Abstract
Manganese (Mn) is an essential dietary nutrient but excess or accumulations can be toxic. Disease states, like manganism, are associated with overexposure or accumulation of Mn and are due to the production of reactive oxygen species, free radicals and toxic metabolites, alteration of mitochondrial function and ATP production and depletion of cellular antioxidant defense mechanisms. This review focuses on all of the preceding mechanisms and the scientific studies that support them as well as provides an overview of the absorption, distribution, and excretion of Mn and the stability and transport of Mn compounds in the body.
Keywords: Manganese, reactive oxygen species, mitochondrial dysfunction, manganism, DA autoxidation
Introduction
Manganese (Mn) serves as a constituent of metalloenzymes and as an enzyme activator so the absorption and excretion of Mn is tightly controlled in order to maintain stable tissue Mn levels for essential reactions. The most widely known enzyme requiring Mn is manganese superoxide dismutase (MnSOD), whose primary function is detoxification of superoxide free radicals. Mn2+ is a central component of some metalloenzymes and an activator of many metal-enzyme complexes. Mn activates these enzymes by binding the protein directly or by acting through an intermediate interaction with a substrate, like ATP, to initiate a conformational change and activate enzymatic activity [1]. Mn3+ is found in the essential enzymes manganese catalase and MnSOD, both of which break down oxidants using the Mn3+ in their reactive catalytic centers. Catalase converts hydrogen peroxide into oxygen and water, aiding in reducing oxidative stress. MnSOD is involved in the dismutation of superoxide in mitochondria to decrease oxidative stress. Oxidative stress is also significant in Mn-induced dopaminergic (DAergic) neurodegeneration. Mn is an essential nutrient and as such it is required as part of a healthy diet; however exposure to excess levels yields toxicity. Mn is available as a nutritional supplement as well as a component in most infant formulas and total parenteral nutrition given to some neonates.
The primary source of Mn toxicity is occupational exposures, which are chiefly inhalational. Workplace exposure to Mn-containing fumes or dusts can be a concern in the iron and steel, ferromanganese, dry-cell battery, welding and smelting industries with the highest concern in the welding industry. Exposure to high levels of Mn can lead to a condition referred to as manganism, characterized by behavioral changes, including slow and clumsy movements, tremors, difficulty walking and facial muscle spasms. Slowed hand movements, irritability, aggressiveness and hallucinations are other symptoms of exposure that may precede manganism, and patients may be asymptomatic for months or years following exposure.
Mn toxicity is mediated, at least in part, by the autoxidation or turnover of intracellular catecholamines, production of free radicals, reactive oxygen species and toxic metabolites, depletion of cellular antioxidant defense mechanisms and alterations of mitochondrial function and ATP production. These mechanisms will be discussed in this review. Evidence supporting Mn-induced cell death through the mechanisms described above will be presented as well as a subsection on the use of mitochondrially-targeted antioxidants as therapeutics.
Mn Stability and Transport
Mn has several valence states; with Mn3+ most commonly found in enzymes and Mn2+ in the diet [2]. The valence of Mn can be changed within the body and studies suggest that Mn3+ is more cytotoxic than Mn2+, due to higher oxidative reactivity [3]. Enhanced oxidative stress induction by Mn3+ was supported by a study in rats given either Mn2+ or Mn3+ [4]. Mn2+ does not readily bind to sulfhydryl (-SH) groups or amines, and does not vary in its stability constants for endogenous ligands glycine, cysteine, riboflavin, and guanosine [2].
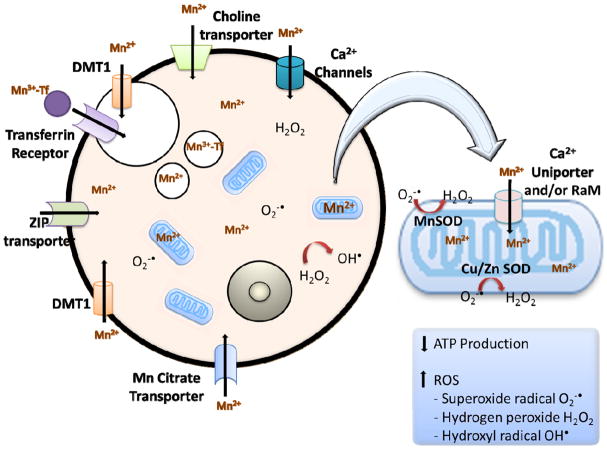
Mn2+ enters the portal circulation from the gastrointestinal tract and binds to plasma alpha2-macroglobulin or albumin. It is then secreted in the bile, and some is oxidized to Mn3+ by ceruloplasmin. The plasma carrier protein for Mn3+ is transferrin, and Mn3+ entry into neurons occurs via transferrin receptor-mediated endocytosis and/or the divalent metal transporter (DMT-1) [5–7]. In the case of Mn toxicity DMT-1 facilitates the accumulation of Mn in DA-rich areas [8]. There are several studies that suggest that Mn can cross the blood-brain barrier through the choline transporter [9, 10], voltage-gated and store-operated calcium channels [11, 12], ionotropic glutamate receptor Ca2+ channels [13] and a Mn citrate transporter [14]. Additional evidence points to Mn transport through the Zn transporters, ZIP8 and ZIP14 [15, 16], and a through ATP13A2, a P-type transmembrane ATPase protein [17].
