Abstract
PCBs have long been known to affect dopamine (DA) function in the brain. The current study used an amphetamine behavioral sensitization paradigm in rats developmentally exposed to PCBs. Long-Evans rats were given perinatal exposure to 0, 3, or 6 mg/kg/day PCBs and behavioral sensitization to d-amphetamine (AMPH) was assessed in one adult male and female/litter. Non-exposed (control) males showed increasing locomotor activity to repeated injections of 0.5 mg/kg AMPH, typical of behavioral sensitization. PCB-exposed males showed greater activation to the initial acute AMPH injection, but sensitization occurred later and was blunted relative to controls. Sensitization in control females took longer to develop than in the males, but no exposure-related differences were observed. Analysis of whole brain and serum AMPH content following a final IP injection of 0.5 mg/kg revealed no differences among the exposure groups. Overall, these results indicated developmental PCB-exposure can alter the motor-stimulating effects of repeated AMPH injections. Males developmentally exposed to PCBs appeared to be pre-sensitized to AMPH, but quickly showed behavioral tolerance to the same drug dose. Results also revealed the behavioral effect was not due to exposure-induced alterations in AMPH metabolism following PCB exposure.
Keywords: polychlorinated biphenyls, neurotoxicology, behavioral pharmacology, behavioral sensitization, amphetamine
1. Introduction
Polychlorinated biphenyls (PCBs) are neurotoxic, environmental contaminants formerly used as lubricants and dielectric fluids (Erikson, 1986). Over time, PCBs have been dispersed into rivers, lakes, air and soil and have bioaccumulated within the ecosystem. A major concern is that PCBs can cross the placenta and be released during lactation (Jacobson et al., 1984; Sable and Schantz, 2006) thereby exposing the developing fetus/infant to potentially irreversible neuroteratogenic effects.
PCBs have been shown to impair executive function (Sable and Schantz, 2006). In particular, deficits in inhibitory control have been reported following developmental PCB exposure in rats (Holene et al., 1998; Holene et al., 1999; Lilienthal et al., 1990; Sable et al., 2006; Sable et al., 2009; Widholm et al., 2001; Widholm et al., 2004), monkeys (Levin et al., 1988; Mele et al., 1986; Rice, 1997; Rice, 1998), and children (Jacobson and Jacobson, 2003; Stewart et al., 2006). Inhibitory control deficits occur following depletion of dopamine (DA) in the prefrontal cortex (PFC) (Sokolowski and Salamone, 1994) of rats, and a number of studies have shown that PCB exposure reduces brain DA levels (Fonnum et al., 2006), suggesting a possible neurochemical mechanism for the inhibitory control deficits observed following PCB exposure.
With prolonged exposure, PCBs reduce the amount of DA in the extraneuronal space in the striatum of adult rats (Seegal et al., 2002). This reduction is thought to occur as a result of end-product inhibition. Initially, PCBs inhibit the DA transporter (DAT) which is important for DA reuptake (Richardson and Miller, 2004). DAT inhibition results in an initial increase in DA in the extraneuronal space, but too much DA in the synapse is believed to activate presynaptic autoreceptors which then limit DA release. In addition, PCBs have also been shown to inhibit the vesicular monoamine transporter (VMAT) which is important for packaging free DA in the axon terminal into synaptic vesicles. This limited packaging is also believed to contribute to the reduction in extraneuronal DA (Bemis and Seegal, 2004; Mariussen et al., 1999). The PCB exposure studies mentioned above were conducted in adult rodents, but one study measured DA concentrations following in utero and lactational exposure to the non-coplanar PCB congener, 2,4,2′,4′-tetrachlorobiphenyl (PCB 47). Significant decreases in DA levels in the frontal cortex and caudate nucleus were found when the rats were adults (Seegal et al., 1997).
Inhibitory control deficits are concerning because they have been shown to contribute to drug abuse and addiction in humans (de Wit, 2009; Ivanov et al., 2008; Tarter et al., 2007; Verdejo-Garcia et al., 2008; Volkow et al., 2002; Volkow et al., 2004) and animals (Belin et al., 2008; Dalley et al., 2007; Jentsch and Taylor, 1999; Mitchell et al., 2006; Perry et al., 2005; Steketee, 2003; Winstanley et al., 2010). Studies also suggest there may be a relationship between reduced DA activity in certain brain regions and later drug-seeking. For example, lesions of midbrain dopaminergic neurons projecting to the medial prefrontal cortex (mPFC) resulted in the acquisition and maintenance of cocaine and amphetamine (AMPH) self-administration at lower doses than in sham controls (Schenk et al., 1991; McGregor et al., 1996; Weissenborn et al., 1997). Human imaging studies have also revealed detoxified psychostimulant abusers had reduced DA D2 receptors in the prefrontal cortex indicative of decreased DA activity (Volkow et al., 2002), although it was not clear whether these differences contributed to, or were the result of, prior drug use.
Given that developmental PCB exposure results in decreased levels of DA in the prefrontal cortex (Seegal et al., 1997) as well as deficits in inhibitory control (Sable et al., 2006; Sable et al., 2009; Stewart et al., 2006), it seems likely that rats with such PCB exposure would respond differently to psychostimulants than non-exposed controls. One method that has been used to assess sensitivity to psychostimulants is the behavioral sensitization paradigm. Behavioral sensitization, which is the increase in locomotor response after repeated exposure to a psychostimulant (Robinson and Becker, 1982), has been postulated to underlie certain mechanisms of drug addiction because the psychomotor effects of drugs are assumed to share the same neural substrate that mediates the reward circuit (Robinson and Berridge, 2000). Of special significance to the current project is the fact that DA hypofunctionality in the mPFC has been linked to the expression of behavioral sensitization to psychostimulant drugs (Steketee, 2003). The current study used the behavioral sensitization paradigm as a model to test psychostimulant sensitivity in rats developmentally exposed to PCBs. Because a decrease in DA function in the mPFC is associated with behavioral sensitization to psychostimulants (Steketee, 2003), and because developmental PCB exposure has been shown to produce a long-term decrease in DA activity in the mPFC (Seegal et al., 1997), it was hypothesized that developmental PCB exposure would enhance behavioral sensitization to AMPH in these rats when tested as adults.
