Abstract
Tocopherols (vitamin E) and tea polyphenols have been reported to have cancer preventive activities. Large-scale human trials with high doses of alpha-tocopherol, however, have produced disappointing results. This review presents data showing that γ- and δ-tocopherols inhibit colon, lung, mammary and prostate carcinogenesis in animal models, whereas α-tocopherol is ineffective in animal and human studies. Possible mechanisms of action are discussed. A broad cancer preventive activity of green tea polyphenols has been demonstrated in animal models, and many mechanisms have been proposed. The cancer preventive activity of green tea in humans, however, has not been conclusively demonstrated and remains to be further investigated.
Keywords: tocopherols, vitamin E, green tea, polyphenols, cancer prevention
1. INTRODUCTION
Tocopherols are a group of fat-soluble compounds, collectively known as vitamin E, well know for their antioxidative action in protecting cell membrane against reactive lipid radicals [1]. Green tea polyphenols are not nutrients, but have been demonstrated to have antioxidative activities and beneficial health effects. Both groups of compounds have been studied by us and others for their cancer preventive activities in animal models and humans. A literature search on PubMed found 3,848 articles on “Vitamin E and cancer,” 859 articles on “Tocopherols and cancers” and 1,646 articles on “Green tea and cancer.” The mechanisms of these actions are rather complex, way beyond a simple “antioxidant” action. The results of human studies on these agents are even more complex and inconclusive. Scientists and the general public are facing massive amounts of, and sometimes confusing information. In this article, we will use mainly data from our laboratories to compare and contrast the actions of these two group of compounds with the purpose of critically analyzing the information to enhance the basic understanding of these topics. We plan to illustrate the importance of studying the activities of different forms of vitamin E as well as the bioavailability issues and redox properties of tea polyphenols. It also discusses the cautions needed to be applied in translating information from experimental systems in vitro to animal models and human populations. Directions for future research are also discussed.
2. STRUCTURES AND FUNCTION OF TOCOPHEROLS
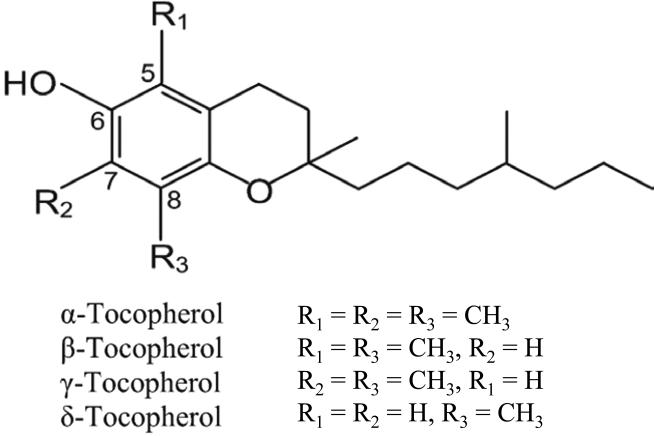
Tocopherols, the major forms of vitamin E, are a family of fat-soluble phenolic compounds. Each tocopherol contains a chromanol ring system and a phytyl chain containing 16 carbons. Depending on the number and position of methyl groups on the ring, they exist as α-, β- γ-, or δ-tocopherols (α-, β-, γ-, and δ-T) [1]. The structures of these tocopherols are shown in Figure 1. α-T is trimethylated at the 5-, 7- and 8-positions of the chromanol ring, whereas γ-T is dimethylated at the 7- and 8-positions, and δ-T is methylated at the 8-position. The unmethylated carbons at 5- and 7-positions are electrophilic centers that can effectively trap reactive oxygen and nitrogen species (RONS). The formation of 5-nitro-γ-T, 5-nitro-δ-T, 7-nitro-δ-T, and 5,7-dinitro-δ-T has been reported [2]. The hydrocarbon tail and ring structure provide the lipophilicity for tocopherols to be incorporated into the lipid bilayers of biological membranes. The phenolic group in the chromanol moiety effectively captures lipid free radicals by one electron reduction. The resulting tocopherol phenoxy radical can be reduced by ascorbic acid or glutathione to regenerate the tocopherol molecule. This is a well established physiological antioxidant mechanism for vitamin E to protect the integrity of biological membranes.
Figure 1.
Structures of tocopherols
Tocopherols are widely occurring in dietary oils, such as corn, soybean, sesame and canola oils, as well as nuts. In these oils, γ-T is 3-5 times more abundant than α-T, and δ-T is also abundant in some oils. Upon ingestions, these tocopherols are transported to the liver via the lymphatic system. In the liver, the α-T transfer protein preferentially transfers α-T to very low-density lipoproteins, which carry tocopherols to the blood; γ-T is not effectively, and δ-T is even less effectively, transferred by this mechanism [1]. Therefore, α-T is the most abundant form of vitamin E in the blood and nonhepatic tissues; γ-T levels are lower and δ-T levels are even lower. The γ-T and δ-T in the liver, however, are actively metabolized through side-chain degradation by the ω-oxidation/β-oxidation pathway [1]. Whereas the biological activities of α-T have been extensively studied, the cancer preventive activities of γ-T and δ-T are just beginning to be investigated.
3. TOCOPHEROLS AND CANCER IN HUMANS
Tocopherols, as effective antioxidants, have been proposed to protect against carcinogenesis; however, this proposal is supported by some epidemiological studies. For example, of the three reported cohort studies on lung cancer, two studies found a significant inverse association between dietary intake of vitamin E and the risk of lung cancer [3]. In both studies, the protective effects were found in current smokers, suggesting a protective effect of vitamin E against oxidative insults from cigarette smoking. In four case-control studies on lung cancer, three studies found lower serum α-T levels in lung cancer patients than in matched controls [3]. In a case-control study, Mahabir et al. observed that the odds ratios of lung cancer for increasing quartiles of dietary α-T intake were 1.0, 0.63, 0.58, and 0.39, respectively (P for trend < 0.0001) [4]. The authors suggested that α-T accounts for 34-53% reduction in lung cancer risk [4]. Since the intake of γ-T was also increased in proportion to the α-T intake, the beneficial effect could also be due to the more abundant γ-T or the combined effects of all forms of tocopherols.