Mn Administration, Distribution and Excretion
Adults consume between 0.7 and 10.9 mg of Mn per day [18] with normal ranges of Mn at 4–15μg/L in blood, 1–8μg/L in urine and 0.4–0.85μg/L in serum. Mn complexes with other substances, resulting in low plasma and tissue concentrations, making it difficult to assess overexposure [19]. Mn2+ is more readily cleared than Mn3+. High iron diets have been shown to suppress Mn absorption, aiding in protection from toxicity, and deficiencies in other essential nutrients, such as magnesium (Mg), may increase the toxicity of Mn [20–24]. It has also been shown that DMT-1 and Mn levels increase with low brain iron concentrations [25]. Mn can readily cross both the blood-brain and placental barriers.
The route of exposure governs the distribution, metabolism, and neurotoxicity of Mn [26, 27]. Only a small fraction of ingested Mn is normally absorbed into the body and in response to high dietary Mn, gastrointestinal absorption is reduced [28]. Several studies have shown that animals with high dietary Mn have increased liver metabolism and excretion of Mn into bile and pancreatic fluids [29–33]. This suggests that ingested Mn is tightly regulated probably due to its essentiality in the diet. In addition to the influence of other nutrients, genetics and disease states can also influence Mn concentrations in the body. In one study of individuals with chronic hepatic encephalopathy, elevated blood Mn concentrations and brain MRI changes consistent with elevated Mn levels have been reported [34]. Following inhalational exposure, increased Mn absorption from the respiratory tract, slower blood clearance of absorbed Mn, and direct olfactory or trigeminal presynaptic nerve endings transport aid in more efficient delivery of Mn to the brain [26].
Suarez and colleagues set out to determine if Mn3+ bound to transferrin (MnTf) is responsible for mediating the neurotoxicity of Mn. They compared Mn bound to pyrophosphate (MnPPi) and MnTf in human neuroblastoma cells (SH-SY5Y). Parameters studied were mitochondrial dehydrogenase activity, cell growth and survival. MnPPi was more toxic than MnTf in their cell line. Membrane permeability was significantly altered in cells exposed to Mn PPi, whereas MnTf did not change membrane permeability significantly. They concluded that while both complexes inhibited mitochondrial enzyme activity, MnPPi was more toxic in affecting morphology, cell number and general membrane permeability [35].
In a study using adult rats, the biological half-life of Mn in the brain was between 51 and 74 days [36]. In humans given intravenous 54Mn tracer doses, an elimination half-time of 53 days was reported from the brain [37]. In accordance, a study in macaque monkeys given MnCl2 via an implanted subcutaneous osmotic minipump reported an elimination half-time of 53 days [38]. This group also exposed two cynomolgus monkeys to aerosolized 54MnCl2 via an endotracheal tube and observed an estimated half-life of elimination of greater than 220 days from the head [38]. The slow elimination time may be due to the elimination of Mn from the skull and not from the brain itself. Neonatal rats given Mn orally throughout lactation had Mn concentrations similar to controls when assessed at PND 73, suggesting that the half-life of elimination from young adult rat brain occurs prior to PND 73 [39], which is consistent with human findings [37]. They also found that brain delivery of inhaled Mn is influenced by particle solubility as MnSO4 was more rapidly cleared from the rat lung than Mn phosphate or Mn tetroxide [39].
Cotzias and colleagues (1968) reported that Mn elimination was dose-dependent in miners exposed to Mn dusts [37]. Absolute amounts of Mn absorbed from Mn-containing dusts are unknown. Two additional studies showed enhanced biliary Mn excretion following inhalation [40, 41]. Smaller particles can also directly enter the blood stream through the gastrointestinal epithelial lining following mucociliary elevator clearance from the respiratory tract [2].
Mn is transported to the liver, conjugated to bile and then passed to the intestine for fecal excretion. Reabsorption through the enterohepatic circulation is possible and Mn can also be detected in small amounts in urine, sweat and breast milk [42]. Absorption, distribution and excretion following dermal exposure have not been documented.
Brain Mn Targets
The distinct neurological effects seen with Mn toxicity are due to its preferential uptake by the brain. Entry of Mn to the brain occurs in one of three ways: (1) from the nasal mucosa to the brain olfactory bulb through olfactory neural connections; (2) from the blood through capillary endothelial cells of the blood-brain barrier; and (3) from the blood to the cerebral spinal fluid via the choroid plexuses [43]. In the cell, Mn is found primarily in the mitochondria in both neurons and astrocytes, in the nucleus and in synaptosomes [44–47]. In the brain, the striatum, globus pallidus and substantia nigra are reported target sites, with the globus pallidus as the primary site for Mn accumulation and toxicity [19, 48]. In addition to cell death, several studies have reported pathologic changes in the globus pallidus [48–50]. The damage has been reported to be the result of dis-inhibition of inputs to the globus pallidus [51] and the high rate of oxidative phosphorylation in this region may also account for its susceptibility. The DAergic system is a major target of Mn. Various studies, even those dating back as early as the 1920’s documented the susceptibility of the globus pallidus to Mn-induced cell death [52–55]. More currently, a study of rhesus monkeys chronically exposed to Mn dusts reported decreased levels of dopamine (DA) in several brain regions, including the caudate and globus pallidus [56]. In another study, rhesus monkeys that received Mn through intravenous injection exhibited gliosis of the globus pallidus and substantia nigra as well as an extrapyramidal syndrome, a condition reflective of deregulated DA release and reuptake [49]. Two independent studies in primates, using either intravenous injection or inhalation showed widespread Mn accumulation in the brain via T1-weighted MRI [57, 58]. In the inhalational studies, Dorman and colleagues, observed Mn accumulation in the globus pallidus ranging from 1.6 to 6.0 fold relative to controls [57]. Following intravenous injection, Guilarte reported 5 fold increases in Mn in the globus pallidus [58]. Mn preference for the DAergic system was also corroborated by a study that linked Mn exposure in welders to a higher prevalence of Parkinsonian symptoms and reduced [18F]fluoro-L-DOPA (FDOPA) in asymptomatic welders [59].
