cocaine dose × phendimetrazine treatment interaction (F8,27 = 2.5, p<0.05). Post-hoc analysis revealed that 1.0 mg/kg/h phendimetrazine significantly decreased choice of 0.032 mg/kg/injection cocaine (Panel 2D). For the number of choices completed per component (Panel 2E) and per session (Panel 2F), mixed model analysis demonstrated no significant main effects or a significant interaction. Thus, tolerance developed to the rate-decreasing effects produced by phendimetrazine during the initial 7 days of treatment such that these effects were no longer apparent at the end of 14 treatment days. Although 4 monkeys completed the 14-day treatment with 0.32 mg/kg/h phendimetrazine, only 3 out of 5 monkeys completed all 14 treatment days with 1.0 mg/kg/h phendimetrazine. One monkey lost catheter patency on day 13 during treatment with 1.0 mg/kg/h phendimetrazine and thus the mean of treatment days 12 and 13 were used in the analysis and presentation of the data shown in Panels 2D-F. The fifth monkey lost catheter patency on day 11 and his data for 1.0 mg/kg/h phendimetrazine were excluded from the analysis for both the 7 and 14-day endpoints. Cocaine choice dose-effect functions returned to pre-treatment levels after 3 days (range 1-4) following termination of (+)-phendimetrazine.
3.3 Effects of (+)-phenmetrazine on cocaine choice
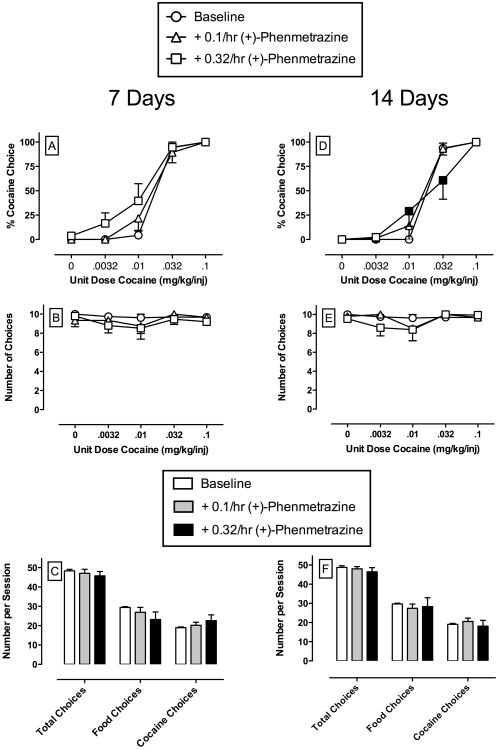
Figure 3 shows the effects of continuous 14-day treatment with (+)-phenmetrazine. During the first 7 days of phenmetrazine treatment for percent cocaine choice (Panel A), mixed model analysis demonstrated a significant effect of cocaine dose (F4,16 = 161.0, p<0.05) and phenmetrazine treatment (F2,8 = 4.0, p<0.05), but no significant interaction. Post-hoc analysis revealed no significant effects of phenmetrazine treatment at any cocaine dose. At the end of 14 days, mixed model analysis demonstrated a significant main effect of cocaine dose (F4,16 = 126.5, p<0.05) and a significant cocaine dose × phenmetrazine treatment interaction (F8,24 = 2.4, p<0.05). Post-hoc analysis revealed that treatment with 0.32 mg/kg/hr phenmetrazine significantly increased choice of 0.01 mg/kg/injection cocaine and significantly decreased choice of 0.032 mg/kg/injection cocaine (Panel D). All 5 monkeys examined completed 14-day phenmetrazine treatments with both doses. A higher dose of phenmetrazine was not studied due to evidence from other studies suggesting emergence of stimulant-associated toxicity at doses >0.32 mg/kg/h (Banks et al., 2011b, 2013; Negus et al., 2009). Cocaine choice dose-effect functions returned to pre-treatment levels after 3 days (range 2-4) following termination of (+)-phenmetrazine.
Figure 3.
