Abstract
Processing of a target stimulus may be inhibited if its location has just been cued, a phenomenon of spatial attention known as inhibition of return (IOR). Here, we demonstrate a striking effect wherein items that have just been the focus of reflective attention (internal attention to an active representation) are also inhibited. Participants saw two items, followed by a cue to think back to (refresh, direct reflective attention toward) one item, and then had to identify either the refreshed item, the unrefreshed item, or a novel item. Responses were significantly slower for previously refreshed items than unrefreshed items, although refreshed items were better remembered on a later memory test. Control experiments replacing the refresh event with a second perceptual presentation did not show similar effects. These results suggest that reflective attention can produce an inhibition effect for attended items that may be analogous to IOR effects in perceptual attention.
Introduction
Adequately processing the flood of incoming information bombarding our senses would be impossible without a selection mechanism—attention—to restrict the flow. Given the large number of studies of perceptual attention, it is perhaps natural to associate “attention” with sensory processing, particularly vision. However, just as one cannot simultaneously examine every stimulus in the visual field, it is similarly impossible to simultaneously think every thought that a situation might trigger. Psychologists dating back to William James (1890) have noted that attention must operate within both the external/perceptual and internal/reflective domains (e.g., M. K. Johnson et al., 2005). Yet studies comparing perceptual and reflective attention are still few compared to the many studies of perceptual attention alone; and the extent to which phenomena and mechanisms of perceptual attention also operate within, or have homologues/analogues in, the reflective domain of thought and memory is still relatively unknown (for review: Chun, Golomb, & Turk-Browne, 2011; Chun & M. K. Johnson, 2011; Lepsien & Nobre, 2006).
One such perceptual attention phenomenon is inhibition of return (IOR; Posner & Cohen, 1984; Posner, Rafal, Choate, & Vaughan, 1985). IOR is characterized by slower response times (RTs) to a stimulus presented at a location where an attention-capturing cue was presented several hundred milliseconds earlier, compared to an uncued location. This inhibition of orienting to previously attended locations is proposed to facilitate “foraging” for novel information to aid visual search or more efficiently explore visual environments (Klein, 2000; Klein & MacInnes, 1999).
One might thus propose that an IOR-like mechanism could facilitate thought as well as perception. Such a mechanism could encourage foraging among thoughts, preventing perseveration on single ideas, potentially enhancing creativity, and keeping the stream of consciousness flowing. To test whether an IOR-like phenomenon may occur in reflection, we examined a simple reflective attention process that is a close analogue to perceptual selective attention.
Refreshing is defined as the act of thinking back to and foregrounding an active mental representation (e.g., of a just-presented stimulus; Chen & Cowan, 2009; Higgins & Johnson, 2009; M. K. Johnson et al., 2005; M. K. Johnson, Reeder, Raye, & Mitchell, 2002; Raye, Johnson, Mitchell, Reeder, & Greene, 2002). Refreshing shares some neural characteristics with perceptual attention. For example, refreshing and perceptual attention activate a similar, partially overlapping frontoparietal network, and both can modulate activity in visual cortical areas relevant to the target item (M. R. Johnson & M. K. Johnson, 2009; M. R. Johnson, Mitchell, Raye, D’Esposito, & M. K. Johnson, 2007; Lepsien & Nobre, 2007; Roth, Johnson, Raye, & Constable, 2009).
As noted, perceptual attention can inhibit as well as facilitate target responses depending on task circumstances. Similarly, refreshing can have both positive and negative effects. Refreshing can increase long-term memory for refreshed versus unrefreshed items (e.g., M. K. Johnson et al., 2002) and produce repetition attenuation in visual cortex for later presentations of refreshed items (Yi, Turk-Browne, Chun, & Johnson, 2008). However, refreshing can also reduce short-term reflective and perceptual access to unrefreshed items (Higgins & Johnson, 2009). The Higgins and Johnson study did not test the effects of refreshing on immediate access to the refreshed items themselves, so it does not speak to whether an IOR-like mechanism could temporarily reduce the accessibility of refreshed representations. The present study sought to examine the impact of reflective attention on subsequent perception of both refreshed and unrefreshed items.
We demonstrate here that refreshing can have a marked negative impact on the speed of subsequently identifying refreshed items. These effects occur for both words and picture stimuli (Experiments 1,3) and appear to be specific to refreshing as opposed to perceptual representation (Experiments 2,4).