Manganism and PD share in their etiology common cellular mechanisms such as preferential accumulation of Mn within mitochondria resulting in oxidative stress [60], and selective DAergic neurotoxicity [61] (Table 1). Manganism commonly occurs in response to acute Mn exposures, whereas PD likely reflects long-term exposure to relatively low Mn exposures. Manganism features less frequent and kinetic tremor, or no tremor vs. patients with PD. Acute high Mn exposures also lead to dystonias and a “cock-walk” with symptoms becoming progressive and irreversible. In addition to affecting the basal ganglia, manganism is also known to affect other brain regions, including the cortex and hypothalamus and at the morphological level leading to neuronal loss and reactive gliosis in the globus pallidus and substantia nigra pars reticulata (SNpr) in the absence of Lewy bodies, which are a hallmark of PD. Furthermore, in manganism, damage to the striatum (caudate nucleus and putamen) and subthalamic nucleus may occur, while the substantia nigra pars compacta (SNpc) is generally spared whilst PD is predominantly characterized by neuronal loss in the SNpc.
Table 1.
Similarities and Differences between PD and Manganese Toxicity.
| Parkinson’s Disease | Manganese Toxicity | Reference | |
|---|---|---|---|
| Genetic Determination | Yes, can be autosomal dominant or autosomal recessive. Approximately 10–20% of cases linked to genetic causes | No | [199] |
| Selective DA sensitivity | Yes | Yes- however several studies have pointed to the selectivity of glutamate and possibly GABA | [57–59] |
| Brain regions affected | Substancia nigra pars compacta (SNpc) primarily and striatum | Globus pallidus primarily but striatum, and substancia nigra may also be involved | [6, 19, 199] |
| Mitochondrial dysfunction/ oxidative stress | Yes | Yes | [85, 199] |
| L-DOPA responsive | Yes | Generally unresponsive to L-DOPA however, some motor symptoms reversed but with major side effects | [52] |
| Symptoms | Emotional and cognitive decline, bradykinesia, rigidity, tremors and postural instability. | Extrapyramidal syndrome | [19, 49, 200, 201] |
| Slowed hand movements, irritability, aggressiveness and hallucinations. | |||
| Later stages: masked-face, forward-flexed posture, gait freezing, shuffling steps, GI issues | Later stages: Rigidity, tremor, gait disturbances (slow and clumsy movements), hypokinesia, facial muscle spasms, propensity to fall backward when pushed, less frequent resting tremor, more frequent dystonia, a “cock-walk” collectively referred to as ‘manganism’ |
Mn and autoxidation of catecholamines and other neurotransmitters
The effects of Mn on DA metabolism have received considerable attention due to preferential accumulation of Mn in the substantia nigra, globus pallidus and striatum, all DA-rich brain regions that are sensitive to oxidative injury. The free radicals that originate from DA autoxidation have been postulated to cause degeneration of DAergic cells [62–64]. Mn3+ is a much more potent oxidizing agent than Mn2+ and has been shown to readily oxidize DA, leading to higher concentrations of damaging DA quinone products [65, 66]. Mn-induced toxicity resulted from the production of hydrogen peroxide and DA quinone and the depletion of DA due to its oxidation [66]. This study also tested ascorbic acid and thiamine as potential antioxidants and results showed that they completely inhibited DA oxidation however they did so in both the absence and presence of Mn, suggesting a complicated mechanism [66]. The oxidization of DA by Mn3+, which does not require oxygen, results in decreased overall DA. The resulting quinone can initiate superoxide radicals by the reduction to the semiquinone, by NADH or NADPH-dependent flavoproteins, which is then readily oxidized by molecular oxygen to form superoxide radicals. The reaction, which is irreversible, can be prevented by NADH, GSH, and ascorbic acid [67]. The mitochondria are import cellular targets in Mn-induced apoptosis in DAergic cells [44].
Desole and colleagues (1994) observed that 22mg Mn/kg/day administered orally in 6-month-old rats resulted in increased concentrations of the DA oxidation product, DOPAC, and uric acid but overall DA levels were unchanged. Higher doses of Mn significantly decreased concentrations of DA, DOPAC, glutathione and ascorbic acid and increased uric acid concentrations in the striatum compared to controls [68]. These results provided support for Mn oxidization of DA.
In a C. elegans study investigating the effects of Mn on intra and extracellular DA, investigators showed that Mn causes a dose-dependent degeneration of DAergic neurons, which required the presence of the reuptake transporter, DAT-1. Knockdown of this transporter abolished loss of GFP-fluorescence in Mn-induced DAergic GFP-labeled neurons. Toxicity was prevented by loss of tyrosine hydroxylase function and loss of the vesicular monoamine transporter, which normally aids DAergic neurons in releasing DA at the synaptic cleft, there was increased tolerance to Mn indicating that DA synthesis is required for DAT-1- dependent Mn toxicity and that extracellular DA, and not intracellular DA, is involved in Mn neurotoxicity [69].