Effects of continuous 14-day treatment with (+)-phenmetrazine (0.1 – 0.32 mg/kg/h) on choice between cocaine and food in rhesus monkeys (n=5). Filled symbols indicate significantly different (p < 0.05) from baseline (saline control) within a cocaine dose. Other details as in Figure 1.
3.4 Effects of d-amphetamine on cocaine choice
Figure 4 shows the effects of continuous 14-day treatment with d-amphetamine. During the first 7 days of d-amphetamine treatment, mixed model analysis demonstrated significant main effects of cocaine dose (F4,15.9 = 62.5, p <0.05) and a significant cocaine dose × d-amphetamine treatment interaction (F8,32.7 = 3.0, p <0.05) on percent cocaine choice (Panel 4A). Post-hoc analysis revealed that treatment with 0.1mg/kg/h d-amphetamine significantly decreased choice of 0.032 mg/kg/injection cocaine. For the number of choices completed per component (Panel 4B), mixed model analysis demonstrated a main effect of d-amphetamine treatment (F2,10 = 12.4, p <0.05). Post-hoc analysis revealed that 0.1mg/kg/h d-amphetamine significantly decreased the number of choices completed during all 5 components of the choice session. For the number of choices completed per session, one-way RM ANOVA demonstrated a significant main effect of d-amphetamine treatment on total choices (F2,15 = 15.4, p<0.05) and cocaine choices (F2,15 = 12.9, p<0.05). Post-hoc analysis revealed that 0.1 mg/kg/h d-amphetamine significantly decreased both total and cocaine choices (Panel 4C).
At the end of 14 days of d-amphetamine, mixed model analysis demonstrated a significant main effect of cocaine dose (F4,19.7 = 91.4, p<0.05) and a significant cocaine dose × d-amphetamine treatment interaction (F8,37.2 = 4.7, p <0.05) for percent cocaine choice (Panel 4D). Post-hoc analysis revealed that 0.1/h d-amphetamine significantly decreased choice of both 0.032 and 0.1 mg/kg/injection cocaine. For the number of choices completed per component (Panel 4E), there was a significant cocaine dose × d-amphetamine treatment interaction (F8,40 = 2.5, p <0.05). Post-hoc analysis revealed that 0.1mg/kg/h d-amphetamine significantly decreased the number of choices completed per component when unit cocaine doses of 0 – 0.032 mg/kg/injection were available. For the number of choices completed per session (Panel 4F), one-way RM ANOVA demonstrated a significant main effect of d-amphetamine treatment on cocaine choices (F2,15 = 16.3, p<0.05). Post-hoc analysis revealed that 0.1 mg/kg/h d-amphetamine significantly decreased both total and cocaine choices. All 6 monkeys completed 14-day d-amphetamine treatments with both doses. Cocaine choice dose-effect functions returned to pre-treatment levels after 3 days (range 1-7) following termination of d-amphetamine.
4. Discussion
The aims of the present study were 1) to determine the efficacy of 14-day continuous phendimetrazine treatment to decrease cocaine vs. food choice in nonhuman primates, and 2) to compare the efficacy of phendimetrazine to the efficacy of its primary active metabolite phenmetrazine and the clinically available schedule II monoamine releaser d-amphetamine. There were two main findings. First, phendimetrazine produced initial decreases in total and food choices, but tolerance developed to this effect. Over the course of 14-days of phendimetrazine treatment, cocaine choice was significantly though modestly decreased. Second, these effects of phendimetrazine on cocaine choice were qualitatively similar to effects produced by chronic treatment with either its active metabolite phenmetrazine or with d-amphetamine. Overall, the results of the present study support further preclinical and clinical research on the schedule III anorectic phendimetrazine as a candidate medication for cocaine dependence because of its clinical availability and lower controlled substance classification compared to d-amphetamine or phenmetrazine.