Because α-T is the most abundant form of tocopherols in blood and tissues and has the highest activity in the classical fertility-restoration assay, α-T is generally considered to be “the vitamin E” [1]. Therefore, many studies on vitamin E have been conducted with α-tocopheryl acetate. The results from several large-scale intervention studies with α-T, however, have been disappointing [5-8]. For example, in the recent Selenium and Vitamin E Cancer Prevention Trial (SELECT), taking 400 IU of all-rac-α-tocopheryl acetate or 200 μg selenium (from L-selenomethionine) or both, daily for an average of 5.5 years, did not prevent prostate or other cancers [7]. In the recently published results on the follow-up (for 7-12 years) of this study, subjects receiving α-tocopheryl acetate had a hazard ratio of 1.17 for developing prostate cancers [8]. The headline in the new media that “vitamin E increases prostate cancer risk” is alarming. It was noted that, in the SELECT, the α-T supplement caused a 50% decrease in median plasma γ-T levels [7]. Since γ-T has been suggested to have strong anti-inflammatory and cancer preventive activities [3,9-13], the α-T-caused decrease in blood and tissue levels of γ-T may partially contribute to the increased prostate cancer risk. Another possible interpretation of the lack of cancer prevention effect of α-T is that supplementation of a nutrient to a population that is already adequate in this nutrient may not produce any beneficial effect. The exact reasons for these negative results from the SELECT and other trials are not known. Nevertheless, the disappointing outcome of these large-scale trials reflects our insufficient understanding of the biological activities of tocopherols and points to the need for systematic studies of the disease preventive activities of the different forms of tocopherols.
4. INHIBITION OF TUMORIGENESIS BY DIFFERENT FORMS OF TOCOPHEROLS IN ANIMAL MODELS
Previous cancer prevention studies in different animal models, mainly with α-T, have obtained inconsistent results [3]. On the other hand, recent studies from our research team at Rutgers University have demonstrated the inhibition of cancer formation and growth in the lung, colon, mammary gland, and prostate by a tocopherol mixture that is rich in γ-T (named γ-TmT) [14-22]. γ-TmT is a by-product in the distillation of vegetable oil and usually contains (per g) 130 mg α-T, 15 mg β-T, 568 mg γ-T, and 243 mg δ-T. Some of these studies have been summarized in a recent commentary [23] and are discussed in the following sections.
4.1. Inhibition of lung tumorigenesis and tumor growth by γ-TmT, γ-T and δ-T
We treated A/J mice (6 weeks old) with a tobacco carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1- butanone (NNK), plus benzo[a]pyrene (B[a]P), a ubiquitous environmental pollutant, at doses of 2 μmol each by oral gavage weekly from Weeks 1 to 8. At Week 19, the mice in the control group (on the AIN93M diet) developed 21 tumors per mouse [14]. Treatment of the mice with 0.3% γ-TmT in the diet, during the entire experimental period, lowered the tumor multiplicity by 30%. γ-TmT treatment also significantly reduced the average tumor volume and tumor burden by 50% and 55%, respectively [14]. In a second study, lung tumorigenesis was induced by NNK (i.p. injection of 100 mg/kg on Week 1 and 75 mg/kg on Week 2). Treatment with the 0.3% γ-TmT diet during the carcinogen-treatment period, the post-initiation period, or the entire experimental period reduced the tumor multiplicity by 18%, 20%, or 30%, respectively. Moreover, the tumor burden was significantly reduced by γ-TmT treatment given during the tumor initiation period or the entire experimental period, by 36% and 43%, respectively [14].
In the NNK plus B[a]P-treated model, dietary γ-TmT treatment significantly increased the apoptotic index (based on cleaved-caspase-3 positive cells) from 0.09% to 0.25% in the lung tumors, without affecting apoptosis in non-tumorous lung tissues. γ-TmT treatment also significantly decreased the percentage of cells with positive immunostaining for 8-hydroxydeoxyguanosine (8-oxo-dG) (from 26% to 17%), a marker for oxidative DNA damage, as well as for phospho-histone 2AX (γ-H2AX) (from 0.51% to 0.23%), a reflection of double-strand break-induced DNA repair. The plasma levels of prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) were markedly elevated in the tumor-bearing mice and the levels were decreased by γ-TmT treatment. The anti-angiogenic activity of dietary γ-TmT was shown by the reduced microvessel density (CD31-labeled capillary clusters and blood vessels) in the peripheral area of the lung adenomas [14]. These results suggest the pro-apoptotic antioxidative, anti-inflammatory and anti-angiogenic activities of γ-TmT [14].
When 0.3% γ-TmT was given to NCr nu/nu mice in the diet one day after implantation of human lung H1299 cells (1 × 106 cells injected s.c. per site), an inhibition of xenograft tumor growth was observed [14]. After 6 weeks, the tumor size and weight were significantly reduced by 56% and 47%, respectively, as compared to those of the control group. The γ-TmT treatment also caused a 3.3-fold increase in apoptotic index as well as a 52% decrease in 8-oxo-dG-positive cells and a 57% decrease in γ-H2AX-positive cells in the xenograft tumors. Strong cytoplasmic staining of nitrotyrosine was observed in xenograft tumors, and the staining intensity was decreased by 44% in mice that received γ-TmT. The γ-TmT treatment also reduced the plasma LTB4 level by 36% [14]. In a similar experiment, the effectiveness of different forms of tocopherols in the inhibition of H1299 xenograft tumor growth was compared [15]. Pure δ-T was most effective, showing dose-response inhibition when given at 0.17% and 0.3% in the basal AIN-93M diet. γ-TmT and pure γ-T were less effective, but α-T was not effective. Studies of H1299 cells in culture also showed that δ-T was more effective than γ-TmT and γ-T in inhibiting cell growth, whereas α-T was not effective [14].
In another transplanted tumor study, dietary 0.1% and 0.3% γ-TmT were found to inhibit the growth of subcutaneous tumors (formed by injection of murine lung cancer CL13 cells, which were derived from NNK-induced lung tumors in A/J mice) in A/J mice by 54% and 80%, respectively, on Day 50 [16].