Mn-induced DA oxidation and cell death of neurons in the globus pallidus is likely the result of dis-inhibition of inputs to the globus pallidus [51] and the high rate of oxidative phosphorylation in this region. Although the globus pallidus accumulates a higher concentration of Mn than other regions, it is important to note that other parts of the brain that have lower accumulation may still be vulnerable and neurotransmitters other than DA can be affected. Mn has been shown to cause reduced glutamine synthetase (GS) in the globus pallidus, which is important for conversion of glutamate to glutamine [70]. Additionally, a handful of studies in rodents have shown increased glutamate levels in the brain after exposure to Mn [71–73]. Another study looking specifically at brain regions showed increased glutamate in frontal cortex but decreased glutamate in the globus pallidus [74]. Glutamate transporters have been shown to be decreased in in vitro studies [22] and primates showed increased mRNA expression and decreased protein of two glutamate transporters and decreased GS in multiple brain regions [70]. Multiple studies looking at γ-aminobutyric acid (GABA) have presented conflicting data [71–73, 75–78], leaving the exact role of GABA in Mn toxicity somewhat unclear. For example, several groups using rodents showed Mn induced increases in striatal GABA levels [71–73, 77, 79, 80] however, decreases in GABA levels in the striatum and frontal cortex were also reported by several groups [76, 81]. Still other studies have reported no effect on Mn on GABA levels [75, 82]. A short-term study in Welders showed no effect on Mn fumes on GABA located in DAergic regions of the brain [83]. MRI studies of presymptomatic smelters exposed to Mn showed Mn accumulation in globus pallidus and elevated GABA levels in the thalamus and adjacent basal ganglia via MRI [84]. Contradictory conclusions about the role of GABA are likely due to differences in route and duration of exposure, age at time of exposure and/or the contribution and alteration of other neurotransmitter systems.
Reactive oxygen species
Mn is known to enhance production of reactive oxygen species (ROS), which contributes to Mn toxicity. ROS is the name commonly given to highly reactive oxygen radicals and peroxides generated by a number of mechanisms within cells. The most common of these ROS are superoxide radical, hydrogen peroxide and hydroxyl radical. Common sites of production of these ROS include the mitochondrial electron transport chain (ETC), NADPH oxidases, enzymatic activation of cytochrome p450, xanthine oxidase and cyclooxygenase 1 and 2; however, for most types of cells the greatest production is by the mitochondrial ETC [85–88]. In mitochondria the superoxide radical (O2−•) is generally the initial ROS species formed. Superoxide radical can be converted into hydrogen peroxide (H2O2) by superoxide dismutase. In the mitochondrial matrix MnSOD catalyzes this reaction, while in the intermembrane space and cytosol it is catalyzed by copper/zinc superoxide dismutase (Cu/Zn SOD). In the presence of iron or other transition metals such as Mn, H2O2 can be converted into the very reactive hydroxyl radical (OH•) [89].
The seven known sites of superoxide production in mitochondria are: the flavin site in complex I (site IF), the ubiquinone-binding sites in complexes I and III (sites IQ and IIIQ0, respectively), glycerol 3-phosphate dehydrogenase, the electron transferring flavoprotein:Q oxidoreductase of fatty acid beta-oxidation (ETFQOR) and pyruvate and α-ketoglutarate dehydrogenases [90]. Most of the work on mitochondrial production of ROS has focused on superoxide production at complexes I [91–97] and III [92, 93, 95, 98]. All of the mitochondrial sites produce O2− • in the matrix space while site IIIQo and glycerol 3-phosphate can also produce O2− • in the intermembrane space; i.e., the space between the inner and outer mitochondrial membranes [90, 96]. Of these mitochondrial sites those associated with ubiquinone binding at complexes I and III in the ETC have the largest maximal rates of O2− • production [90]. A simple, intuitive view of O2− • production by the ETC would picture electrons moving between ETC complexes and between binding sites such as flavins, iron-sulfur proteins and cytochromes within each complex. Some of these sites would be near the protein-lipid interface between the ETC complex and the phospholipid of the membrane. Because molecular oxygen (O2) partitions to about 3 times higher concentration within the phospholipid membrane than in the aqueous portions of the cell [94], this O2 occasionally captures an electron from a nearby binding site in the ETC complex and forms O2− •.
Mitochondrial O2− • production has been shown to increase as the membrane potential (Δψ) or electrochemical proton gradient (ΔμH) increases [92, 93, 95, 96]. ATP production by oxidative phosphorylation or mild uncoupling decreases these gradients and significantly decreases ROS production. Other factors that increase mitochondrial ROS production include uptake of cations such as Ca2+ and Mn2+ [99–102], induction of the mitochondrial permeability transition (MPT) [103, 104], inhibition of the ETC [90, 94], and hyperglycemia [105].
While it is not known definitively where normal mitochondrial O2− • production occurs in vivo [90], this would generally take place during ATP production and, therefore, at decreased Δψ and ΔμH. Under these conditions, O2− • production probably occurs at most of the mitochondrial sites with most of the O2− • produced at complexes I and III [90, 94].
Most of the mitochondrial production of O2− • occurs in the mitochondrial matrix with only a small fraction being produced in the intermembrane space [90, 94]. Furthermore, since O2− • is a charged species, it does not easily penetrate membranes. However, the O2− • in the matrix is quickly converted into H2O2 by Mn SOD, and H2O2 readily crosses the membrane [94]. Therefore, the H2O2 produced from mitochondrial matrix O2− • can readily move into the intermembrane space, the cytosol and even the nucleus of the cell.