Experiment 1
Method
Nine paid participants (2 male, mean age 21.3 years) from Yale University took part in Experiment 1a. An additional 20 participants (12 male, mean age 19.2 years) from Ohio State University (OSU) took part in Experiment 1b for course credit. Procedures for all experiments were approved by the corresponding university’s institutional review board.
In the main task (Figure 1A), participants first saw two words presented onscreen for 1500ms which they were instructed to read silently, followed by a brief blank-screen delay (500ms) and then an arrow pointing to the location of one of the two just-presented words (1500ms). The arrow cued participants to think back to the indicated item and say it aloud. 100ms after the arrow’s offset, participants saw a final word on each trial (1500ms) which could be either a re-presentation of the refreshed word (refreshed probe condition), a re-presentation of the word that was initially presented but not refreshed (unrefreshed probe), or a novel word (novel probe), and were instructed to read the word aloud as quickly and accurately as possible. The inter-trial interval was 3000ms.
Figure 1. Task diagrams.
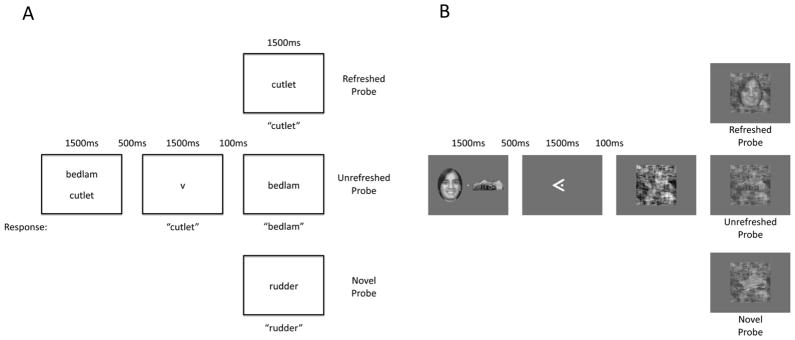
A: Design for Experiment 1. Participants first saw two words, followed by an arrow cue instructing them to think back to (refresh) one of the just-presented words and speak it aloud. After that, a probe word was presented, and participants were instructed to speak it aloud as quickly and accurately as possible. Probes could either be a representation of the refreshed word (refreshed probe) or the unrefreshed word (unrefreshed probe), or a previously unseen novel word (novel probe). The design of Experiment 2 was similar, except that instead of an arrow cue, the word itself was presented onscreen for participants to read aloud. B: Design for Experiment 3. Participants first saw two pictures, followed by an arrow cue instructing them to briefly visualize (refresh) one of the just-presented pictures. A series of scrambled noise images then gradually faded away to reveal either the refreshed item (refreshed probe), the unrefreshed item (unrefreshed probe), or a previously unseen novel item (novel probe). Participants were instructed to press a “stop” button as soon as they detected the probe picture underneath the noise.
The task comprised 144 trials (48 per condition). Stimulus lists were equated (all ps > 0.8) for word length, frequency, number of phonemes, number of syllables, and average time to read the word aloud (Balota et al., 2007); all conditions and lists were fully counterbalanced across participants. Responses were spoken into a microphone and recorded digitally. The digital recording was analyzed using a custom Matlab (MathWorks, Natick, MA) script that detected sounds exceeding a specified amplitude and duration threshold (generally half the standard deviation of the entire recording’s amplitude for at least 100ms) and allowed manual adjustment of the word onset if automatic word detection failed or was triggered early by non-speech sounds. Some recordings with excessive low-frequency background noise were high-pass filtered before processing (cutoff frequency = 100Hz). Trials where participants misspoke, stammered, or spoke too quietly to be detected were discarded (1a: 2.9%, 1b: 8.9%).