4.1 Stability of cocaine choice
Consistent with previous nonhuman primate studies from our laboratory (Banks et al., 2011b, 2013; Negus, 2003) and others (Czoty and Nader, 2012; Woolverton and Balster, 1981), when low unit cocaine doses were available as the alternative to food, food was primarily chosen. As the unit cocaine dose increased, monkeys reallocated their responding from the food-to the cocaine-associated key such that high unit cocaine doses maintained almost exclusive cocaine choice. Furthermore, the results of the present study demonstrate the stability of the cocaine choice dose-effect curve during 14-days of continuous saline administration and provide a foundation upon which to compare the effects of novel compounds on this stable choice behavior.
4.2 Effects of phendimetrazine, phenmetrazine and amphetamine on cocaine choice
The goal of any candidate medication for the treatment of cocaine dependence should be not only to reduce cocaine-taking behavior, but also to promote a reallocation of behavior towards activities maintained by more adaptive reinforcers (Vocci, 2007). Towards this end, preclinical choice procedures may be especially useful during medication development to determine whether a given experimental manipulation produces this critical reallocation of behavior (Banks and Negus, 2012; Haney and Spealman, 2008). During 14 days of treatment with phendimetrazine and d-amphetamine, monkeys responded for fewer cocaine injections and for more food pellets during availability of 0.032 mg/kg/injection cocaine. Furthermore during d-amphetamine treatment, there was also a significant decrease in cocaine choice and associated increase in food choice during availability of the highest unit dose of 0.1 mg/kg/injection cocaine. Lastly, monoamine releaser-induced decreases in cocaine choice were especially robust in some monkeys. For example, phendimetrazine and d-amphetamine decreased cocaine choice to <50% across all cocaine doses in 1/4 and 3/6 monkeys, respectively (see supplemental figures 1 for individual results 2). Thus, phendimetrazine and d-amphetamine promoted reallocation of behavior from cocaine choice to food choice when high cocaine doses were available. Moreover, the similarity in effects produced by phendimetrazine and d-amphetamine on cocaine choice in this study suggests that phendimetrazine may also serve as a Schedule III alternative to d-amphetamine as a candidate agonist medication for clinical treatment of cocaine dependence. However, the potentially desirable effects of phendimetrazine and d-amphetamine on cocaine choice were accompanied by two other effects that are likely to function as negative influences on clinical utility of these compounds.
First, although phendimetrazine and d-amphetamine decreased high-dose cocaine choice, there were initial and marginal increases in low-dose cocaine choice in some monkeys after 7 days of treatment, and increased choice of 0.01 mg/kg/injection cocaine was significant even for 7 days of phendimetrazine treatment and 14 days of phenmetrazine treatment. A previous study with 7-day treatment regimens did not observe phenmetrazine-induced increases in low-dose cocaine choice, although increases in cocaine choice were observed in some monkeys with other releasers (e.g. methamphetamine, (Banks et al., 2011b, 2013)). Similarly, acute d-amphetamine pretreatment increased low-dose cocaine self-administration under both concurrent and fixed-ratio schedules in rats (Barrett et al., 2004; Thomsen et al., 2013). Taken together, these results raise the possibility that treatment with monoamine releasers may transiently stimulate cocaine-maintained behavior early in treatment.
Second, monoamine releaser-induced decreases in cocaine choices were also accompanied by initial disruption of overall choice behavior, as indicated by decreases in the total number of choices completed. Thus, d-amphetamine produced the greatest decrease in cocaine choice, but also the greatest decrease in total responding. Conversely, phenmetrazine produced the smallest effects on cocaine choice, but also the smallest effects on total responding It is possible that higher phenmetrazine doses may have produced greater decreases in cocaine choice. Higher doses were not tested in the present study because the highest dose tested (0.32 mg/kg/h) produced stereotyped behaviors (e.g., hair plucking), and in previous studies (Banks et al., 2011b, 2013; Negus et al., 2009), higher doses decreased total responding and produced other undesirable effects. Overall, these data suggest that emergence of undesirable stimulant effects may limit the clinical utility of dopamine/norepinephrine-selective monoamine releasers to decrease cocaine self-administration. However, some degree of tolerance develops to these undesirable effects develops during chronic treatment, and transient expression of these effects may be a manageable risk in cocaine-dependent patients already exposed to some degree of stimulant toxicity by virtue of their cocaine use.