4.2. Inhibition of Colon Tumorigenesis by γ-TmT, γ-T and δ-T
Previous studies concerning the effect of α-T on colon carcinogenesis have yielded mostly negative results [3]. Recently, we studied the effect of γ-TmT in the colons of mice that had been treated with azoxymethane (AOM) and dextran sulfate sodium (DSS) [17]. γ-TmT treatment (0.3% in the diet) resulted in a significantly lower colon inflammation index (52% of the control) on Day 7, and reduced the number of colon adenomas (to 9% of the control) on Week 7. γ-TmT treatment also resulted in higher apoptotic indexes in adenomas, lower PGE2, LTB4, and nitrotyrosine levels in the colon, and lower PGE2, LTB4, and 8-isoprostane levels in the plasma on Week 7. In the second experiment, with AOM/DSS-treated mice sacrificed on Week 21, dietary γ-TmT treatment significantly inhibited adenocarcinoma and adenoma formation in the colon (to 17-33% of the control). In the third experiment, mice received dietary treatment with 0%, 0.1%, and 0.3% γ-TmT in the AIN 93M basal diet. One week later, 1% DSS was given to mice in drinking water for one week to induce inflammation, and a dose-dependent anti-inflammation was also observed [17]. These studies demonstrate the anti-inflammatory and anti-carcinogenic activities of γ-TmT in the colon. In another study, the inhibitory activities of α-T, γ-T, δ-T and γ-TmT were compared in an AOM-induced colon carcinogenesis model in rats [22]. δ-T was the most effective in inhibiting the formation of aberrant crypt foci (ACF) and high grade dysplastic ACF. γ-TmT and γ-T had slightly lower activities, but α-T was ineffective [22]. This study is the first clear demonstration of the higher cancer preventive activity of δ-T than γ-T, and the ineffectiveness of α-T, in an animal carcinogenesis model.
4.3. Inhibition of mammary carcinogenesis by tocopherols
In previous studies on mammary carcinogenesis, four studies showed a protective effect of α-T, but one study showed no effect [3]. Recently, we demonstrated that dietary administration of 0.1%, 0.3% or 0.5% γ-TmT dose-dependently suppressed N-methyl-N-nitrosourea (NMU)-induced mammary tumor development and growth in rats [18,19]. The inhibitory action was associated with increased expression of p21, p27, peroxisome proliferator activated receptor-γ (PPAR-γ) and cleaved caspase-3; whereas protein kinase B (AKT) and the estrogen-dependent signaling pathways in mammary tumors were significantly decreased by γ-TmT treatment [19]. Furthermore, in NMU-treated rats, dietary γ-TmT, γ-T and δ-T decreased cell proliferation and increased apoptosis in these mammary tumors, but α-T was not effective [24].
4.4. Inhibition of prostate carcinogenesis by tocopherols
Previous studies on the effects of α-T and its synthetic analogs on prostate carcinogenesis and xenograft cancer growth have not yielded consistent results [3]. Recent work showed that 0.1% γ-TmT in the diet inhibited prostate carcinogenesis in the transgenic adenocarcinoma of the mouse prostate (TRAMP) model [21]. During the development of prostate cancer in the TRAMP mouse, loss of nuclear factor-erythroid 2-related factor 2 (Nrf2) expression was observed, and γ-TmT treatment prevented the loss [21]. Takahashi et al. demonstrated that γ-T (0.005 or 0.01% in the diet), but not α-T, decreased the number of adenocarcinomas in the ventral lobe in the transgenic rat for adenocarcinoma of prostate model [25], and the inhibitory action was associated with enhanced apoptosis (activation of caspases). We recently demonstrated the dose-dependent inhibition of LNCaP prostate cancer growth by γ-TmT (0.1%, 0.3% and 0.5% in the diet) in a xenograft tumor model in severe combined immunodeficiency (SCID) mice [20].
5. DOES VITAMIN E PREVENT OR PROMOTE CANCER?
We propose that, at the nutritional level, all forms of vitamin E are cancer preventive. This concept is consistent with many observations that the dietary intake or plasma levels of α-T and other tocopherols are inversely associated with cancer risk, especially among smokers [3,4,26,27]. At the supra-nutritional levels, however, α-T is not cancer preventive, which has been shown in several recent cancer prevention trials [5-8]. This concept is consistent with many studies in animal models, by others and us, showing the lack of cancer preventive activity of α-T supplementation [3,15,22]. Recent results further showed that δ-T, γ-T and γ-TmT are cancer preventive in animal models [3,14-24], and we propose that γ-T and δ-T are cancer preventive in humans as well [23]. This concept may help to explain the enhanced prostate cancer risk in subjects who took daily supplementation of 400 IU of α-T in the SELECT [8]. This supplementation caused a 50% decrease in the median plasma γ-T level [7], and this may decrease the cancer preventive effect of γ-T. High concentrations of α-T may also decrease the cancer preventive activity of γ-T or δ-T by competing for its binding to proteins that are important for cancer prevention, but this possibility remains to be demonstrated. A common mechanism of the cancer preventive action of tocopherols appears to be the trapping of RONS. In addition to trapping RONS, δ-T and γ-T can be efficiently converted to side-chain metabolites, which retain the intact chromanol ring and may possess cancer preventive activities. Other mechanisms remain to be studied.
6. CHEMISTRY AND BIOCHEMISTRY OF TEA POLYPHENOLS
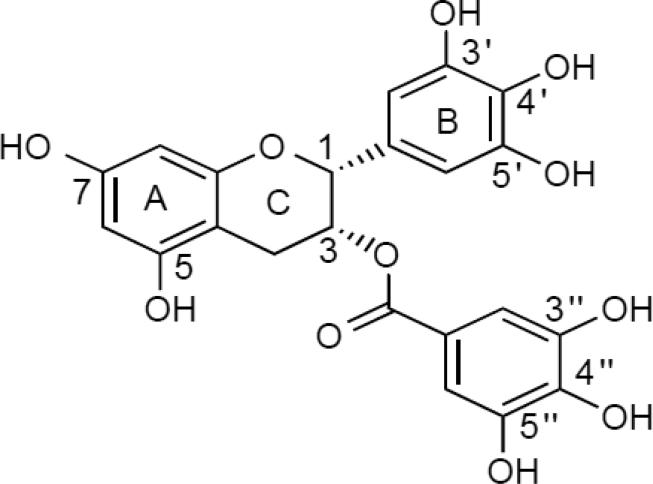
Tea, made from the leaves of the plant Cadamellia sinensis, is a popular beverage worldwide. By heating and drying the tea leaves, their characteristic constituents, catechins, are preserved. After brewing in hot water, about one-third of the materials are water extractable, of which 30–40% (by dry weight) are polyphenols, known as catechins. (–)-Epigallocatechin-3-gallate (EGCG) is the major catechin in tea, and (–)-epigallocatechin (EGC), (–)-epicatechin-3-gallate (ECG), and (–)-epicatechin (EC) are also present in significant amounts [28,29]. The structure of EGCG is shown in Figure 2.