2. Method
2.1. Animals
Nulliparous female Long Evans rats, approximately 60 days of age, were purchased from Harlan (Indianapolis, IN) in two cohorts spaced about 6 months apart. The rats were maintained in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). Specifically, rats were individually housed in a temperature- and humidity- controlled room (22 degrees C, 40–55% humidity) on a 12-hour light-dark cycle (lights on at 0800 h) in ventilated, plastic shoebox cages with corn-cob bedding. Standard rat chow and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign and were in accordance with the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals (National Institutes of Health, 2002) and The Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003).
2.2. Exposure
After one week of adaptation, the rats were assigned to exposure groups evenly distributed by body weight, and began receiving daily PCB doses of 0, 3, or 6 mg/kg body weight. Exposure began 28 days prior to mating and continued until pups were weaned on postnatal day (PND) 21. The PCB mixture was formulated to mimic the congener profile found in walleye—a popular sport-caught fish—taken from the Fox River in northeast Wisconsin (Kostyniak, et al., 2005). The mixture consisted of: 35% Aroclor 1242 (Monsanto Lot KB 05-415); 35% Aroclor 1248 (AccuStandard Lot F-110); 15% Aroclor 1254 (Monsanto Lot KB 05-612); and 15% Aroclor 1260 AccuStandard Lot 021-020). The PCB mixture was diluted in corn oil (Mazola®) and pipetted onto one-half of a vanilla wafer cookie (Keebler Golden Vanilla Wafers®) at a volume of 0.4 mL/kg. Corn oil vehicle alone was pipetted onto cookies for rats in the control group. Cookies were fed to the female rats daily at approximately 1100 hr. Doses were adjusted daily to account for weight gain. Consumption of the cookies was visually confirmed. Dams generally ate the cookies within 15 minutes.
2.3. Breeding, pregnancy, and weaning
Four weeks after the initial PCB exposure, each female was paired with an unexposed male Long-Evans rat (Harlan, Madison, WI) in a hanging wire cage for eight consecutive days. Females were returned to their home cage briefly each day for PCB dosing, and were monitored twice daily for the presence of a sperm plug to determine gestational day (GD) 0. Of the 37 dams that began the study (cohort 1 = 18 dams; cohort 2=19 dams), all were found with a sperm plug. All dams within the first cohort were pregnant and had usable litters. Within the second cohort, there was one control dam, one dam dosed with 3 mg/kg/day PCBs, and two dams dosed with 6 mg/kg/day PCBs that did not get pregnant. In addition, there were also two control dams, and one dam in each of the PCB dosing groups that had litters that were too small to use (<7 pups).
On the day of parturition (PND 0), the pups were examined for gross abnormalities, the number of stillbirths was noted, and the pups were sexed and weighed. On PND 2, litters were culled to 10 pups (5 females and 5 males when possible); cross-fostering extra pups of the same age and from the same treatment group was done to bring small litters that had at least 7 pups up to 10 pups total. Cross-fostered pups were marked by ear clip and were not used as subjects in the study. The gestational and developmental outcomes of the dams and of pups that were littermates to those used in this study are reported elsewhere (Sable et al., 2011).
On PND 21, the dam from each litter was euthanized via overexposure to CO2, her liver weighed, and the number of implantation sites recorded. One male and female pup per litter were also euthanized via overexposure to CO2, and organ weights (brain, liver, and thymus) were obtained. When available, four pups from each litter were retained − 1 male and 1 female for behavioral sensitization testing (reported here) and 1 male and 1 female for drug discrimination testing (see Sable et al., 2011). Pups retained on the day of weaning were housed in same-litter, same-sex pairs with food and water available ad libitum until PND 60. Between PND 7 and PND 60, pups were weighed weekly. Nine male-female littermate pairs previously exposed to 6 mg/kg/day PCBs completed behavioral sensitization testing, while 10 littermate pairs completed testing for the control and 3 mg/kg/day PCB groups.
2.4. Drugs
D-amphetamine sulfate (Sigma; St. Louis, MO) was dissolved in 0.9% sterile saline to a concentration of 0.5 mg/mL for the 0.5 mg/kg dose. Dosages were selected based on pilot research in our lab and previous use in similar experiments of behavioral sensitization (Hall et al., 2008; Mathews et al., 2008). All AMPH doses were freshly mixed prior to each day of administration and maintained in areas of low light to prevent photodecomposition.
2.5. Apparatus
Behavioral testing was conducted in two identical automated plexiglass locomotor activity chambers (40.64 × 40.64 × 38.10 cm; San Diego Instruments, San Diego, CA) linked to a computer. Each chamber was equipped with a 16 × 16 array of horizontal infrared photobeams located 3.5 cm above the floor and spaced 2.54 cm apart which made a grid on the floor of the chamber. The photobeams divided each chamber into 289 equally sized zones. Flex/Open-field software (San Diego Instruments) recorded the number of photobeam breaks by sampling the status of all photobeams every 100 milliseconds. The software compared the x-y position of the rat to that recorded previously: an ambulation was recorded if at least one previously interrupted photobeam was no longer interrupted, and at least one new, previously uninterrupted photobeam was interrupted. Rearing was recorded via a second array of 16 photobeams located 13 cm above the floor and spaced 2.54 cm apart.
2.6. Procedure
2.6.1. Food restriction
Beginning at PND 60, food access was restricted to reduce body weight to about 85% of free-feeding weight. Food was restricted so that food rewards could be used as motivation for the cage mates of these rats which were tested in a drug discrimination task as well as to promote consistent body weight and better health in the adult animals (Masoro, 2009). Food restriction has been used routinely in our lab and there is no evidence that it confounds PCB-mediated effects.
2.6.2. Behavioral sensitization testing
Behavioral sensitization testing started when the rats were approximately 75 days of age. Each session of activity testing consisted of a pre-injection habituation period and a post-injection test period. Each session began by placing the rat into the chamber where activity as indicated by photobeam breaks was recorded for 30 mins (consisting of six, 5-min blocks). After the pre-injection period, the rat was given an intraperitoneal (IP) injection of either saline (on day 1 exclusively) or of 0.5 mg/kg AMPH (on days 2–6, 10) at which point the rat was immediately placed back into the activity box for the post-injection test period which lasted 60 mins (consisting of twelve, 5-min blocks). The rats remained in their home cages for 3 days (days 7–9) during which time no drug treatment was given and no testing was conducted. Cages were thoroughly cleaned between sessions and the chambers were tested daily to confirm they were in working order.