The results of the present study should also be considered in context of a recent clinical trial examining the effects of d-amphetamine in combination with topiramate in cocaine-dependent individuals stratified according to their severity of cocaine use (Mariani et al., 2012). In this clinical trial, treatment with d-amphetamine and topiramate was least effective in light cocaine users and most effective in the heaviest cocaine users (Mariani et al., 2012). Cocaine access conditions in the present study were limited to 2 h/day and a maximum cocaine intake of approximately 1.4 mg/kg during the choice session, and the efficacy and safety of agonist medications like phendimetrazine to decrease cocaine choice may also vary as a function of the intensity of cocaine use. Thus, a potential future direction would be to assess the efficacy of phendimetrazine and d-amphetamine under conditions of more extensive cocaine consumption.
4.3 Implications for monoamine releasers as candidate “agonist-based” medications
Although concerns about the deployment of a medication that has abuse potential itself in a population of drug abusers are clearly justified, two other points are worth considering in regard to the potential administration of monoamine releasers to cocaine-dependent subjects. First, cocaine-dependent individuals displayed a blunted response to amphetamine-induced dopamine release compared to healthy controls as measured by positron emission tomography (Martinez et al., 2007). If increased dopamine release is associated with abuse potential (Di Chiara and Imperato, 1988), then results from Martinez et al (2007) suggest that amphetamine may have less abuse potential in cocaine-dependent individuals compared to healthy volunteers. Moreover, these results might suggest that phendimetrazine would have even less abuse potential than d-amphetamine or phenmetrazine in cocaine-dependent individuals, given that phendimetrazine has been shown to produce no significant increases in dopamine levels (Rothman et al., 2002). Second, the results of Martinez et al (2007) and Volkow et al (1997) suggest a hypodopaminergic state in cocaine-dependent individuals, and dopamine-selective monoamine releasers may serve as medications that can normalize this hypodopaminergic state. These two points are hypotheses regarding the clinical utility of monoamine releasers as pharmacotherapies for cocaine dependence and will require testing in both preclinical and clinical research settings.
Supplementary Material
Acknowledgments
We appreciate the technical assistance of Jennifer Gough and Crystal Reyns.
Role of Funding Source: Funding for this study was provided by National Institutes of Heath grants R01-DA026946 and R01-DA012790 from the National Institute on Drug Abuse, National Institutes of Health. NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi
Contributors: Blough, and Negus designed the study. Blough synthesized (+)-phendimetrazine. Banks, Blough, and Negus wrote the manuscript. All authors have contributed to and have approved the final manuscript
AUTHOR DISCLOSURES: Conflict of Interest: of the authors have any conflicts of interest to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J Exp Anal Behav. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks M, Roma P, Folk J, Rice K, Negus SS. Effects of the delta-opioid agonist SNC80 on the abuse liability of methadone in rhesus monkeys: a behavioral economic analysis. Psychopharmacology. 2011a;216:431–439. doi: 10.1007/s00213-011-2235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Role of phenmetrazine as an active metabolite of phendimetrazine: evidence fromstudies of drug discrimination and pharmacokinetics in rhesus monkeys. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.10.026. epub 2012 Dec 1 http://dx.doi.org/10.1016/j.drugalcdep.2012.10.026. [DOI] [PMC free article] [PubMed]