Figure 2.
Structure of (-)-epigallocatechin-3-gallate (EGCG)
Black tea is produced by a process known as fermentation, in which the tea leaves are crushed to promote enzymatic oxidation and subsequent condensation of tea polyphenols, leading to the formation of oligomeric polyphenols (theaflavins) and polymeric polyphenols (thearubigins). Black tea contains 2–6% theaflavins, >20% thearubigins, and 3–10% catechins in the water-extractable materials. Tea leaves also contain 2–5% caffeine in the water-extractable material of green, oolong, and black tea.
Because of the polyphenolic structure, catechins are strong antioxidants by quenching reactive oxygen species (ROS) and by chelating metal ions to prevent the formation of ROS. Catechins can also generate ROS via auto-oxidation in solution, such as in cell cultural medium. The phenolic groups are also donors for H-bonding; for example, EGCG has 8 phenolic groups serving as donors of H-bonding. The large sphere of hydration due to H-bonding and a molecular weight of 458 of EGCG limit its bioavailability [30]. The strong H-bonding ability also enables EGCG to bind many proteins with high affinity [31]. As reviewed previously, some of these proteins have been proposed as targets of EGCG for inhibitory actions against cancer cells [31]. For example, vimentin binds to EGCG with a Kd of 3.3 nM, and functional studies showed that EGCG inhibited the phosphorylation of vimentin at serine 50 and serine 55 with IC50 = 17 μM [32]. The difference in effective concentrations is probably due to the nonspecific binding of EGCG to other proteins, which compete with the target protein. Binding of EGCG to the 67 kDa laminin receptor with a dissociation constant (Kd) value of 0.04 μM was observed using a surface plasmon resonance assay, and this was proposed to be a mechanism for the anti-cancer actions of EGCG [33]. A study with NMR spectroscopy showed the direct binding of tea polyphenols to the BH3 pocket of anti-apoptotic B-cell lymphoma-2 (Bcl-2) proteins with an inhibition constant (Ki) of 0.33–0.49 μM [34]. However, higher EGCG concentrations were needed to induce apoptosis [34]. A recent study demonstrated the binding of EGCG to both the WW and PPIase domains of peptidyl prolyl cis/trans isomerase (Pin 1) [35]. Biochemical studies showed a dissociation constant of 21.6 μM, and this was proposed as the mechanism for the cancer preventive activities of EGCG.
7. INHIBITION OF CARCINOGENESIS IN ANIMAL MODELS BY TEA POLYPHENOLS
In contrast to tocopherols, tea polyphenols are not nutrients. Some of the tea polyphenols, such as EGCG, have only limited bioavailabilities. Nevertheless, as reviewed previously [28,31], the evidence for the cancer preventive activities of green tea and its constituents in animal models is compelling. Most of the studies have been conducted with green tea and green tea polyphenols, especially the most abundant and biologically active EGCG.
7.1. Inhibition of lung tumorigenesis in rodent models
The inhibitory effects of tea preparations against lung tumorigenesis have been demonstrated in at least 20 studies [31,36]. Most studies used NNK or B[a]P to induce carcinogenesis. Oral administration of extracts or solutions of green tea, black tea, EGCG, EGC or theaflavins significantly decreased lung tumorigenesis in rats, mice, or hamsters [31,36]. Treatment of A/J mice with extracts of green or black tea also inhibited the spontaneous formation of lung tumors and rhabdomyosarcomas. In addition, oral administration of green tea infusion reduced the number of lung colonies of mouse Lewis lung carcinoma cells in a metastasis model.
Chung and coworkers demonstrated that caffeine effectively inhibited NNK-induced lung tumorigenesis in rats, and that the inhibitory effect of 2% black tea (containing 680 ppm caffeine) against lung tumorigenesis was due to caffeine [37]. This conclusion is different from the experiments with A/J mice, which demonstrated the inhibition of lung tumorigenesis by decaffeinated green and black tea preparations [38]. A possible explanation of this difference is that the systemic bioavailability of tea polyphenols in mice is much higher than in rats [30]. In our recent study, administration of 0.5% Polyphenon E (PPE, a standardized green tea polyphenol preparation containing 65% EGCG, 25% other catechins, and 0.6% caffeine) or 0.044% caffeine in the drinking water, to tumor-bearing A/J mice (induced by a single dose of NNK administered 20 weeks earlier) for 32 weeks, inhibited the progression of lung adenomas to adenocarcinomas [39]. PPE and caffeine treatment inhibited cell proliferation, enhanced apoptosis, and decreased levels of c-JUN and phospho-extracellular regulated kinase1/2 (ERK1/2) in adenocarcinomas. These effects were not observed in the normal lung tissues, suggesting that the effect is selective against tumor cells. These results demonstrate the broad inhibitory activity of tea preparations in the inhibition of lung neoplasia at different stages of carcinogenesis.
For enhancing the cancer preventive activity of tea polyphenols, the synergistic inhibitory action of PPE in combination with a cholesterol-lowering agent, atorvastatin (trade name Lipitor), against NNK-induced lung carcinogenesis in A/J mice, has been demonstrated [40]. Atorvastatin is an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. The synergistic action of this combination was also demonstrated in human lung cancer H1299 and H460 cells. In both the cell lines and the mouse lung tumors, downregulation of the anti-apoptotic proteins myeloid cell leukemia sequence 1 (MCL1) and B-cell lymphoma-extra large (BCL-XL) and induction of apoptosis were associated with the synergistic inhibitory action [40]. It remains to be determined whether such synergistic actions between atorvastatin and tea polyphenols occur in humans.