Each of the three primary ROS species, O2− •, H2O2 and OH• can cause damage to nucleic acids, proteins and phospholipids within mitochondria and within cells [106, 107]. Mitochondrial DNA is particularly sensitive to ROS damage because of the lack of protective histones in mitochondria and the lack of repair mechanisms such as those found in the nucleus [106, 107]. Furthermore, mitochondrial ROS can contribute to induction of the mitochondrial permeability transition (MPT), which can, in turn, induce apoptosis [95, 108–112]. This will be discussed in more detail below.
While we do not have experimental evidence for the mechanisms through which intramitochondrial Ca2+ or Mn increase ROS production, we know that intramitochondrial Ca2+ activates a series of sites within the mitochondrion which increase the rate of ATP production by oxidative phosphorylation including activation of three dehydrogenases in the TCA cycle which catalyze increased production of NADH [113–115]. Activation of these dehydrogenases increases the NADH/NAD+ ratio and the metabolic rate, which should, in itself lead to a higher rate of ROS production. Mn2+ binds to almost every Ca2+ binding site and could increase ROS production by simply functioning as a Ca2+ analog. However, we also know that Mn2+ inhibits the rate of ATP production through inhibition sites in the electron transport chain, the TCA cycle and at other loci [60, 116, 117]. The known sites of mitochondrial production of O2− • represent loci at which the probability of electron capture by O2 is relatively high [90]. Any inhibition caused by Mn2+ binding to intramitochondrial sites which cause a buildup of occupancy in these sites of O2− • production could also cause an increase in ROS production [90].
Not all ROS effects are negative; ROS have also been shown to be important in cell signaling [118]. For example, H2O2 is important in stabilizing Hif-1a, which is probably the most important factor in inducing the changes that enable a cell to survive hypoxia [119, 120]. H2O2 has also been found to activate mitochondrial uncoupler proteins (UCP’s), which through uncoupling effects decrease Δψ and ΔμH, and thereby decrease ROS production [121]. ROS signaling has also been implicated in a number of activities which can be beneficial to the cell, including: activation of transcription factors [122] including nuclear factor 2 (erythroid-derived 2)(Nrf2), which protects against ROS damage [123, 124]; cell proliferation and regulation of the cell cycle [125, 126]; regulation of apoptosis [127]; regulation of metalloproteinase [128]; and activation of protein kinases and phosphatases [128, 129].
There are mechanisms present both in the cell cytosol and in mitochondria that protect against ROS-induced damage. One of the most effective protective molecules is the short peptide glutathione (γ-L-glutamyl-L-cysteinyl glycine or GSH) [130]. GSH is made in the cytosol and transported into the mitochondrion. The enzyme catalase, present in both the mitochondrial matrix and the cytosol, can convert H2O2 to H2O and O2, eliminating the ROS danger. Cytochrome c is not only important as part of the ETC, but can also serve a protective function. It is present in the intermembrane space at concentrations greater than those needed for its electron transport function, and it can scavenge O2− • radicals and return the electrons to complex IV of the ETC [131]. Similarly, ubiquinol (reduced co Q10), when present in excess, has been reported to scavenge hydroperoxyl radical [132], which like ubiquinol is lipophilic, and return the electron to the ETC. Finally, while mitochondrial DNA does not have protective histones and elaborate repair mechanisms, as the nucleus does, mitochondria do contain many copies of mitochondrial DNA so that if one is damaged by ROS there are many other copies to take its place.
It has been shown that antioxidants, particularly mitochondrially-targeted antioxidants can ameliorate a range of specific disease conditions associated with increased ROS production and extend the lives of test animals which show these conditions [133–143], however the results of experiments designed to show that treatment with antioxidants leads to longer lifespan generally have not been convincing [144]. Autophagy is a process through which a cell breaks down and reuses components of misfolded proteins and malfunctioning organelles. Many antioxidants inhibit basal and induced autophagy [145]. Since induction of autophagy is associated with increased lifespan, the answer to this apparent inconsistency may lie in the inhibitory effects of antioxidants on autophagy [145]. Essentially, antioxidants only extend lifespan in cases where increased production of ROS contributes to cell death.
Mitochondria, the mitochondrial permeability transition, and apoptosis
Mitochondria in higher eukaryotes carry out many important cellular functions including production of over 90% of the cell’s ATP by oxidative phosphorylation [146], cytosolic [Ca2+] buffering [111, 147], partial control of apoptosis [148–150], β oxidation of fatty acids [151], a role in the urea cycle [151], and a role in the synthesis and metabolism of iron-containing proteins [152, 153]; however, oxidative phosphorylation was undoubtedly their initial and is now their most important function. Since intramitochondrial [Ca2+] is understood to be a major factor in controlling the rate of ATP production by oxidative phosphorylation [113–115, 154], and since it also plays a major role in inducing the mitochondrial permeability transition (MPT) (described below), mitochondrial Ca2+ transport takes on added importance. Mitochondria contain an elaborate system for Ca2+ sequestration and control consisting of two mechanisms of influx, the Ca2+ uniporter and the rapid mode or RaM, and two mechanisms of efflux, the Na+-dependent and the Na+-independent efflux mechanisms [147]. An additional mechanism, a mitochondrial ryanodine receptor, has also been reported in heart mitochondria [155–157]. In addition, a very unusual mitochondrial mechanism called the MPT, which will be discussed in more detail below, also affects and is affected by intramitochondrial [Ca2+]. Because Mn2+ almost always binds to Ca2+ binding and transport sites, the mitochondrial Ca2+ transport mechanisms are important to Mn toxicity as well. The role of [Ca2+] in activating ATP production by oxidative phosphorylation and the mechanisms of mitochondrial Ca2+ transport is important in Mn toxicity for several reasons. First, the mitochondrial Ca2+ uniporter has been shown to transport Mn2+ as well as Ca2+ [158–161]. Second, many studies on Mn toxicity have indicated Mn interference with energy metabolism [60, 76, 117, 162–167] and inhibition of oxidative phosphorylation [60, 116, 117, 167]. Finally, Mn2+ has been found not to be transported out of mitochondria via the Na+-dependent efflux mechanism, which is by far the dominant mechanism in brain mitochondria, but only to be transported outward by the Na+-independent efflux mechanism, which is much less active in brain mitochondria [168]. This means that once Mn2+ is sequestered within brain mitochondria, the target tissue for Mn toxicity by the rapid uniporter mechanism, it is very difficult for the Mn2+ to be transported out again. This also explains the relatively long half-life of Mn within the brain.