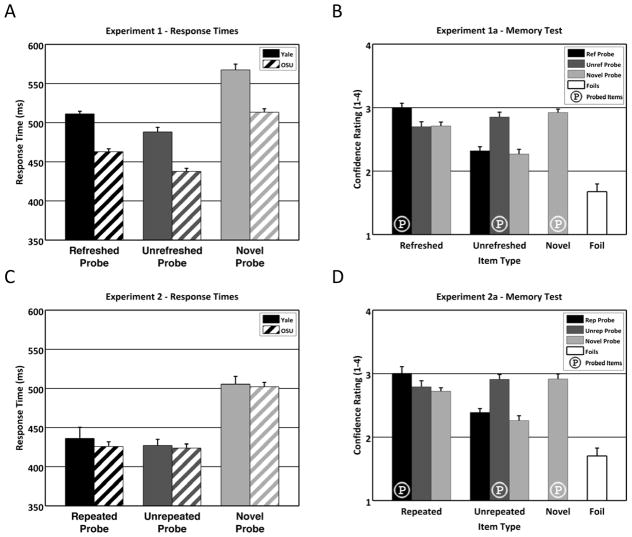
After the main task, participants in Experiment 1a performed an unrelated working memory task with alphabet letters for approximately 20 minutes before receiving a surprise memory test. All 336 words presented in the main task were pseudo-randomly intermixed with 336 foil words (672 trials total). The memory test included 4 main item types: Refreshed words, unrefreshed words, target words, and foils (the four groups of bars in Figure 2B). These can be further subdivided by which condition of the main task they originally appeared in. Refreshed words may have been presented again as probes (in the refreshed probe condition), or they may have been refreshed but not probed (in either the unrefreshed probe or novel probe conditions). Likewise, unrefreshed words may have occurred as probes (in the unrefreshed probe condition) or not (refreshed probe or novel probe conditions). Novel words were seen only in the novel probe condition (the single column of novel items in Figure 2B).
Figure 2. Results from Experiments 1 and 2.
A: Response times for Experiment 1a (Yale) and 1b (OSU). Participants were slower to respond to refreshed probes (1a: 511ms, 1b: 463ms) than unrefreshed probes (1a: 488ms, 1b: 438ms; an IOR-like effect) and slowest to respond to novel probes (1a: 567ms, 1b: 513ms). B: Memory test results for Experiment 1a. Participants indicated higher confidence in remembering all old items versus foils. Probed items (indicated with circled letter ‘P’) were remembered better than non-probed items, but most importantly, refreshed items (leftmost group of bars) were remembered better than unrefreshed items (second group of bars from left). C: Response times for Experiment 2a (Yale) and 2b (OSU). Participants were faster to respond to both repeated (2a: 436ms, 2b: 426ms) and unrepeated probes (2a: 427ms, 2b: 424ms) than novel probes (2a: 505ms, 2b: 502ms), but did not differ in their response times to repeated versus unrepeated probes. D: Memory test results for Experiment 2a. Despite the lack of a response time difference between repeated and unrepeated probes in Experiment 2, the memory test showed the same pattern of results as in Experiment 1a, in particular better memory for repeated (leftmost group of bars) than unrepeated items (second group of bars from left). Error bars in all panels were generated using Morey’s (2008) correction to Cousineau’s (2005) method for creating intuition-fitting error bars for within-subjects comparisons.
In each test trial, a word was presented centrally along with the question “Have you seen this word before?” Participants answered via keypress “Definitely No,” “Maybe No,” “Maybe Yes,” or “Definitely Yes.” Responses were converted into numerical confidence ratings ranging from 1 (confident the item was new) to 4 (confident the item was old). Participants in Experiment 1b performed only the main task.
Results
A one-way repeated-measures ANOVA of probe RTs for the three conditions (Figure 2A) was significant in both Experiment 1a (F2,16 = 49.05, p < 10−6, η2 = 0.86) and 1b (F2,38 = 90.62, p < 10−14, η2 = 0.83). Paired t-tests between all pairs of conditions were also significant (all individual ps < 0.002) in both experiments. Participants responded more slowly to novel probes than to probes that had appeared in the initial display (whether refreshed or unrefreshed), and more slowly to refreshed probes than unrefreshed probes. One might predict items that had just been refreshed and spoken aloud to be more strongly activated and thus show a stronger priming effect (faster RTs); however, the presence of the reverse pattern suggests an effect of reflective attention analogous to IOR.
In contrast, the long-term memory test in Experiment 1a showed an advantage for refreshed items over unrefreshed items (Figure 2B). All types of old items had higher confidence ratings (were better remembered) than foils (all individual t8 > 6.4, p < 0.0002, two-tailed paired t-tests). In addition, probed items (collapsing across refreshed, unrefreshed, and novel probes) were remembered better than non-probed items (collapsed across condition; t8 = 5.86, p < 0.0005). However, to test the primary hypothesis regarding the effects of refreshing, we conducted a 2 (refreshed/unrefreshed words) × 2 (probed/non-probed words) repeated-measures ANOVA, collapsing non-probed words across relevant conditions (e.g., refreshed non-probed words were trials in which the probe was novel or the unrefreshed item; unrefreshed non-probed words were trials in which the probe was novel or the refreshed item). The ANOVA showed significant main effects of refreshing (F1,8 = 26.16, p < 0.001, ηp2 = 0.77) and probe status (F1,8 = 25.91, p < 0.001, ηp2 = 0.76). Planned comparisons indicated that refreshed items were remembered better than unrefreshed items for both probed words (t8 = 2.79, p < 0.03) and non-probed words (t8 = 4.10, p < 0.005). There was also an interaction of probe status and refreshing (F1,8 = 5.40, p < 0.05), due to a greater benefit of refreshing for non-probed words than probed words. Confidence ratings for novel probes were numerically between refreshed probes and unrefreshed probes, but not significantly different from either (p = 0.33 versus refreshed probes, p = 0.28 versus unrefreshed probes).