7.2. Inhibition of tumorigenesis of the oral-digestive tract
Inhibitory effects of tea against tumorigenesis in the oral cavity, esophagus, stomach, small intestine, and colon have been shown in more than 30 studies [31,36]. For example, tea preparations were shown to inhibit 7,12-dimethylbenz[a]anthracene (DMBA)-induced carcinogenesis in the hamster cheek pouch and N-nitrosomethylbenzylamine-induced rat esophageal carcinogenesis in our studies. EGCG also inhibited tumorigenesis in rat stomach and forestomach induced by N-methyl-N′-nitro-N-nitrosoguanidine. Black tea polyphenols (Polyphenon B), given at 0.05% in the diet, also effectively inhibited forestomach tumor formation; this inhibition was associated with increased apoptosis as well as reduced cell proliferation, inflammation and angiogenesis [31,36].
The inhibitory effects of tea and tea polyphenols against intestinal tumorigenesis have been consistently witnessed in mouse models [31,36]. For example, we observed that administration of EGCG at 0.02%-0.32% in drinking water dose-dependently inhibited spontaneous small intestinal tumorigenesis in ApcMin/+ mice, while caffeine did not show an inhibitory effect [41]. The inhibition was associated with increased levels of E-cadherin on the plasma membrane, as well as decreased levels of nuclear β-catenin, c-Myc, phospho-Akt, and phospho-ERK1/2 in tumors [41]. Administration of green tea extracts (0.6% in drinking fluid) also inhibited the formation of AOM-induced ACF in CF-1 mice on a high-fat diet [42]. Recently, Shimizu et al. [43] demonstrated the inhibition of AOM-induced ACF formation in male C57BL/KsJ-db/db mice by EGCG (0.01% and 0.1% in drinking water) through suppressing the activities of the insulin-like growth factor (IGF)/IGF-1 receptor (IGF-1R) axis. The elevated levels of IGF-1R, phospho-IGF-1R, phospho-glycogen synthase kinase-3β (GSK-3β) and β-catenin in the colonic mucosa were decreased by treatment with EGCG [43]. Also decreased were the plasma levels of IGF-1, insulin, triglyceride, cholesterol, and leptin.
In rat models, the effects of tea preparations on colon tumorigenesis have not been consistent [31,36]. This inconsistency in colon carcinogenesis is rather surprising, because the intestine is considered to be a promising site for chemoprevention with polyphenols that have low systemic bioavailability [30]. Orally ingested EGCG has only limited systemic bioavailability, with most of it passing through the colon; and the absorbed EGCG is excreted mostly through the bile into the intestine. Our recent animal study showed that, after the injection with AOM, the treatment of rats with PPE, 0.12 or 0.24% in the diet for 8 weeks, dose-dependently decreased the total number of ACF per rat. The inhibitory activity was associated with decreased levels of nuclear β-catenin and cyclin D1, and increased levels of retinoid X receptor α (RXRα), in the ACF with high-grade dysplasia [44]. After treatment with 0.24% PPE for 34 weeks, the incidence of adenocarcinoma decreased from 57% to 23%, and the multiplicity of adenocarcinoma and adenoma decreased by 80% and 45%, respectively (Yang, C.S. et al., unpublished). The loss of RXRα expression was observed in colonic dysplastic ACF, adenomas, and adenocarcinoma, but the RXRα expression was (partially) retained in PPE treated rats in these lesions.
7.3. Inhibition of prostate tumorigenesis in mouse models
Administration of a green tea polyphenol infusion (0.1% in drinking water) to the TRAMP mice for 24 weeks markedly inhibited prostate cancer development and distant site metastases [45,46]. The inhibition was associated with increased apoptosis, decreased cell proliferation, decreased IGF-1 levels, and restored IGF binding protein 3 (IGF-BP3) levels in both serum and the dorso-lateral prostate [45,46]. These changes were associated with reduced levels of phosphotidylinositol 3-kinase (PI3K) as well as phosphorylated forms of AKT, ERK1 and ERK2. The treatment also significantly decreased levels of angiogenic and metastatic markers [46]. These results suggest that the inhibition of the IGF-1 signaling pathway, vascular endothelial growth factor A (VEGFA), and matrix metalloproteinases contribute to the cancer prevention activity of green tea polyphenols. Caporali et al. [47] reported similar inhibitory activities of orally administered green tea catechins on prostate tumor formation in the TRAMP model. The IGF-1 signaling pathway appears to be a key target for the inhibition; it is not clear whether tea polyphenols inhibit IGF-1 pathway by a direct or indirect action of tea polyphenols in the prostate or by systemic actions.
7.4. Inhibition of tumorigenesis in the mammary glands and other organs
There are at least 10 studies on possible inhibitory effects of tea against mammary tumorigenesis, but the results were inconsistent [31,36]. In our study, even at a high dose of 1,000 mg/kg b.w./day, i.g., EGCG only slightly (non-significantly) reduced mammary tumor incidence and multiplicity in rats treated with NMU [48]. The lack of robust inhibition of mammary tumorigenesis [36] is likely to be due to low bioavailability of tea polyphenols in the mammary tissues. The observed low inhibitory effect of tea on mammary tumorigenesis may also be due to an indirect action of tea. For example, Rogers et al. [49] showed no significant inhibitory effect of black tea administered during the promotion stage of DMBA-induced mammary tumorigenesis in rats maintained on the AIN76 diet. However, in rats on a high-fat diet, black tea was found to reduce the tumor number and size. The results suggest that black tea may decrease fat absorption and body fat levels, which subsequently influence estrogen metabolism and mammary tumorigenesis.
Green tea has shown to inhibit bladder carcinogenesis induced by N-(4-hydroxybutyl)-N-butyl-nitrosamine (OH-BBN) in rats [50,51]. In our study, PPE was administered (100 or 250 mg/kg b.w./day, intragastrically) to rats at 126 days of age (1 week after the final dose of OH-BBN of a total of 16 doses in 8 weeks). Palpable urinary bladder tumor incidence was reduced from 59% (control) to 40% (by 100 mg/kg) or 18% (by 250 mg/kg) [48].