2.6.3. Brain/serum AMPH content
After behavioral testing was complete all rats were given a final ip injection of 0.5 mg/kg AMPH, 10 min prior to euthanasia via live decapitation. Whole brain and plasma samples were collected, rapidly frozen in liquid nitrogen, and stored at −80°C until analysis. AMPH content was determined via GC/MS-NCI in a randomly selected subset of animals (n=5 litters/exposure group) using a modification of published procedures (Varian application notes M1992 & M3163, and Wu et al., 2008). Approximately 0.3 g of serum was diluted with 0.1 M phosphate buffer (pH 6.0) and spiked with 100 ng of deuterated internal standard (amphetamine-d5). Sample extraction and cleanup utilized a Bond Elut Certify 130 mg cartridge (Varian Inc., Walnut Creek, CA), conditioned with methanol and 0.1 M phosphate buffer. The cartridge was rinsed with deionized water followed by 0.1 M acetic acid, and vacuum-dried, washed with methanol and again vacuum-dried. After elution with a mixture of dichloromethane-isopropanol-ammonium hydroxide (78:20:2, v/v/v), 50 μl of methanol-hydrochloric acid (99:1, v/v) was added to the eluate, dried under nitrogen, and reconstituted in ethyl acetate and heptafluorobutyric anhydride (HFBA), and derivatized at 70°C for 30 min. One ml of 1M phosphate buffer (pH7.0) was then added, mixed and centrifuged. The organic layer was transferred to GC vial and analyzed by GC/MS-NCI.
For brain samples, approximately 0.2 g of tissue was homogenized in 0.1 M phosphate buffer (pH 6.0), then sonicated for 15 min. The homogenate was spiked with 100 ng of deuterated internal standard and centrifuged. The supernatant was loaded on Bond Elut Certify 500 mg cartridge pre-conditioned with methanol followed by 0.1 M phosphate buffer. The remaining procedures were similar to those described for serum samples.
GC/MS-NCI analysis was carried out with an Agilent 6890 GC/5973 MS with a capillary column (DB-5MS, 15 m × 0.25 mm i.d., 0.10 μm film thickness; Agilent J&W, Palo Alto, CA). The injection temperature was 250°C and helium gas flow rate was 1.0 ml/min. The oven temperature was held at 60°C for 1 min, then increased to 290°C at 30°C/min and held at 290°C for 3.33 min. Selected ions were monitored in SIM mode at ions 311 (AMPH derivatized with HFBA) and 316 (amphetamine-d5 derivatized with HFBA). A sample blank and two QC spike samples prepared in the same sample matrix were included in each batch run. Concentrations were determined from a standard curve. The LOD and LOQ of the analyses for serum samples were 4.2 ng/g and 14.1 ng/g and for brain samples 7.9 ng/g and 26.3 ng/g, respectively.
2.7. Data analysis
All statistical analyses were conducted using SPSS for MS Windows (version 15.0, SPSS Inc.; Chicago, IL) with statistical significance for the omnibus analyses set at p<0.05. In the case of some repeated measures factors, a sphericity violation was noted. In such cases, a Greenhouse-Geisser correction was used to reduce the risk of a Type I error if ε was < 0.75 and a Huynh-Feldt correction was used when ε was > 0.75 (Rogan et al., 1979). Analyses requiring such correction are reported in the results using the appropriate adjusted degrees of freedom. Cohort was initially included as a factor in all analysis. When no cohort effects were found, the data were combined and this factor removed to simplify the design.
In the interest of brevity, only significant exposure-related results are presented. When significant main effects of exposure or exposure-related interactions were obtained from the omnibus analyses, additional post hoc Dunnett t-tests were conducted on the data from each testing day to determine if the PCB groups were different from the control group on that day. Given that the Dunnett’s procedure alters the critical value to maintain experimental-wise error = 0.05 (Maxwell and Delaney, 2000), no adjustments to the significance level were made. Post hoc simple contrasts were also conducted across treatment days for each exposure group in order to determine on which days sensitization occurred. To control for the increased risk of Type I error that accompanies multiple simple contrasts, a Bonferroni correction was used (=0.05/5 contrasts) and the significance level adjusted to p≤0.01.
The habituation data collected prior to the very first saline injection were analyzed via a 3 (exposure group) x 2 (sex) x 6 (5-min block) mixed ANOVA where exposure group and cohort were between-subjects factors, and sex (nested within litter), and 5 minute blocks of time were repeated measures factors. This was done in order to determine if there was a difference between groups in the novelty response to the testing chamber. The remaining habituation data collected prior to AMPH injections were analyzed in a separate analysis using the same factors as the novelty data and including an additional repeated measures factor of “day” to represent the 6 days on which habituation was measured prior to AMPH administration. The data collected post-AMPH injection were analyzed in the same way as the pre-injection data except that there were twelve, 5-min blocks. Finally, a sensitization score calculated by subtracting the total number of photobeam breaks on day 2 from the total number on day 10 was determined for each animal as an indicator of the magnitude of behavioral sensitization exhibited on the last day of testing. The sensitization scores for the males and females were analyzed separately using a one-way ANOVA for exposure group.
The whole brain and plasma AMPH data were analyzed separately via a 3 (exposure group) x 2 (sex) mixed ANOVA where exposure group and cohort were between-subjects factors, and sex (nested within litter) was a repeated measures factor.
3. Results
3.1. Habituation
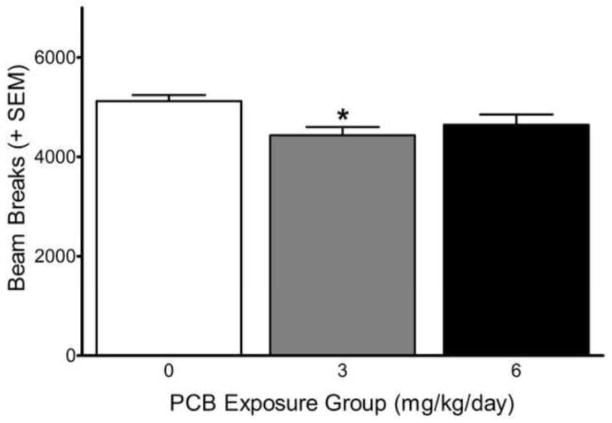
Analysis of the photobeam breaks on the first day of testing (prior to injection of saline) revealed a signficant main effect of exposure [F(2,23)=4.19, p=0.028] without a significant exposure x block interaction. As can be seen in Figure 1, rats given 3 mg/kg/day PCBs were less active than non-exposed controls (p=0.012). On days 2–6 and 10, analysis of the photobeam breaks during the 30 min habituation period prior to injection of AMPH revealed no exposure-related effects (data not shown).