- Banks ML, Blough BE, Negus SS. Effects of monoamine releasers with varying selectivity for releasing dopamine/norepinephrine versus serotonin on choice between cocaine and food in rhesus monkeys. Behav Pharmacol. 2011b;22:824–836. doi: 10.1097/FBP.0b013e32834d63ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Stevens Negus S. Interaction between behavioral and pharmacological treatment strategies to decrease cocaine choice in Rhesus monkeys. Neuropsychopharmacology. 2013;168:395–404. doi: 10.1038/npp.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci. 2012 doi: 10.1155/2012/281768. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47:256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Chiodo KA, Läck CM, Roberts DCS. Cocaine self-administration reinforced on a progressive ratio schedule decreases with continuous d-amphetamine treatment in rats. Psychopharmacology. 2008;200:465–473. doi: 10.1007/s00213-008-1222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Woolverton WL, Schuster CR, Johanson CE. Anorectics: effects on food intake and self-administration in rhesus monkeys. Alcohol Drug Res. 1987;7:351–361. [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Martelle JL, Nader MA. Prolonged attenuation of the reinforcing strength of cocaine by chronic d-amphetamine in Rhesus monkeys. Neuropsychopharmacology. 2011;36:539–547. doi: 10.1038/npp.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Nader MA. Effects of dopamine D2/D3 receptor ligands on food-cocaine choice in socially housed male cynomolgus monkeys. J Pharmacol Exp Ther. 2013;344:329–338. doi: 10.1124/jpet.112.201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL. Sustained release d-amphetamine reduces cocaine but not “speedball”-seeking in buprenorphine-maintained volunteers: a test of dual-agonist pharmacotherapy for cocaine/heroin polydrug abusers. Neuropsychopharmacology. 2010;35:2624–2637. doi: 10.1038/npp.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Brady JV, Bradford LD. Predicting the abuse liability of drugs with animal drug self-administration procedures: psychomotor stimulants and hallucinogens. In: Thompson T, Dews PB, editors. Advances in Behavioral Pharmacology. New York: Academic Press; 1979. pp. 164–208. [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen KM, Raunio H, Rautio J. Prodrugs—from serendipity to rational design. Pharmacol Rev. 2011;63:750–771. doi: 10.1124/pr.110.003459. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Pavlicova M, Bisaga A, Nunes EV, Brooks DJ, Levin FR. Extended-release mixed amphetamine salts and topiramate for cocaine dependence: a randomized controlled trial. Biol Psychiatry. 2012;72:950–956. doi: 10.1016/j.biopsych.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in Rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS, Baumann MH, Rothman RB, Mello NK, Blough BE. Selective suppression of cocaine- versus food-maintained responding by monoamine releasers in Rhesus monkeys: benzylpiperazine, (+)phenmetrazine, and 4-benzylpiperidine. J Pharmacol Exp Ther. 2009;329:272–281. doi: 10.1124/jpet.108.143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Katsnelson M, Vu N, Partilla JS, Dersch CM, Blough BE, Baumann MH. Interaction of the anorectic medication, phendimetrazine, and its metabolites with monoamine transporters in rat brain. Eur J Pharmacol. 2002;447:51–57. doi: 10.1016/s0014-2999(02)01830-7. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Sevak RJ, Hays LR. Cocaine choice in humans during D-amphetamine maintenance. J Clin Psychopharmacol. 2010;30:152–159. doi: 10.1097/JCP.0b013e3181d21967. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Thorndike EB. Effect of rate of delivery of intravenous cocaine on self-administration in rats. Pharmacol Biochem Behav. 2009;93:375–381. doi: 10.1016/j.pbb.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Rathnayaka N, Green C, Moeller FG, Dougherty AE, Grabowski J. Combination of modafinil and d-Amphetamine for the treatment of cocaine dependence: a preliminary investigation. Front. 2012 doi: 10.3389/fpsyt.2012.00077. Psychiatry epub ahed of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Rush CR. Agonist replacement for stimulant dependence: a review of clinical research. Curr Pharm Des. 2013 doi: 10.2174/138161281940131209142843. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Negus SS, Caine SB. Cocaine - food chocie in rats: environmental manipulations and effects of amphetamine. J Exp Anal Behav. 2013;99:211–233. doi: 10.1002/jeab.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci FJ. Can replacement therapy work in the treatment of cocaine dependence? And what are we replacing anyway? Addiction. 2007;102:1888–1889. doi: 10.1111/j.1360-0443.2007.02014.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Balster RL. Effects of antipsychotic compounds in Rhesus monkeys given a choice between cocaine and food. Drug Alcohol Depend. 1981;8:69–78. doi: 10.1016/0376-8716(81)90088-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.