Catechins are not the only cancer preventive constituents in tea; caffeine has also been shown to inhibit lung and skin carcinogenesis in mouse models [37,39,52]. The mechanism of action of caffeine in the inhibition of skin tumorigenesis has been thoroughly studied and discussed by Conney et al [52].
8. POSSIBLE MECHANISMS OF ACTION OF TEA POLYPHENOLS AND HUMAN RELEVANCE
Many mechanisms have been proposed for cancer prevention by tea constituents, and this subject has been reviewed recently [31]. For example, EGCG has been proposed to bind to different molecular targets, inhibit the activities of many key enzymes, and inhibit several receptor-dependent signaling pathways [31]. EGCG is known for its antioxidative actions, but it can also produce ROS, especially in vitro [53]. It is uncertain whether all of these proposed mechanisms are relevant to cancer prevention in vivo. Apparently, mechanisms derived from cancer prevention studies in animal models are likely to be more relevant. These include the induction of apoptosis in different animal models, inhibition of the phosphorylation of c-JUN and ERK1/2 in lung tumorigenesis model, suppression of phospho-AKT and nuclear β-catenin levels in colon cancer models, inhibition of the IGF/IGF-1R axis in colon and prostate cancer models, and suppression of VEGF-dependent angiogenesis in lung and prostate cancer models [31,39,41,43,46,47]. It is still unclear whether these molecules are direct targets for EGCG or downstream events of the primary action. In theory, in vitro experiments could provide more information on the detailed mechanisms. It is reasonable to assume that the high affinity binding proteins reported in the literature [31] could serve as initial targets, but this point remains to be substantiated in animal models. From the limited human studies that are available, action of tea constituents in reducing oxidative stress and enhancing the elimination of carcinogens could also be important [54,55].
In contrast to the strong evidence for the cancer preventive activity of tea constituents in animal models, results from epidemiological studies have not been consistent concerning the cancer preventive effect of tea consumption in humans [56]. The different results between animal and human studies is likely to be due to the lower quantities of human tea consumption as compared to the doses used in animal studies. In animal studies, the doses of tea preparations and the experimental conditions are set to maximize the opportunity to detect a cancer prevention effect. In humans many life style, genetic polymorphism and other confounding factors reduce the power of epidemiological studies for detecting a cancer preventive effect. Smoking appears to be a strong confounding factor. For example, in a case-control study on the effect of green tea consumption on esophageal cancer in Shanghai by Gao et al. [57], a protective effect was only observed in non-smokers, mostly women. A recent systematic review of epidemiological studies in Japan on green tea consumption and gastric cancer indicated no overall preventive effect of green tea in cohort studies. However, a small consistent risk reduction was found in women, and the result was confirmed by pooling data of six cohort studies [58]. A possible explanation is that women were mostly non-smokers, whereas most men were smokers in this Japanese population. The relationship between tea consumption and cancer risk may become more clear if we quantify the tea consumption better, correct for confounding factors, and consider genetic polymorphisms of the populations.
9. CONCLUDING REMARKS
In this article, we have discussed two groups of phenolic compounds. Both of them are antioxidants, but their biological actions are way beyond their antioxidative activities. With tocopherols, we illustrated the importance of studying specific forms. We propose that at the nutritional level, all tocopherols are cancer preventive; either α-T or tocopherol mixtures contribute to cancer prevention. At the supra-nutritional levels, α-T is not effective in cancer preventive, based on human studies and some laboratory studies [3,5-8,15,22]. Our proposal that supra-nutritional levels of γ-T and δ-T are cancer preventive is based mainly on laboratory studies in animal models [3,14-24]. It would be important to conduct prevention trials with pure δ-T, γ-T or tocopherol mixtures that are rich in γ-T and δ-T to demonstrate their cancer preventive activities in human populations.
In studying tocopherols, tea polyphenols and other chemopreventive agents, cautions need to be applied in translating results from animal studies to human populations. Animal studies are usually designed to demonstrate the cancer prevention effects using doses higher than the levels of human consumption, without many of the confounding factors involved in human studies. Even more difficult is the use of information from cell line studies to predict cancer preventive activities in animals and humans. This is clearly seen in the case of the tea polyphenol EGCG, which has limited bioavailability and easily oxidized in cell culture medium to generate ROS. Such auto-oxidation is not like to occur in vivo. Therefore, predictions from cell line studies to in vivo situation could be problematic.
With the strong evidence provided by laboratory studies for the cancer preventive activities of tea constituents, even though the results from epidemiological studies are not conclusive, tea preparations can still be used for the prevention of certain types of human cancer. Results from laboratory studies discussed above could aid us to design the optimal conditions for cancer prevention trials and prospective studies as well as to accurately interpret results from epidemiological studies.
In future intervention studies with either tocopherols or tea polyphenols, it would be important to measure baseline blood levels of tocopherols or polyphenols as well as the levels of the intervening agents at different time points of the trials. Individuals may absorb or eliminate these agents differently. These results may help to interpret the outcome of the trials. Individuals with low baseline levels of tocopherols may gain more benefit from the intervention with tocopherol, or even with tea polyphenols, if antioxidative action is a key mechanism of action.