Figure 1.
Total number of photobeam breaks during the 30 min of habituation prior to saline administration on testing day 1. *Significant difference from 0 mg/kg PCB exposure group (p=0.012).
3.2. Post-injection
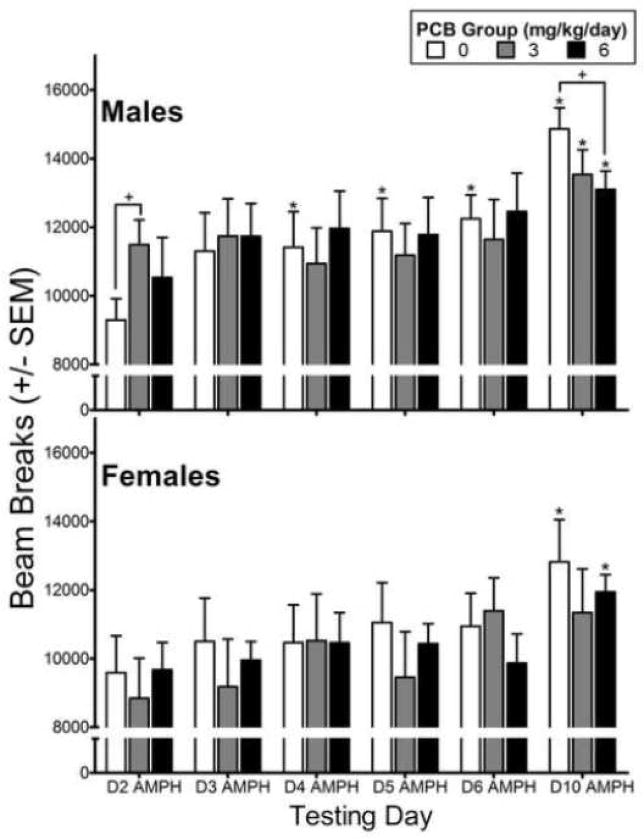
Analysis of the photobeam breaks on days 2–6 and 10 during the 60 min period following injection of 0.5 mg/kg AMPH revealed a significant interaction of exposure x sex x day [F(10,115)=1.94, p=0.047]. As can be seen in Figure 2 (top panel), males who were developmentally exposed to PCBs were more activated by the first injection of AMPH relative to controls, but this difference was significant only for the 3 mg/kg/day PCB group (p=0.035). Relative to the first AMPH injection on day 2, the control males were significantly more active by the third injection of AMPH (day 4) indicating sensitization had already occurred. In contrast, sensitization in the PCB groups did not appear until after the sixth injection of AMPH which occurred on day 10. The level of sensitization in both PCB groups following the final AMPH injection was significantly blunted relative to the level seen in control males, but the difference was significant only for the 6 mg/kg/day group (p=0.038). In the females (Figure 2, bottom panel), the post-AMPH activity levels of the controls and 6 mg/kg PCB rats were not significantly different from day 2 until day 10, indicating sensitization occurred much later than in the male rats. In addition, a statistically significant level of behavioral sensitization to AMPH was not observed in the females exposed to 3 mg/kg PCBs.
Figure 2.
Total number of photobeam breaks for the males (top panel) and females (bottom panel) during 60 min post-injection of 0.5 mg/kg amphetamine (AMPH) across testing days as a function of exposure group. +Significant difference from 0 mg/kg/day PCB group (p<0.05) on that testing day; *Significant difference from day 2 (p≤0.01).
3.3. Sensitization scores
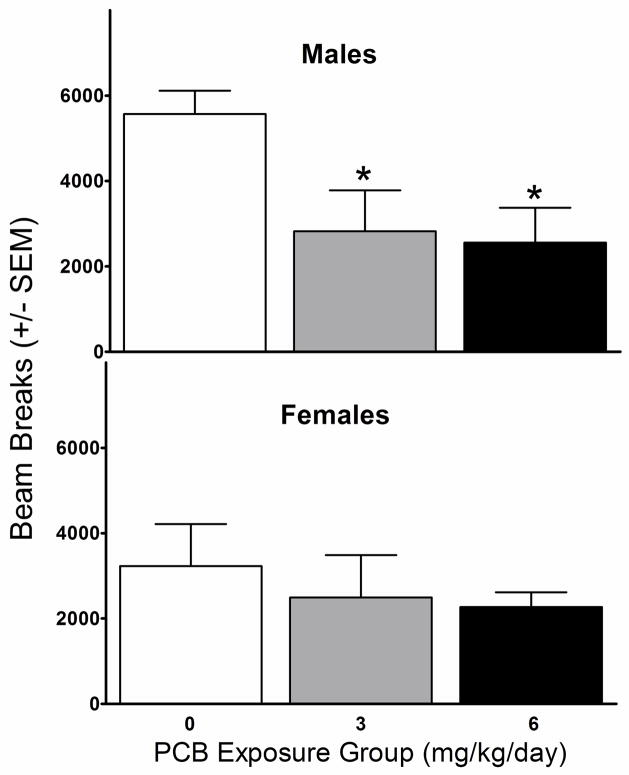
Analysis of the sensitization scores for the males revealed a main effect of exposure [F(2,23)=5.54, p=0.011]. As can be seen in Figure 3 (top panel), males in the 3 (p=0.033) and 6 (p=0.023) mg/kg PCB groups exhibited a significantly lower sensitization score than their non-exposed counterparts. No exposure-related effects were observed on the female sensitization scores (Figure 3, bottom panel).
Figure 3.
Sensitization scores (= day 10 beam breaks − day 2 beam breaks) for the males (top panel) and females (bottom panel) as a function of exposure group. *Significant difference from 0 mg/kg/day PCB group (p<0.05).
3.4. Brain/Serum AMPH Levels
Separate analysis of the brain and serum AMPH content following the final 0.5 mg/kg IP injection of AMPH revealed the main effect of exposure and the exposure x sex interaction were not significant for either analyses. The main effect of sex approached significance for AMPH content in brain [F(1,12)=3.85, p=0.073], but was not significant in serum. These data are presented in Table 1.
TABLE 1.