Experiment 2
Experiment 2 was designed to confirm that Experiment 1’s results were due to reflective attention, and not merely the consequence of having said one (refreshed) word aloud and not the other (unrefreshed) word.
Method
Nine paid participants (all female, mean age 21.7 years) from Yale took part in Experiment 2a. An additional 21 participants (12 male, mean age 18.7 years) from OSU took part in Experiment 2b for course credit. The design was identical to that of Experiment 1, with one critical change: In the main task, instead of an arrow cuing participants to refresh a just-presented word and say it aloud, the word itself was printed onscreen. The duration of the second event (in this case the repeated word) was reduced from 1500ms to 1300ms, and the delay between the second event and probe increased from 100ms to 300ms, to make the transition between the repeated word and the probe more obvious while keeping all stimulus onset asynchronies (SOAs) identical to those of Experiment 1. Participants were instructed to read the initial word pair silently to themselves, and then read aloud the two subsequently presented words as quickly and accurately as possible. The same stimulus lists, equipment, retention interval filler task, and memory test were used as in Experiment 1. The analogue of Experiment 1’s refreshed probe condition was the repeated probe condition, and the analogue of the unrefreshed probe condition was the unrepeated probe condition. Participants in Experiment 2b performed only the main task. 5.0% of trials were discarded in Experiment 2a; 6.8% were discarded in 2b.
Results
A one-way repeated-measures ANOVA of probe RTs for the three conditions (Figure 2C) was significant in both Experiment 2a (F2,16 = 15.17, p < 0.0005, η2 = 0.66) and 2b (F2,40 = 68.63, p < 10−12, η2 = 0.77). Paired t-tests showed that the significant ANOVA result was due only to repeated (2a: t8 = 3.61, p < 0.01; 2b: t20 = 9.52, p < 10−8) and unrepeated (2a: t8 = 9.16, p < 0.0001; 2b: t20 = 11.11, p < 10−9) probes being faster than novel probes. Critically, RTs for the repeated probe and unrepeated probe conditions did not differ (2a: p = 0.61; 2b: p = 0.80). This indicates that merely saying a word aloud is insufficient to induce an IOR-like effect, and suggests that Experiment 1’s effect indeed resulted from reflective attention. To confirm this difference between Experiments 1 and 2, we combined the Yale and OSU participant groups and performed a mixed 2 (Experiment: 1 vs. 2) × 2 (Condition: refreshed/repeated vs. unrefreshed/unrepeated) ANOVA. The critical interaction between Experiment and Condition was significant (F1,57 = 6.03, p < 0.02, ηp2 = 0.10), confirming the difference between the two experiments and suggesting that the lack of an IOR-like effect in Experiment 2 was unlikely to be due to insufficient statistical power. (We also performed a 3-way ANOVA with the additional factor of location [Yale/OSU] to determine whether it was appropriate to combine those groups. There was no significant interaction with location [all p > 0.19] and only a weak trend for a main effect [p = 0.09], with OSU participants responding somewhat faster overall. Thus, we felt it appropriate to combine the groups. However, the critical interaction was also significant in the larger OSU sample alone [F1,39 = 6.00, p < 0.02, ηp2 = 0.13]).
However, as expected, Experiment 2a’s memory test results (Figure 2D) were similar to those of Experiment 1a. Old items had higher confidence ratings than foils (all individual t8 > 5.7, p < 0.0005), and probed items were remembered better than non-probed items (t8 = 5.95, p < 0.0005). Furthermore, the 2 (repeated, unrepeated) × 2 (probed, non-probed) repeated-measures ANOVA showed main effects of repetition (F1,8 = 14.48, p < 0.006, ηp2 = 0.64) and probe status (F1,8 = 25.86, p < 0.001, ηp2 = 0.76) and a significant interaction (F1,8 = 5.71, p < 0.05, ηp2 = 0.42), with a greater benefit of repetition for non-probed words than probed words, all analogous to the effects in Experiment 1a. Planned comparisons showed that repeated items were remembered significantly better than unrepeated items for non-probed words (t8 = 4.51, p < 0.002), and numerically but not significantly better for probed words (p = 0.38). The similar memory test results suggest again that the lack of an IOR-like RT effect for repeated items was not due to insufficient power or poorer attention in Experiment 2.