Acknowledgments
FUNDING SOURCES
This work was supported by US NIH grants CA120915, CA122474 and CA133021, and the John Colaizzi Chair Endorsement Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Traber MG, Vitamin E. In: Present Knowledge in Nutrition. 9th Edition Bowman BA, Russell RM, editors. ILSI Press; Washington DC: 2006. pp. 211–219. [Google Scholar]
- 2.Patel A, Liebner F, Netscher T, Mereiter K, Rosenau T. Vitamin E chemistry. Nitration of non-alpha-tocopherols: products and mechanistic considerations. J Org Chem. 2007;72:6504–6512. doi: 10.1021/jo0706832. [DOI] [PubMed] [Google Scholar]
- 3.Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong AN, Yang CS. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010;31:533–542. doi: 10.1093/carcin/bgp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahabir S, Schendel K, Dong YQ, Barrers SL, Spitz MR, Forman MR. Dietary alpha-, beta-, gamma- and delta-tocopherols in lung cancer risk. Int J Cancer. 2008;123:1173–1180. doi: 10.1002/ijc.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. Jama. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 6.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Sesso HD, Buring JE. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr., Baker LH, Coltman CA., Jr. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein EA, Thompson IM, Jr., Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL, Jr., Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner KH, Kamal-Eldin A, Elmadfa I. Gamma-tocopherol--an underestimated vitamin? Ann Nutr Metab. 2004;48:169–188. doi: 10.1159/000079555. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 11.Campbell S, Stone W, Whaley S, Krishnan K. Development of gamma-tocopherol as a colorectal cancer chemopreventive agent. Crit Rev Oncol Hematol. 2003;47:249–259. doi: 10.1016/s1040-8428(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 12.Hensley K, Benaksas EJ, Bolli R, Comp P, Grammas P, Hamdheydari L, Mou S, Pye QN, Stoddard MF, Wallis G, Williamson KS, West M, Wechter WJ, Floyd RA. New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic Biol Med. 2004;36:1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Mol Aspects Med. 2007;28:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu G, Xiao H, Li G, Chen K-Y, Hao J, Loy S, Yang CS. γ-tocopherols-rich mixture of tocopherols inhibits chemically-induced lung tumorigenesis in A/J mice and xenograft tumor growth. Carcinogenesis. 2010;31:687–694. doi: 10.1093/carcin/bgp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li GX, Lee MJ, Liu AB, Yang Z, Lin Y, Shih WJ, Yang CS. delta-tocopherol is more active than alpha - or gamma -tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev Res (Phila) 2011;4:404–413. doi: 10.1158/1940-6207.CAPR-10-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert JD, Lu G, Lee MJ, Hu J, Yang CS. Inhibition of lung cancer growth in mice by dietary mixed tocopherols. Mol Nutr Food Res. 2009;53:1030–1035. doi: 10.1002/mnfr.200800438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ju J, Hao X, Lee MJ, Lambert JD, Lu G, Xiao H, Newmark HL, Yang CS. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prev Res (Phila Pa) 2009;2:143–152. doi: 10.1158/1940-6207.CAPR-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HJ, Ju J, Paul S, So JY, DeCastro A, Smolarek AK, Lee MJ, Yang CS, Newmark HL, Suh N. Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-g. Clin Can Res. 2009;15:4242–4249. doi: 10.1158/1078-0432.CCR-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suh N, Paul S, Lee HJ, Ji Y, Lee MJ, Yang CS, Reddy BS, Newmark HL. Mixed tocopherols inhibit N-methyl-N-nitrosourea-induced mammary tumor growth in rats. Nutr Cancer. 2007;59:76–81. doi: 10.1080/01635580701419022. [DOI] [PubMed] [Google Scholar]
- 20.Zheng X, Cui X-X, Khor TO, Huang Y, DiPaola RS, Goodin S, Lee M-J, Yang CS, Kong A-N, C.A. H. Inhibitory effect of a γ-tocopherol-rich mixture of tocopherols on the formation and growth of LNCaP prostate tumors in immunodefficient mice. Cancers. 2012;3:3762–3772. doi: 10.3390/cancers3043762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barve A, Khor TO, Nair S, Reuhl K, Suh N, Reddy B, Newmark H, Kong AN. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int J Cancer. 2009;124:1693–1699. doi: 10.1002/ijc.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan F, Li GX, Liu AB, Lee M-J, Yang Z, Chen Y-K, Lin Y, Shih W, Yang CS. delta- and gramma -Tocopherols, but no alpha-tocopherols, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prev. Res. 2012;5:644–654. doi: 10.1158/1940-6207.CAPR-11-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang CS, Suh N, Kong AN. Does vitamin E prevent or promote cancer? Cancer prevention research. 2012;5:701–705. doi: 10.1158/1940-6207.CAPR-12-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smolarek AK, Suh N. Chemopreventive activity of vitamin E in breast cancer: a focus on gamma- and delta-tocopherol. Nutrients. 2011;3:962–986. doi: 10.3390/nu3110962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi S, Takeshita K, Seeni A, Sugiura S, Tang M, Sato SY, Kuriyama H, Nakadate M, A. K., Maeno Y, Nagao M, Shirai T. Suppression of prostate cancer in a transgenic rat model via gamma-tocopherol activation of caspase signaling. Display Settings. 2009;69:644–651. doi: 10.1002/pros.20915. [DOI] [PubMed] [Google Scholar]
- 26.Helzlsouer KJ, Huang HY, Alberg AJ, Hoffman S, Burke A, Norkus EP, Morris JS, Comstock GW. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst. 2000;92:2018–2023. doi: 10.1093/jnci/92.24.2018. [DOI] [PubMed] [Google Scholar]
- 27.Huang HY, Alberg AJ, Norkus EP, Hoffman SC, Comstock GW, Helzlsouer KJ. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am J Epidemiol. 2003;157:335–344. doi: 10.1093/aje/kwf210. [DOI] [PubMed] [Google Scholar]
- 28.Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 29.Sang S, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacol Res. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52(Suppl 1):S139–151. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- 31.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ermakova S, Choi BY, Choi HS, Kang BS, Bode AM, Dong Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J Biol Chem. 2005;280:16882–16890. doi: 10.1074/jbc.M414185200. [DOI] [PubMed] [Google Scholar]