Amount of Amphetamine in Whole Brain and Serum 10 Minutes After Injection with 0.5 mg/kg Amphetamine (IP) For Each PCB Exposure Group
| PCB Exposure Group (mg/kg/day) | Brain (ng/g) | Serum (ng/g) |
|---|---|---|
| 0 (Control; 5 litters) | ||
| MALE | 750.9 ± 181.0 | 134.4 ± 30.8 |
| FEMALE | 796.0 ± 109.9 | 135.7 ± 19.2 |
| 3 (5 litters) | ||
| MALE | 670.6 ± 120.8 | 110.3 ± 13.6 |
| FEMALE | 976.5 ± 173.4 | 155.4 ± 20.7 |
| 6 (5 litters) | ||
| MALE | 755.2 ± 180.5 | 129.8 ± 25.2 |
| FEMALE | 1091.9 ± 102.7 | 153.8 ± 6.6 |
Note. Means (± SEM). PCB =Polychlorinated biphenyls
4. Discussion
Overall, it was demonstrated that the behavioral response of PCB-exposed Long-Evans rats to repeated injections of AMPH was blunted in comparison to that of unexposed controls, and that the effects of PCB exposure on behavioral sensitization to AMPH were sex-specific. PCB males (particularly those in the 3.0 mg/kg group) showed a greater locomotor response to the first, acute injection of 0.5 mg/kg AMPH. This finding suggested these males were pre-sensitized to AMPH by perinatal PCB exposure. PCB exposure has been shown to decrease DAT expression (Bemis and Seegal, 2004; Caudle et al., 2006; Fonnum et al., 2006; Lyng et al., 2007; Richardson and Miller, 2004; Seegal et al., 2010) such that, arguably, the ratio of DATs engaged in AMPH-induced reverse transport (versus those involved in typical DA reuptake) would be higher in PCB-exposed rats, thereby resulting in greater synaptic accumulation of DA and pronounced locomotor activation. The enhanced acute response to AMPH in the PCB-exposed males might explain the smaller degree of behavioral sensitization seen after repeated AMPH administration. Sensitization did not appear in the PCB-exposed males until after the final injection of 0.5 mg/kg AMPH. This was delayed relative to control males who exhibited sensitization after the third injection. Furthermore, although the males in both PCB-exposed groups did show sensitization on the last day of testing, the level of AMPH-induced sensitization was significantly higher in the control males.
The higher acute response in PCB-exposed males followed by the blunted sensitization response suggests that the pharmacological response to AMPH was weakened with repeated administration of the drug in these rats. There are several potential mechanims that may underlie this effect. First, the blunted sensitization response may represent an ongoing depletion of presynaptic DA stores in the brain. Specifically, PCB-exposed males may not have as much DA available in presynpatic stores to be released after repeated AMPH injections, leading to decreased activation by AMPH and reduced behavioral sensitization. This may be related to decreased expression of VMAT2 as has been reported following PCB exposure in adult animals (Bemis and Seegal, 2004; Richardson and Miller, 2004). VMAT inhibition may have prevented the packaging of free DA in the axon terminal into synaptic vesicles for later release. In addition, the resulting accumulation of DA in the terminal may possibly have activated a negative feedback mechanim that could also have resulted in less DA synthesis (Richardson and Miller, 2004; Bemis and Seegal, 2004; Mariussen et al., 1999; Cerrito and Raiteri, 1980). PCB exposure has also been reported to reduce DA synthesis in a more direct manner by inhibiting tyrosine hydroxylase (Cerrito and Raiteri, 1980; Kumer and Vrana, 1996) which is the first enzyme in the biochemical formation of DA (Cooper et al., 1996).
A difference in the density of neurons caused by developmental PCB exposure could also potentially explain the difference in AMPH behavioral sensitization. However, cortical neurons treated with the PCB mixture Aroclor 1254 for 48 hrs at concentrations ranging from 0.01 – 10 μM did not exhibit an increase in apoptosis (Howard et al., 2003). Like the mixture used in the current study, Aroclor 1254 is a mixture of predominately non-coplanar congeners (Howard et al., 2003; Kostyniak et al., 2005). Both mixtures have relatively low (but similar) aryl hydrocarbon receptor (AhR) activity and high (but similar) ryanodine receptor (RyR) activity (Kostyniak et al., 2005). Interestingly, gestational and lactational exposure to 1 and 6 mg/kg/day Aroclor 1254 in the maternal diet has been shown to produce age-dependent alterations in the rate of dendritic growth of hippocampal pyramidal neurons with no evidence of general atrophy (Lein et al., 2007; Wayman et al., 2012; Yang et al., 2009). In PCB-exposed animals, dendritic length and complexity of branching were decreased relative to non-exposed controls at PND 22, but increased compared to non-exposed controls on PND 60 (Lein et al., 2007). The PCB-induced changes in pyramidal neuron dendritogenesis appear to be dependent upon RyR activity as cultured hippocampal neurons exposed to 200 nM PCB 95 (a congener with high affinity for the RyR) for 48 hours produced dendritic growth similar to that of Aroclor 1254 described earlier, while exposure to the same concentration of PCB 66 (which has limited RyR activity) over the same time frame did not (Wayman et al., 2012). Similar results were found when in vivo exposure to PCB 95 was used. Gestational and lactational exposure to 0.1 and 1 mg/kg/day PCB 95 via the dam also resulted in dendritic grown in hippocampal pyramidcal neurons in PND 31 offspring (Wayman et al., 2012). As the mixture used in the current study has been shown to have high RyR activity (Kostyniak et al., 2005), it is also possible that altertions in dendritic ontogeny somehow contributed to the change in AMPH behavioral sensitization in PCB-exposed animals.
Finally, another possible mechanism that may have contributed to the observed differences in AMPH behavioral sensitization reported here relates to differences in the expression/function of D1-like (D1-Rs) and D2-like (D2-Rs) receptors resulting from prior developmental PCB exposure. Previous research addressing this question is limited and to date has only examined perinatal exposure to individual PCB congeners but not environmentally relevant PCB mixtures. For example, Coccini et al. (2011) reported that exposure to PCB 153 (which is a di-ortho, non-coplanar PCB congener) from GD 7 – PND 21 reduced the density of D1-Rs in both cortex and striatum of PCB-exposed males (but not exposed females) at weaning (PND 21) with expression at PND 36 equivalent to what was observed in non-exposed males. D2-Rs density in cortex (but not striatum) was higher in the males at both time points, but only at PND 36 for the females. They also reported that D1-Rs affinity was not affected by exposure to PCB 153, while D2-Rs affinity decreased only in the cortex for both sexes at both time points. A similar study examining the effects of developmental exposure to the coplanar congener PCB 77 revealed no PCB-exposure related differences D1-Rs or D2-Rs expression or affinity when measured in the offspring after they reached adultood (Harer et al., 2001). Taken together, these results indicate changes in D1-Rs or D2-Rs expression and affinity are possible following PCB exposure, but what effects are observed likely depend on the mixture/congeners examined, sex, brain region examined, and the age of the animal at the time samples are collected.