Experiment 3
Experiment 3 tested whether the effects seen in Experiment 1 also occurred for non-word stimuli.
Method
Twenty-two paid participants (5 male, mean age 20.9 years) from Yale took part in Experiment 3a. An additional 29 participants (10 male, mean age 19.6 years) from OSU took part in Experiment 3b for course credit; one other participant was excluded as an outlier for having overall RTs over three standard deviations slower than the group mean. The task (Figure 1B) was conceptually similar to Experiment 1’s main task, but used both different materials and a different probe measure. Participants first saw two items (drawn from a set of chair, face, house, or shoe stimuli; Newman & Norman, 2010) presented side by side for 1500ms, followed by a 500ms delay and then an arrow pointing to the location of one of the just-presented items (1500ms). The arrow cued participants to think back to the indicated item and briefly visualize it. 100ms after the arrow’s offset, a series of noise images (formed by phase-scrambling pieces of randomly selected picture stimuli) flashed onscreen at 30Hz. A probe image faded into view underneath the changing noise images (starting at 10% opacity and fading in at the rate of 60% opacity per second); probes could be either a re-presentation of the refreshed picture (refreshed probe condition), a re-presentation of the picture that was initially presented but not refreshed (unrefreshed probe condition), or a novel picture (novel probe condition). Participants were instructed to press a “stop” button under their non-dominant index finger as soon as they detected the probe picture. When they did, the probe display disappeared and a screen appeared asking them whether the probe was a chair, face, house or shoe. Participants then indicated the category using the four non-thumb fingers of their dominant hand to confirm that they had indeed identified the probe.
The task comprised 216 trials (72 per condition), divided into 3 blocks. The initial presentation always contained two pictures from different categories (e.g., chair-face or chair-shoe, but never two chairs), and in the novel probe condition, the probe’s category was always different from both the refreshed and unrefreshed stimuli. Each block contained equal distributions of each stimulus category in every position; trial orders and the particular stimuli seen in each trial were randomized without replacement. Participants were instructed to maintain central fixation between the onset of the initial stimulus pair and the offset of the probe display. RTs were measured from the onset of the noise images to the participant’s “stop” button press. Trials (3a: 2.3%, 3b: 2.1%) in which participants answered the category question incorrectly were discarded. No memory test was administered.
Results
A one-way repeated-measures ANOVA of probe RTs for the three conditions (Figure 3A) was significant in both Experiment 3a (F2,42 = 15.50, p < 10−5, η2 = 0.42) and 3b (F2,56 = 6.24, p < 0.005, η2 = 0.18). Paired t-tests between all pairs of conditions were also significant (all individual p < 0.05) except for unrefreshed vs. novel probes in Experiment 3b (t28 = 1.45, p = 0.16). Participants responded fastest to novel probes, followed by unrefreshed probes, and slowest for refreshed probes. Thus, the same IOR-like effect (slower responses to refreshed than unrefreshed probes) was seen as in Experiment 1. The fact that novel probes in Experiment 3 had the fastest RTs, whereas in Experiments 1 participants were slowest to respond to novel probes, may result from the difference in stimulus category (pictures/words), probe type (button press/verbal response), or a combination of these factors. The combination seems particularly likely, as the verbal responses (to read the probe word aloud) in Experiments 1–2 would likely be primed by participants’ previously seeing the refreshed and unrefreshed words, whereas in Experiment 3, the time-critical response (a “stop” button press) was the same across all conditions, and thus unlikely to be primed by previously seen stimuli.
Figure 3. Results from Experiments 3 and 4.