Mitochondria can undergo a dramatic change in permeability of the inner membrane caused by the opening of a very large pore called the permeability transition pore (PTP). The process is referred to as inducing the MPT [108, 110, 111, 169, 170]. Opening of the PTP rapidly dissipates ΔμH and blocks oxidative phosphorylation. It is quite remarkable that a process, which dissipates ΔμH and turns off production of over 90% of the cell’s ATP is highly conserved within eukaryotes. This must be because the function of the MPT is very important to the organism. While still speculative, it is likely that the importance of the MPT lies in its relationship to apoptosis and the mitochondrion’s role in control of apoptosis, which is discussed further below [147].
By the middle 1970’s, the MPT had been described in mitochondria as a process which causes rapid swelling and ultrastructural changes, and also allows NADH and NADPH to directly enter the mitochondrial matrix, something which they cannot otherwise do [171–173]. This transition was originally referred to as the Ca2+-induced MPT, because intramitochondrial Ca2+ was always required for its induction. Hunter and Haworth noted that what was known about the MPT could be explained by the opening of a proteinaceous pore, later called the PTP [174, 175]. The opening of the PTP was found to have the following characteristics: 1) it could be induced by Ca2+ alone or by smaller amounts of Ca2+ in the presence of other agents called “inducing agents” [111]; 2) common endogenous inducing agents include inorganic phosphate, oxaloacetate and acetoacetate, while Mg2+, ADP and ATP act as endogenous inhibitors of PTP opening [108, 111, 170]; 3) molecules with a molecular weight less than 1,500 Daltons penetrate the PTP rapidly [175], while even larger molecules can slowly penetrate the PTP [176]; 4) PTP opening is greatly aided by production of ROS [108, 110, 111, 169, 170], whereas, acidic pH and a high mitochondrial Δψ are potent inhibitors of PTP opening [177, 178]. Commonly used non-endogenous inducing agents include phenylarsine oxide, tert butyl hydroperoxide, atractyloside and carboxyatractyloside, while commonly used inhibitors are cyclosporin A, bongkrekate, adenine nucleotides and chelation of Ca2+ [111, 170].
The discovery that the immunosuppressant cyclosporin A (cys A) was a potent inhibitor of PTP opening [179, 180] was followed by studies establishing that this inhibition occurs by its binding to a mitochondrial matrix protein, cyclophilin D (cyp D). This led to efforts to determine the makeup of the pore protein and the development of the hypothesis that the crucial step in pore opening was the Ca2+-induced binding of cyp D to the adenine nucleotide translocase (ANT). Evidence showed that cys A inhibited this process by binding to cyp D, therefore keeping it from binding to ANT [112, 181, 182]. Later Wallace’s laboratory demonstrated the MPT in mitochondria from the livers of newborn mice that had been genetically modified to lack the ANT, thereby establishing that the ANT was not required for the MPT [183]. Many still support the hypothesis that the Ca2+-induced binding of cyp D to the pore protein is the crucial step in PTP opening with the modification that a set of mitochondrial membrane proteins and not just the ANT can form the basis for the pore [184].
The MPT is viewed as an important component of the mechanisms which give mitochondria a significant role in control of apoptosis or programmed cell death, and ROS are viewed as playing a significant role in induction of the MPT and therefore in apoptosis. Unlike necrosis, apoptosis preserves components of cells for reuse by other cells, usually macrophages [150]. Characteristics that are commonly observed in apoptosis include contraction of cell volume rather than the swelling seen in necrosis, chromatin condensation, nuclear fragmentation, blebbing of the plasma membrane, and the formation of apoptotic vesicles containing cellular material that can be taken up and reused by the surrounding cells [150]. In recent years the relatively clear distinction between apoptotic and necrotic cell death has been blurred somewhat. It is presently believed that many types of cell death include both apoptotic and necrotic characteristics [95].
Mn, ROS, the Mitochondria and Apoptosis
In terms of electron structure, Mn2+ ion has a half filled d shell, giving it spherical symmetry similar to that of Ca2+ and Mg2+, and its ionic radius is intermediate between that of Ca2+ and Mg2+ [160, 161]. For these reasons, it binds to almost every Ca2+ or Mg2+ binding site, and can often either substitute for Ca2+ or Mg2+ in biological processes or act as an inhibitor of these processes. Therefore, it comes as no surprise that Mn2+ can interfere with Ca2+’s role in activating ATP production by oxidative phosphorylation.