No PCB-related differences in locomotor activation were seen following the first injection of 0.5 mg/kg AMPH in the females. Sensitization for the control females took longer to develop than in the control males, and was not statistically significant until the final injection of 0.5 mg/kg AMPH. Females given 6 mg/kg PCBs exhibited sensitization similar to that of control females, while females given 3 PCB mg/kg did not exhibit sensitization. No exposure-related differences in the degree of sensitization were observed in the females following the final injection of 0.5 mg/kg AMPH and no differences in the sensitization scores were seen among the female exposure groups. Thus, unlike in the males, PCB exposure in the females did not drastically alter the time course or strength of AMPH behavioral sensitization.
The differential effects of PCBs on males versus females in the current study are not unusual as there are other studies in the PCB literature that report neurobehavioral differences between PCB-exposed males and females. For example, Sable et al. (2009) reported that exposure to the same PCB mixture used in the current study produced decreased performance on an operant inhibitory control task known as differential reinforcement of low rates of responding (DRL), with the males appearing to be more affected than the females. In addition, AMPH was less disruptive to DRL responding in PCB exposed males relative to control males, while the response to AMPH did not differ between PCB-exposed females and control females. Likewise, Holene et al. (1998) showed that lactational exposure to the specific PCB congener, PCB 153, caused decreased performance only in males on a fixed-interval task which also requires inhibitory control for efficient responding.
Aromatase, which converts testosterone to estradiol, has an important role in sexual differentiation of the male brain (Dickerson and Gore, 2007; Roselli, 2007). Aromatase levels in the rat brain peak during the perinatal period, with males having higher levels than females (Lauber et al., 1997). Prenatal exposure to PCBs has been shown to reduce aromatase activity in the brains of newborn male rat pups (Hany et al., 1999). Interestingly, alteration in aromatase during early development has been shown to influence DA concentrations in the prefrontal cortex (Stewart and Rajabi, 1994). Thus, differences in aromatase activity between the males and females during exposure, may have contributed to the observed sex differences in behavioral sensitization. Future research is needed to examine this potential mechanism more closely.
Importantly, AMPH levels in brain and plasma samples did not differ significantly as a function of PCB exposure group, so there was no evidence to suggest that the PCB and control groups were responding to different active doses of the drug in the brain. Given that PCB-exposed male rats showed a greater acute response to the 0.5 mg/kg dose of AMPH, the results also indicate that the blunted sensitization to 0.5 mg/kg AMPH in the PCB-exposed rats is not the result of a right-ward shift in their dose-response to AMPH.
In conclusion, developmental exposure to PCBs can lead to a reduction in the motor-stimulating effects typically observed following repeated AMPH injections. This change in behavioral sensitization may be the result of alterations in DA function. PCBs have been consistently reported to disrupt DA activity in the brain. The results of studies examining the relationship between developmental PCB exposure and the behavioral response to psychostimulants are important, because they provide information about whether this exposure has the potential to enhance psychostimulant addiction risk later in life.
Highlights.
Long Evans rats were perinatally exposed to 0, 3, or 6 mg/kg/day PCBs.
One male and female/litter were tested for behavioral sensitization to 0.5 mg/kg amphetamine.
PCB-exposed males inhibited greater locomotor activation to the first injection of amphetamine.
Amphetamine behavioral sensitization was attenuated in PCB-exposed males.
No exposure-induced alterations in AMPH metabolism following PCB exposure were found.
Acknowledgments
This research was funded by grants from NIEHS (K99 ES015428, R00 ES015428, R01 ES015687, and T32 ES007326). The authors would like to thank Ms. Mindy Howe for her outstanding technical assistance, Dr. Jeffrey Sable for his programming support, and Dr. Larry Hansen for his help in formulating the PCB mixture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Emily Poon, Email: epoon@illinois.edu.
Supida Monaikul, Email: smonaik2@illinois.edu.
Paul J. Kostyniak, Email: pjkost@buffalo.edu.
Lai Har Chi, Email: lchi@buffalo.edu.
Susan L. Schantz, Email: schantz@illinois.edu.
References
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol Sci. 2004;80:288–295. doi: 10.1093/toxsci/kfh153. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Delea KC, Guillot TS, Wang M, Pennell KD, Miller GW. Polychlorinated biphenyl-induced reduction of dopamine transporter expression as a precursor to Parkinson’s disease-associated dopamine toxicity. Toxicol Sci. 2006;92:490–499. doi: 10.1093/toxsci/kfl018. [DOI] [PubMed] [Google Scholar]
- Cerrito F, Raiteri M. Dopamine biosynthesis is regulated by the amine newly recaptured by dopaminergic nerve endings. Eur J Pharmacol. 1980;68:465–470. doi: 10.1016/0014-2999(80)90421-5. [DOI] [PubMed] [Google Scholar]
- Coccini T, Roda E, Castoldi AF, Poli D, Goldoni M, Vettori MV, Mutti A, Manzo L. Developmental exposure to methylmercury and 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153) affects cerebral dopamine D1-like and D2-like receptors of weanling and pubertal rats. Arch Toxicol. 2011;85:1281–1294. doi: 10.1007/s00204-011-0660-y. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. 7. Oxford University Press; New York: 1996. [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Gore AC. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord. 2007;8:143–159. doi: 10.1007/s11154-007-9048-y. [DOI] [PubMed] [Google Scholar]
- Erikson M. Analytical Chemistry of PCBs. Butterworth Publishers; Stoneham, MA: 1986. [Google Scholar]
- Fonnum F, Mariussen E, Reistad T. Molecular mechanisms involved in the toxic effects of polychlorinated biphenyls (PCBs) and brominated flame retardants (BFRs) J Toxicol Environ Health A. 2006;69:21–35. doi: 10.1080/15287390500259020. [DOI] [PubMed] [Google Scholar]