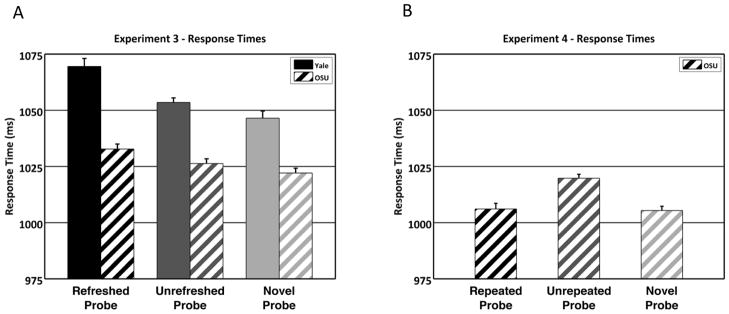
A: Response times for Experiment 3a (Yale) and 3b (OSU). As in Experiment 1, participants were slower to respond to refreshed items (3a: 1069ms, 3b: 1033ms) than unrefreshed items (3a: 1053ms, 3b: 1026ms). However, in this experiment, participants were fastest (rather than slowest) to respond to novel probes (3a: 1046ms, 3b: 1022ms), likely due to changes in the probe response between Experiments 1 and 3 (see main text). B: Response times for Experiment 4. In this experiment, participants were actually faster to respond to repeated probes (1006ms) than unrepeated probes (1020ms). Responses to novel probes (1005ms) were also faster than those to unrepeated probes, but did not differ from repeated probes. Error bars in all panels were generated using Morey’s (2008) correction to Cousineau’s (2005) method for creating intuition-fitting error bars for within-subjects comparisons.
Experiment 4
As Experiment 2 did for Experiment 1, Experiment 4 tested whether Experiment 3’s IOR-like effect was indeed specific to the case of reflective attention, and could not be induced simply by a perceptual re-presentation of a stimulus.
Method
Twenty-nine undergraduates from OSU (18 male, mean age 19.7 years) participated in Experiment 4 for course credit; one additional participant was excluded as an outlier for having overall RTs over three standard deviations slower than the group mean. The design was identical to that of Experiment 3, except that instead of the arrow cuing participants to refresh one just-presented picture, the picture itself was shown onscreen. Participants were instructed simply to view and pay attention to the presented pictures, and then respond to the probe stimulus as in Experiment 3. The same stimuli, timings, and equipment were used as in Experiment 3b. Error trials (2.0%) were again discarded.
Results
A one-way repeated-measures ANOVA of probe RTs for the three conditions (Figure 3B) was significant (F2,56 = 15.13, p < 10−5, η2 = 0.35). Paired t-tests showed that in the critical comparison between repeated and unrepeated probes, participants actually responded faster to repeated probes (t28 = 4.29, p < 0.0005). Participants also responded faster to novel than unrepeated probes (t28 = 6.55, p < 10−6), and RTs did not differ between repeated and novel probes (p = 0.83). This suggests once again that mere perceptual repetition is insufficient to drive the IOR-like effect observed in Experiment 3, and in fact the opposite effect was observed in Experiment 4.
To confirm the difference between Experiments 3 and 4, we combined the Experiment 3a and 3b participant groups and performed a mixed 2 (Experiment) × 2 (Condition: refreshed/repeated vs. unrefreshed/unrepeated) ANOVA. The critical interaction between Experiment and Condition was significant (F1,78 = 35.16, p < 10−7, ηp2 = 0.31). This confirms the difference between the results of Experiments 3 and 4, and coupled with the fact that participants responded significantly slower to unrepeated than repeated probes in Experiment 4, suggests again that the lack of an IOR-like effect in Experiment 4 was not due to insufficient statistical power or participants’ failure to attend to the task. (As Experiment 4 was conducted only at OSU, we could not run a three-way ANOVA to determine whether location [Yale/OSU] affected the interaction; however, it was also significant in the OSU sample alone [F1,56 = 20.77, p < 0.0001, ηp2 = 0.27]).
Discussion
These studies demonstrate that participants are slower to respond to a word (Experiment 1) or picture (Experiment 3) that was just the target of internal/reflective attention than one that was not. Importantly, this effect does not occur when words or pictures are simply shown again without participants reflectively accessing an active memory representation (Experiments 2,4).
This apparent short-term inaccessibility of recently refreshed representations stands in contrast to the long-term memory benefit we observed for refreshed versus unrefreshed words (Experiment 1a), which replicates previous findings of better memory for refreshed versus unrefreshed items (e.g., M. K. Johnson et al., 2002). Thus, whatever mechanisms underlie the impairment in responding to refreshed items at the short-term (~1sec) time scale do not persist forever, but eventually (by ~20min later, in this study) cross over into a long-term memory benefit. Of course, the differences in time scale and task (the implicit initial probe versus a later explicit recognition test) make it difficult to directly compare one form of short-term impairment to another form of long-term facilitation; thus, future studies may find it helpful to employ a more implicit long-term test and/or a shorter retention interval in order to investigate the transition from impairment to facilitation more fully.