Using a C. elegans model, Benedetto and colleagues demonstrated extracellular Mn-induced oxidation of synaptic DA, generating ROS and leading to lipid peroxidation [69]. In their study, SKN-1 (mammalian NRF2 homolog) and BLI-3, proteins important for antioxidant/oxidant homeostasis, were linked to the Mn-induced toxicity. Loss of function of SKN-1 increased sensitivity to Mn, and Mn was able to induce expression of antioxidant proteins through SKN-1 [69]. BLI-3 is a dual oxidase involved in di-tyrosine bond formation in the worm cuticle; pathogen-induced ROS production and loss of this gene resulted in no increase in ROS production by Mn [69].
Gavin and colleagues, using isolated mitochondria and concentrations of Mn2+ greater than 1μM, showed evidence that Mn2+ causes impairment of energy metabolism [60, 168]. In another study of Mn inhibition of energy metabolism, Brouillet and colleagues measured ATP and lactate levels following injection of 2 μM Mn in the rat striatum. They reported N-methyl-D-aspartate (NMDA) excitotoxic lesions as well as a 51% reduction of ATP and a 97% increase in lactate compared to control. Dose-dependent decreases in DA, GABA, and substance P were also noted. These data suggest that oxidative metabolism was impaired following exposure to Mn [76].
Additional support for oxidative stress as a mechanism of toxicity comes from studies on the use of antioxidants to combat Mn-induced ROS. Hazell and colleagues (2006) co-treated rats with N-acetylcysteine (an antioxidant) and 50mg/kg MnCl2 for 4 days. Addition of N-acetylcysteine prevented the pathological changes observed in Mn exposed animals [185]. In another study using catecholaminergic cells, Stredrick and colleagues found that supplementing culture media with glutathione or N-acetylcysteine protected against Mn cytotoxicity [61]. Brenneman et al (1999) measured ROS in the brains of neonatal rats exposed to 22mgMn/kg/day and reported no increase in ROS in the striatum, hippocampus or hindbrain at postnatal day (PND) 21 but a significant increase in ROS levels in the cerebellum. Interestingly, Mn levels were not significantly increased in the cerebellum but were increased in the striatum when measured at PND 49 [186]. Klockgether and Turski reported that the pathophysiology of PD is due to dis-inhibition of glutamatergic inputs to the globus pallidus regulated by differential activation of glutamate receptor subtypes in the basal ganglia [51].
The death of cultured cells and cells in the brains of non-human primates [187] exposed to Mn has largely been described as apoptotic and is often associated with increased ROS production. The primary tools used to measure the increase in ROS are 2′,7′-dichlorofluoroscein (DCF) and its acetate form, DCF-DA. DCF fluorescence was used to show an increase in ROS induced by Mn addition to a primary culture of astrocytes [99] and to a culture of neural stem cells [102], with cell death showing apoptotic characteristics. DCF-DA was used with primary mesencephalic cells prepared from ventral mesencephalic tissues of fetal rat brain to show an ROS increase and cell death with apoptotic characteristics [101]. Latchoumycandane et al reported strong evidence for apoptotic cell death in a rat mesencephalic DAergic N27 cell line. Cytochrome c release, caspase 3 activation, DNA fragmentation and the effects of the apoptosis inhibitors Z-DEVD-FMK were all studied, and showed apoptotic characteristics [188]. Guilarte’s laboratory found signs of apoptosis in the brains of cynomolgus macaques after treatment with 3.3 – 5.0 mg Mn/kg body weight weekly for 10 months [187, 189].
In an effort to study the contribution of Mn to L-DOPA toxicity, Migheli and colleagues used the 3-(4,5-demethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, flow cytometry, fluorescence microscopy and TUNEL staining to measure apoptosis in PC12 (catecholaminergic) cells. They showed that neither exposure to a subtoxic dose (0.2mM) of MnCl2 or 10–20 μM L-DOPA alone affected any of their experimental parameters but apoptotic cell death occurred following MnCl2 and L-DOPA co-exposure [190]. Use of N-acetylcysteine (NAC) inhibited apoptosis and decreases in cell viability [190]. Additionally, Ramesh and colleagues (2002) measured the DNA binding activity of nuclear factor kappa B (NF-κB) after exposure to Mn in PC12 cells [191]. They reported a five-fold induction of NF-κB following Mn exposure for just 30–60 minutes and activation of mitogen activated protein kinase kinase (MAPKK); the authors suggest that this activation could lead downstream to excessive transcription of proteins that contribute to cell death [191]. Kitazawa and colleagues, using the same cell line, highlighted the importance of proteolytic activation of protein kinase Cδ (PKCδ) by caspase-3 in mediating apoptosis following Mn exposure [192]. Mitochondrial membrane depolarization, cytochrome c release, caspase-3 activation and DNA fragmentation were all reported in Mn exposed cells. These events, as well as the proteolytic cleavage of PKCδ, were blocked by overexpression of Bcl-2. Expression of the catalytically inactive mutant PKCδ attenuated apoptosis and administration of active recombinant PKCδ induced DNA fragmentation [192]. Taken together, this evidence points to apoptosis as a mechanism of toxicity and the involvement of PKCδ and caspase-3 in mediating apoptosis following Mn exposure.