- Hall D, Stanis J, Avila HM, Gulley J. A comparison of amphetamine- and methamphetamine-induced locomotor activity in rats: evidence for qualitative differences in behavior. Psychopharmacology (Berl) 2008;195:469–478. doi: 10.1007/s00213-007-0923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hany J, Lilienthal H, Sarasin A, Roth-Harer A, Fastabend A, Dunemann L, Lichtensteiger W, Winneke G. Developmental exposure of rats to a reconstituted PCB mixture or aroclor 1254: effects on organ weights, aromatase activity, sex hormone levels, and sweet preference behavior. Toxicol Appl Pharmacol. 1999;158:231–243. doi: 10.1006/taap.1999.8710. [DOI] [PubMed] [Google Scholar]
- Härer AD, Lilienthal H, Bubser M, Kronthaler U, Mundy WR, Ward TR, Schmidt W, Winterhoff H, Winneke G. Neurotransmitter concentrations and binding at dopamine receptors in rats after maternal exposure to 3,4,3′,4′-tetrachlorobiphenyl: the role of reduced thyroid hormone concentrations. Environ Toxicol Pharmacol. 2001;9:103–115. doi: 10.1016/s1382-6689(00)00069-7. [DOI] [PubMed] [Google Scholar]
- Holene E, Nafstad I, Skaare JU, Krogh H, Sagvolden T. Behavioural effects in female rats of postnatal exposure to sub-toxic doses of polychlorinated biphenyl congener 153. Acta Paediatr Suppl. 1999;88:55–63. doi: 10.1111/j.1651-2227.1999.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Holene E, Nafstad I, Skaare JU, Sagvolden T. Behavioural hyperactivity in rats following postnatal exposure to sub-toxic doses of polychlorinated biphenyl congeners 153 and 126. Behav Brain Res. 1998;94:213–224. doi: 10.1016/s0166-4328(97)00181-2. [DOI] [PubMed] [Google Scholar]
- Howard AS, Firzpatrick R, Pessah I, Kostyniak P, Lein PJ. Polychlorinated biphenyls induce caspase-dependent cell death in cultured embryonic rat hippocampal but not cortical neurons via activation of the ryanodine receptor. Toxicol Appl Pharmacol. 2003;190:72–86. doi: 10.1016/s0041-008x(03)00156-x. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Schulz KP, London ED, Newcorn JH. Inhibitory control deficits in childhood and risk for substance use disorders: a review. Am J Drug Alcohol Abuse. 2008;34:239–258. doi: 10.1080/00952990802013334. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Fein GG, Jacobson SW, Schwartz PM, Dowler JK. The transfer of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) across the human placenta and into maternal milk. Am J Public Health. 1984;74:378–379. doi: 10.2105/ajph.74.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal exposure to polychlorinated biphenyls and attention at school age. J Pediatr. 2003;143:780–788. doi: 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RF, Olson JR, Helferich JL, Kim KH, Sable HJK, Seegal RF, Pessah IN, Schantz SL. Formulation and Characterization of an Experimental PCB Mixture Designed to Mimic Human Exposure from Contaminated Fish. Toxicol Sci. 2005;88:400–411. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Lauber ME, Sarasin A, Lichtensteiger W. Sex differences and androgen-dependent regulation of aromatase (CYP19) mRNA expression in the developing and adult rat brain. J Steroid Biochem Mol Biol. 1997;61:359–364. [PubMed] [Google Scholar]
- Lein PJ, Yang D, Bachstetter AD, Tilson HA, Harry GJ, Mervis RF, Kodavanti PR. Ontogenetic alterations in molecular and structural correlates of dendritic growth after developmental exposure to polychlorinated biphenyls. Environ Health Perspect. 2007;115:556–563. doi: 10.1289/ehp.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Schantz SL, Bowman RE. Delayed spatial alternation deficits resulting from perinatal PCB exposure in monkeys. Arch Toxicol. 1988;62:267–273. doi: 10.1007/BF00332486. [DOI] [PubMed] [Google Scholar]
- Lilienthal H, Neuf M, Munoz C, Winneke G. Behavioral effects of pre- and postnatal exposure to a mixture of low chlorinated PCBs in rats. Fundam Appl Toxicol. 1990;15:457–467. doi: 10.1016/0272-0590(90)90032-f. [DOI] [PubMed] [Google Scholar]
- Lyng G, Snyder-Keller A, Seegal R. Polychlorinated biphenyl-induced neurotoxicity in organotypic cocultures of developing rat ventral mesencephalon and striatum. Toxicol Sci. 2007;97:128–139. doi: 10.1093/toxsci/kfm027. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Morch Andersen J, Fonnum F. The effect of polychlorinated biphenyls on the uptake of dopamine and other neurotransmitters into rat brain synaptic vesicles. Toxicol Appl Pharmacol. 1999;161:274–282. doi: 10.1006/taap.1999.8806. [DOI] [PubMed] [Google Scholar]
- Mathews I, Mills R, McCormick C. Chronic social stress in adolescence influenced both amphetamine conditioned place preference and locomotor sensitization. Dev Psychobiol. 2008;50:451–459. doi: 10.1002/dev.20299. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Caloric restriction-induced life extension of rats and mice: A critique of proposed mechanisms. Biochim Biophys Acta. 2009;1790:1040–1048. doi: 10.1016/j.bbagen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD. Designing Experiments and Analyzing Data: A Model Comparison Perspective. Lawrence Erlbaum Associates. Inc; Mahwah, NJ: 2000. [Google Scholar]
- McGregor A, Baker G, Roberts DC. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on intravenous cocaine self-administration under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 1996;53:5–9. doi: 10.1016/0091-3057(95)00192-1. [DOI] [PubMed] [Google Scholar]
- Mele PC, Bowman RE, Levin ED. Behavioral evaluation of perinatal PCB exposure in rhesus monkeys: fixed-interval performance and reinforcement-omission. Neurobehav Toxicol Teratol. 1986;8:131–138. [PubMed] [Google Scholar]
- Mitchell SH, Reeves JM, Li N, Phillips TJ. Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcohol Clin Exp Res. 2006;30:429–437. doi: 10.1111/j.1530-0277.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Public Health Service Policy on Humane Care and Use of Laboratory Animals. NIH/Office of Laboratory Animal Welfare; Rockville, MD: 2002. [Google Scholar]
- National Research Council, Institute for Laboratory Animal Research. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academy Press; Washington, D.C: 2003. [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Rice DC. Effects of postnatal exposure of monkeys to a PCB mixture on spatial discrimination reversal and DRL performance. Neurotoxicol Teratol. 1998;20:391–400. doi: 10.1016/s0892-0362(97)00134-7. [DOI] [PubMed] [Google Scholar]