This short-term negative impact on identifying an item whose representation was recently the target of (and presumably enhanced by) reflective attention invites a comparison to the short-term IOR caused by visuo-spatial attention. To our knowledge, no such effect has previously been reported in the IOR literature or other areas of cognitive psychology. Fuentes, Vivas, and Humphreys (1999) reported a somewhat similar “semantic IOR” effect for words, but that effect differs from the IOR-like effects we observed in two critical ways. First, their design involved only perception of the items in question, without a reflective attention cue, whereas our Experiment 2 demonstrated that perceptual repetition without reflective attention did not produce the same IOR-like effect we observed in Experiment 1. Second, Weger and Inhoff (2006) demonstrated that Fuentes et al.’s semantic IOR effect depended on extensive item repetition and a small, homogenous set of items, whereas our IOR-like effects were observed using unique, heterogeneous stimulus sets.
Although both the present effect and traditional IOR effects share the characteristic of slowed responses to an item/location that was recently the focus of attention, the two effects may or may not be mechanistically related. Future studies (e.g., using neuroimaging) would be necessary to establish if the two effects stem from a common neural source, if they result from a similar neural phenomenon (e.g., habituation) occurring at distinct loci, or if their underlying neural sources are unrelated. Complicating the present study’s interpretation is that even traditional IOR is not completely understood; it may arise as a result of activity at multiple levels of the nervous system, and deciding which effects should even be labeled as IOR is subject to debate (for review, see Dukewich, 2009). Thus, “IOR” may be a descriptor for a class of perceptual and reflective attention effects with varying degrees of similarity in their neural or behavioral profiles; alternatively, evidence may arise for a qualitative distinction between “true” IOR and other effects.
Either way, additional studies manipulating the SOA of the probe would help characterize our reflective IOR-like effect in relation to traditional IOR effects. Spatial IOR typically begins 200–300ms after the initial cue and may persist for several seconds (Klein, 2000), with attentional facilitation occurring at shorter SOAs. Although reflective attention can enhance active neural representations in a manner similar to perceptual attentional enhancement (M. R. Johnson & M. K. Johnson, 2009), it is unknown whether reflective attention would similarly facilitate behavioral responses to refreshed stimuli at shorter SOAs before IOR-like effects begin. If demonstrated, such a pattern of facilitation at very short time spans (less than ~250ms) followed by inhibition would support the idea that traditional IOR and our reflective IOR-like effect stem from similar cognitive mechanisms. A related possibility is that IOR’s general pattern of facilitation followed by later inhibition may occur for reflective attention, but at a slower time scale due to differences between exogenously cued perceptual attention and endogenously cued reflective attention. The timing of endogenous reflective attention events is likely to be more variable as well; thus, high-temporal-resolution recording methods such as electroencephalography (EEG) could be helpful not only for describing the general neural profile of reflective IOR-like effects, but also for determining the onsets of internal mental events in order to time-lock probe presentations more precisely relative to them.
Demonstrating facilitation at shorter delays would certainly strengthen the analogy between traditional IOR and the IOR-like slowing we report here, but even if such facilitation does not emerge in future studies, the strong inhibition of subsequent probe processing we have observed for instances of reflective attention (but not perceptual repetition) is nonetheless a striking and noteworthy effect deserving of additional study. Thus, in either event, further investigations of these possibilities should provide a more fine-grained understanding of IOR-like response slowing in both the reflective and the traditional perceptual domains, as well as shedding further light on the relationship and possible overlap between the neurocognitive processes comprising reflective and perceptual attention.