- 33.Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. 2004;11:380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- 34.Leone M, Zhai D, Sareth S, Kitada S, Reed JC, Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003;63:8118–8121. [PubMed] [Google Scholar]
- 35.Urusova DV, Shim JH, Kim DJ, Jung SK, Zykova TA, Carper A, Bode AM, Dong Z. Epigallocatechin-gallate suppresses tumorigenesis by directly targeting Pin1. Cancer Prev Res (Phila) 2011;4:1366–1377. doi: 10.1158/1940-6207.CAPR-11-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ju J, Lu G, Lambert JD, Yang CS. Inhibition of carcinogenesis by tea constituents. Semin Cancer Biol. 2007;17:395–402. doi: 10.1016/j.semcancer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung FL, Wang M, Rivenson A, Iatropoulos MJ, Reinhardt JC, Pittman B, Ho CT, Amin SG. Inhibition of lung carcinogenesis by black tea in Fischer rats treated with a tobacco-specific carcinogen: caffeine as an important constituent. Cancer Res. 1998;58:4096–4101. [PubMed] [Google Scholar]
- 38.Wang ZY, Hong JY, Huang MT, Reuhl KR, Conney AH, Yang CS. Inhibition of N-nitrosodiethylamine- and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced tumorigenesis in A/J mice by green tea and black tea. Cancer Res. 1992;52:1943–1947. [PubMed] [Google Scholar]
- 39.Lu G, Liao J, Yang G, Reuhl KR, Hao X, Yang CS. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 2006;66:11494–11501. doi: 10.1158/0008-5472.CAN-06-1497. [DOI] [PubMed] [Google Scholar]
- 40.Lu G, Xiao H, You H, Lin Y, Jin H, Snagaski B, Yang CS. Synergistic inhibition of lung tumorigenesis by a combination of green tea polyphenols and atorvastatin. Clin Cancer Res. 2008;14:4981–4988. doi: 10.1158/1078-0432.CCR-07-1860. [DOI] [PubMed] [Google Scholar]
- 41.Ju J, Hong J, Zhou JN, Pan Z, Bose M, Liao J, Yang GY, Liu YY, Hou Z, Lin Y, Ma J, Shih WJ, Carothers AM, Yang CS. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (-)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65:10623–10631. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- 42.Ju J, Liu Y, Hong J, Huang MT, Conney AH, Yang CS. Effects of green tea and high-fat diet on arachidonic acid metabolism and aberrant crypt foci formation in an azoxymethane-induced colon carcinogenesis mouse model. Nutr Cancer. 2003;46:172–178. doi: 10.1207/S15327914NC4602_10. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu M, Shirakami Y, Sakai H, Adachi S, Hata K, Hirose Y, Tsurumi H, Tanaka T, Moriwaki H. (-)-Epigallocatechin gallate suppresses azoxymethane-induced colonic premalignant lesions in male C57BL/KsJ-db/db mice. Cancer Prev Res (Phila) 2008;1:298–304. doi: 10.1158/1940-6207.CAPR-08-0045. [DOI] [PubMed] [Google Scholar]
- 44.Xiao H, Hao X, Simi B, Ju J, Jiang H, Reddy BS, Yang CS. Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 rats. Carcinogenesis. 2008;29:113–119. doi: 10.1093/carcin/bgm204. [DOI] [PubMed] [Google Scholar]
- 45.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 47.Caporali A, Davalli P, Astancolle S, D'Arca D, Brausi M, Bettuzzi S, Corti A. The chemopreventive action of catechins in the TRAMP mouse model of prostate carcinogenesis is accompanied by clusterin over-expression. Carcinogenesis. 2004;25:2217–2224. doi: 10.1093/carcin/bgh235. [DOI] [PubMed] [Google Scholar]
- 48.Lubet RA, Yang CS, Lee MJ, Hara Y, Kapetanovic IM, Crowell JA, Steele VE, Juliana MM, Grubbs CJ. Preventive effects of polyphenon E on urinary bladder and mammary cancers in rats and correlations with serum and urine levels of tea polyphenols. Mol Cancer Ther. 2007;6:2022–2028. doi: 10.1158/1535-7163.MCT-07-0058. [DOI] [PubMed] [Google Scholar]
- 49.Rogers AE, Hafer LJ, Iskander YS, Yang S. Black tea and mammary gland carcinogenesis by 7,12-dimethylbenz[a]anthracene in rats fed control or high fat diets. Carcinogenesis. 1998;19:1269–1273. doi: 10.1093/carcin/19.7.1269. [DOI] [PubMed] [Google Scholar]
- 50.Sato D. Inhibition of urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl)-nitrosamine in rats by green tea. Int J Urol. 1999;6:93–99. doi: 10.1046/j.1442-2042.1999.06239.x. [DOI] [PubMed] [Google Scholar]
- 51.Sato D, Matsushima M. Preventive effects of urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl)-nitrosamine in rat by green tea leaves. Int J Urol. 2003;10:160–166. doi: 10.1046/j.1442-2042.2003.00587.x. [DOI] [PubMed] [Google Scholar]
- 52.Conney AH, Zhou S, Lee MJ, Xie JG, Yang CS, Lou YR, Lu Y. Stimulatory effect of oral administration of tea, coffee or caffeine on UVB-induced apoptosis in the epidermis of SKH-1 mice. Toxicol Appl Pharmacol. 2007;224:209–213. doi: 10.1016/j.taap.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–7246. [PubMed] [Google Scholar]
- 54.Schwartz JL, Baker V, Larios E, Chung FL. Molecular and cellular effects of green tea on oral cells of smokers: a pilot study. Mol Nutr Food Res. 2005;49:43–51. doi: 10.1002/mnfr.200400031. [DOI] [PubMed] [Google Scholar]
- 55.Hakim IA, Harris RB, Brown S, Chow HH, Wiseman S, Agarwal S, Talbot W. Effect of increased tea consumption on oxidative DNA damage among smokers: a randomized controlled study. J Nutr. 2003;133:3303S–3309S. doi: 10.1093/jn/133.10.3303S. [DOI] [PubMed] [Google Scholar]
- 56.Yuan JM, Sun C, Butler LM. Tea and cancer prevention: epidemiological studies. Pharmacol Res. 2011;64:123–135. doi: 10.1016/j.phrs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao YT, McLaughlin JK, Blot WJ, Ji BT, Dai Q, Fraumeni JF., Jr. Reduced risk of esophageal cancer associated with green tea consumption. J Natl Cancer Inst. 1994;86:855–858. doi: 10.1093/jnci/86.11.855. [DOI] [PubMed] [Google Scholar]
- 58.Sasazuki S, Tamakoshi A, Matsuo K, Ito H, Wakai K, Nagata C, Mizoue T, Tanaka K, Tsuji I, Inoue M, Tsugane S. Green tea consumption and gastric cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2012;42:335–346. doi: 10.1093/jjco/hys009. [DOI] [PubMed] [Google Scholar]