- Rice DC. Effect of postnatal exposure to a PCB mixture in monkeys on multiple fixed interval-fixed ratio performance. Neurotoxicol Teratol. 1997;19:429–434. doi: 10.1016/s0892-0362(97)87364-3. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Miller GW. Acute exposure to aroclor 1016 or 1260 differentially affects dopamine transporter and vesicular monoamine transporter 2 levels. Toxicol Lett. 2004;148:29–40. doi: 10.1016/j.toxlet.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Behavioral sensitization is accompanied by an enhancement in amphetamine-stimulated dopamine release from striatal tissue in vitro. Eur J Pharmacol. 1982;85:253–254. doi: 10.1016/0014-2999(82)90478-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Rogan J, Keselman H, Mendoza J. Analysis of repeated measurements. Brit J Math Stat Psych. 1979;32:269–86. [Google Scholar]
- Roselli CF. Brain aromatase: roles in reproduction and neuroprotection. J Steroid Biochem Mol Biol. 2007;106:143–150. doi: 10.1016/j.jsbmb.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable HJ, Eubig PA, Powers BE, Wang VC, Schantz SL. Developmental exposure to PCBs and/or MeHg: effects on a differential reinforcement of low rates (DRL) operant task before and after amphetamine drug challenge. Neurotoxicol Teratol. 2009;31:149–158. doi: 10.1016/j.ntt.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable H, Monaikul S, Poon E, Eubig P, Schantz S. Discriminative stimulus effects of cocaine and amphetamine in rats following developmental exposure to polychlorinated biphenyls (PCBs) Neurotoxicol Teratol. 2011;33:255–262. doi: 10.1016/j.ntt.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable HJ, Powers BE, Wang VC, Widholm JJ, Schantz SL. Alterations in DRH and DRL performance in rats developmentally exposed to an environmental PCB mixture. Neurotoxicol Teratol. 2006;28:548–556. doi: 10.1016/j.ntt.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Sable HJK, Schantz SL. Executive function following developmental exposure to polychlorinated biphenyls (PCBs): what animal models have told us. In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment (Frontiers in Neuroscience) CRC Press; Boca Raton, FL: 2006. pp. 147–167. [PubMed] [Google Scholar]
- Schenk S, Horger BA, Peltier R, Shelton K. Supersensitivity to the reinforcing effects of cocaine following 6-hydroxydopamine lesions to the medial prefrontal cortex in rats. Brain Res. 1991;543:227–235. doi: 10.1016/0006-8993(91)90032-q. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Brosch KO, Okoniewski RJ. Effects of in utero and lactational exposure of the laboratory rat to 2,4,2′,4′- and 3,4,3′,4′-tetrachlorobiphenyl on dopamine function. Toxicol Appl Pharmacol. 1997;146:95–103. doi: 10.1006/taap.1997.8226. [DOI] [PubMed] [Google Scholar]
- Seegal R, Marek K, Seibyl J, Jennings D, Molho E, Higgins D, Factor S, Fitzgerald E, Hills E, Korrick S, Wolff M, Haase R, Todd A, Parsons P, McCaffrey R. Occupational exposure to PCBs reduces striatal dopamine transporter densities only in women: a beta-CIT imaging study. Neurobiol Dis. 2010;38:219–225. doi: 10.1016/j.nbd.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, Okoniewski RJ, Brosch KO, Bemis JC. Polychlorinated biphenyls alter extraneuronal but not tissue dopamine concentrations in adult rat striatum: an in vivo microdialysis study. Environ Health Perspect. 2002;110:1113–1117. doi: 10.1289/ehp.021101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski JD, Salamone JD. Effects of dopamine depletions in the medial prefrontal cortex on DRL performance and motor activity in the rat. Brain Res. 1994;642:20–28. doi: 10.1016/0006-8993(94)90901-6. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res Brain Res Rev. 2003;41:203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Cortical mechanisms of cocaine sensitization. Crit Rev Neurobiol. 2005;17(2):69–86. doi: 10.1615/critrevneurobiol.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- Stewart J, Rajabi H. Estradiol derived from testosterone in prenatal life affects the development of catecholamine systems in the frontal cortex in the male rat. Brain Res. 1994;646:157–160. doi: 10.1016/0006-8993(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Sargent DM, Reihman J, Gump BB, Lonky E, Darvill T, Hicks H, Pagano J. Response inhibition during Differential Reinforcement of Low Rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environ Health Perspect. 2006;114:1923–1929. doi: 10.1289/ehp.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Feske U, Vanyukov M. Modeling the pathways linking childhood hyperactivity and substance use disorder in young adulthood. Psychol Addict Behav. 2007;21:266–271. doi: 10.1037/0893-164X.21.2.266. [DOI] [PubMed] [Google Scholar]
- Varian Consumable Products Application Note M1992. Solid phase extraction of amphetamine and methamphetamine.
- Varian Consumable Products Application Note M3163. Amphetamine/methamphetamine post-derivatization clean-up.
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Yang D, Bose DD, Lesiak A, Ledoux V, Bruun D, Pessah IN, Lein PJ. PCB-95 promotes dendritic growth via ryanodine receptor-dependent mechanisms. Environ Health Perspect. 2012;120:997–1002. doi: 10.1289/ehp.1104832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn R, Robbins TW, Everitt BJ. Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacol (Berl) 1997;134:242–257. doi: 10.1007/s002130050447. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Clarkson GB, Strupp BJ, Crofton KM, Seegal RF, Schantz SL. Spatial reversal learning in Aroclor 1254-exposed rats: sex-specific deficits in associative ability and inhibitory control. Toxicol Appl Pharmacol. 2001;174:188–198. doi: 10.1006/taap.2001.9199. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Villareal S, Seegal RF, Schantz SL. Spatial alternation deficits following developmental exposure to Aroclor 1254 and/or methylmercury in rats. Toxicol Sci. 2004;82:577–589. doi: 10.1093/toxsci/kfh290. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YH, Lin KL, Chen SC, Chang YZ. Integration of GC/EI-MS and GC/NCI-MS for simultaneous quantitative determination of opiates, amphetamines, MDMA, ketamine, and metabolites in human hair. J Chromatogr B. 2008;870:192–202. doi: 10.1016/j.jchromb.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Yang D, Kim KH, Phimister A, Bachstetter AD, Ward TR, Stackman RW, Mervis RF, Wisniewski AB, Klein SL, Kodavanti PR, Anderson KA, Wayman G, Pessah IN, Lein PJ. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ Health Perspect. 2009;117:426–435. doi: 10.1289/ehp.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]