References
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, Neely JH, et al. The English Lexicon Project. Behavior Research Methods. 2007;39(3):445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Chen Z, Cowan N. How verbal memory loads consume attention. Memory & Cognition. 2009;37(6):829–836. doi: 10.3758/MC.37.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Golomb JD, Turk-Browne NB. A taxonomy of external and internal attention. Annual Review of Psychology. 2011;62:73–101. doi: 10.1146/annurev.psych.093008.100427. doi:0.1146/annurev.psych.093008.100427. [DOI] [PubMed] [Google Scholar]
- Chun MM, Johnson MK. Memory: Enduring traces of perceptual and reflective attention. Neuron. 2011;72:520–535. doi: 10.1016/j.neuron.2011.10.026. doi:0.1016/j.neuron.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson’s method. Tutorials in Quantitative Methods in Psychology. 2005;1:42–45. [Google Scholar]
- Dukewich KR. Reconceptualizing inhibition of return as habituation of the orienting response. Psychonomic Bulletin & Review. 2009;16(2):238–251. doi: 10.3758/PBR.16.2.238. [DOI] [PubMed] [Google Scholar]
- Fuentes LJ, Vivas AB, Humphreys GW. Inhibitory mechanisms of attentional networks: Spatial and semantic inhibitory processing. Journal of Experimental Psychology: Human Perception and Performance. 1999;25(4):1114–1126. doi: 10.1037/0096-1523.25.4.1114. [DOI] [Google Scholar]
- Higgins JA, Johnson MK. The consequence of refreshing for access to nonselected items in young and older adults. Memory & Cognition. 2009;37(2):164–174. doi: 10.3758/MC.37.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. The principles of psychology. New York, NY: Henry Holt and Company; 1890. [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Cunningham WA, Sanislow CA. Using fMRI to investigate a component process of reflection: prefrontal correlates of refreshing a just-activated representation. Cognitive, Affective, & Behavioral Neuroscience. 2005;5(3):339–361. doi: 10.3758/cabn.5.3.339. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Reeder JA, Raye CL, Mitchell KJ. Second thoughts versus second looks: an age-related deficit in reflectively refreshing just-activated information. Psychological Science. 2002;13(1):64–67. doi: 10.1111/1467-9280.00411. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Johnson MK. Top-down enhancement and suppression of activity in category-selective extrastriate cortex from an act of reflective attention. Journal of Cognitive Neuroscience. 2009;21(12):2320–2327. doi: 10.1162/jocn.2008.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MR, Mitchell KJ, Raye CL, D’Esposito M, Johnson MK. A brief thought can modulate activity in extrastriate visual areas: Top-down effects of refreshing just-seen visual stimuli. NeuroImage. 2007;37(1):290–299. doi: 10.1016/j.neuroimage.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends in Cognitive Sciences. 2000;4(4):138–147. doi: 10.1016/S1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Klein RM, MacInnes WJ. Inhibition of return is a foraging facilitator in visual search. Psychological Science. 1999;10(4):346–352. doi: 10.1111/1467-9280.00166. [DOI] [Google Scholar]
- Lepsien J, Nobre AC. Cognitive control of attention in the human brain: insights from orienting attention to mental representations. Brain Research. 2006;1105(1):20–31. doi: 10.1016/j.brainres.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Nobre AC. Attentional modulation of object representations in working memory. Cerebral Cortex. 2007;17(9):2072–2083. doi: 10.1093/cercor/bhl116. [DOI] [PubMed] [Google Scholar]
- Morey RD. Confidence intervals from normalized data: A correction to Cousineau (2005) Tutorial in Quantitative Methods for Psychology. 2008;4:61–64. [Google Scholar]
- Newman EL, Norman KA. Moderate excitation leads to weakening of perceptual representations. Cerebral Cortex. 2010;20(11):2760–2770. doi: 10.1093/cercor/bhq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis D, editors. Attention and Performance. X. Hillsdale, NJ: Erlbaum; 1984. pp. 531–556. [Google Scholar]
- Posner MI, Rafal RD, Choate LS, Vaughan J. Inhibition of return: Neural basis and function. Cognitive Neuropsychology. 1985;2(3):211–228. doi: 10.1080/02643298508252866. [DOI] [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Reeder JA, Greene EJ. Neuroimaging a single thought: dorsolateral PFC activity associated with refreshing just-activated information. NeuroImage. 2002;15(2):447–453. doi: 10.1006/nimg.2001.0983. [DOI] [PubMed] [Google Scholar]
- Roth JK, Johnson MK, Raye CL, Constable RT. Similar and dissociable mechanisms for attention to internal versus external information. NeuroImage. 2009;48(3):601–608. doi: 10.1016/j.neuroimage.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger UW, Inhoff AW. Semantic inhibition of return is the exception rather than the rule. Perception & Psychophysics. 2006;68(2):244–253. doi: 10.3758/bf03193673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi D, Turk-Browne NB, Chun MM, Johnson MK. When a thought equals a look: refreshing enhances perceptual memory. Journal of Cognitive Neuroscience. 2008;20(8):1371–1380. doi: 10.1162/jocn.2008.20094. [DOI] [PMC free article] [PubMed] [Google Scholar]