A case of the use of mitochondrially-targeted antioxidants
Untargeted antioxidants have been shown to ameliorate Mn-induced damage. Vitamin E was assessed as an antioxidant treatment option and it was found that pretreatment attenuated the Mn-induced increase in cerebral isoprostanes and protected medium spiny neurons from dendritic atrophy and dendritic spine loss [193].
L-DOPA has been tested for Mn-toxicity due to the similarities between manganism and PD. Some of the motor symptoms associated with manganism were reversed by L-DOPA treatment albeit with serious side effects [52]. Treatment with anti-Parkinsonian drugs may be less effective due to the preferential accumulation of Mn in the globus pallidus, rather than the cell death in the substantia nigra seen in PD.
Treatment of cells and isolated mitochondria with Mn2+ has also been found to cause inhibition of ATP production by oxidative phosphorylation [60, 116, 117, 167]. Inhibition of the ETC could cause the proximal part of the ETC to become even more reduced and therefore more likely to produce O2− •, and increases of O2− •, production of this type have been reported [194–197]. These observations as well as those reported above showing an increase in ROS production following Mn treatment [99, 101, 102] suggest that Mn-induced ROS production and probably opening of the PTP are involved with induction of the form of apoptosis leading to cell death in the globus pallidus. If untargeted antioxidants can ameliorate damage in Mn toxicity, how much more clinically effective could drugs that protect against ROS damage, MPT induction and apoptosis at the mitochondrial level be in treating Mn toxicity [198]. A number of different drugs are currently being developed to be used to treat conditions like apoptotic cell death. These drugs are sequestered into mitochondria where they act as reducing agents and detoxify mitochondrial ROS [137–139, 141, 142].
MitoTEMPO, mitoNAC, and MitoQ are part of a series of antioxidants under development and study containing nitroxide, tocopherol and ubiquinone moieties. They are made membrane permeable and mitochondrially-targeted by attaching a positively charged triphenol phosphonium (TPP) moiety. For example, mitoQ is made up of a combination of ubiquinone or coenzyme Q10 (co Q10) and TPP. Analogous to the other members in this series, it can move rapidly across the cell and mitochondrial membranes into mitochondria. Inside the mitochondrion, mito Q can be oxidized by reaction with ROS and then reduced by complex II and reused as an antioxidant. It cannot pass electrons on to complex III as co Q does and so it functions to protect against oxidative damage to the mitochondria [138–140]. Hyperglycemia in diabetes [105], excessive ethanol use [134], and some forms of hypertension [135] are accompanied by an increase in mitochondrial ROS production in some organs. Mito Q was orally administered to mice showing the Akita J model of Type I diabetes over a 12-week period. Interstitial fibrosis and glomerular damage were significantly reduced in the kidneys of treated vs. control animals, showing in this case that mitochondrially-targeted therapies were beneficial in the treatment of diabetic nephropathy [133]. In a four week oral treatment of Mito Q to Sprague-Dawley rats consuming ethanol using the Lieber-Decarli diet vs. controls, it was found that the Mito Q treatment decreased hepatic steatosis in ethanol-treated animals, prevented ethanol-induced formation of 3-NT and 4-HNE, and stabilized HIF1alpha [134]. Stroke-prone, spontaneously hypertensive rats were treated orally with Mito Q for 8 weeks, systolic blood pressure in the treated animals was reduced by approximately 25 mm Hg over the 8-week treatment period [135].
The Szeto-Schiller or SS peptides are a set of mitochondrially-targeted, antioxidant peptides, which have been shown to penetrate the blood-brain barrier and protect brain mitochondria against ROS damage while showing minimal toxicity [141, 142]. The Szeto-Schiller peptide SS-31 has recently been shown to protect against induction of the MPT following a burst of ROS during reperfusion of ischemic kidney tissue reducing apoptosis and necrosis of tubular cells [143].
SkQ1, an antioxidant in the SkQ family, combines plastoquinone, a very effective electron carrier and antioxidant of chloroplasts with decyltriphenolphosphonium to form a cation that easily penetrates membranes [137]. In a number of tests SkQ1 was found to save the lives of young animals after treatments resulting in kidney ischemia, rhabdomyolysis, heart attack, arrhythmia, and stroke and to suppress the appearance of many traits related to senescence [136].
Conclusion
Manganese is an essential nutrient, which can be harmful at higher doses or accumulations. Because of its essentiality, stable tissue levels are maintained making it difficult to determine if overexposure has occurred. As Mn toxicity can cause a number of functional problems, such as manganism, it is imperative that we understand the mechanism(s) of damage to attempt to prevent or counter them. Such mechanisms include the autoxidation of DA, production of free radicals, ROS and toxic metabolites, depletion of cellular antioxidant defense mechanisms and alterations of mitochondrial function and ATP production. Decades of research have also shown that the energy requirements of certain brain regions (i.e. globus pallidus) make them more vulnerable. Future research endeavors should be aimed at not only pinpointing the mechanism(s) underlying the damage but also identifying biomarkers for exposure and treatment strategies.
Figure 1.
Mn Transport
Highlights.
Mn is a key component of the diet, but in excess can be toxic
Mn is a component of enzymes and forms complexes in the body
Mn toxicity is the result of reactive oxygen species, and altered energy metabolism
Therapeutics that target the mitochondria may be useful in combating toxicity
Acknowledgments
The authors wish to acknowledge generous funding from the National Institutes of Environmental Health Sciences: R01-10563, T32-007028 and P30 000267.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael Aschner, Email: michael.aschner@vanderbilt.edu.
Thomas E. Gunter, Email: thomas_gunter@urmc.rochester.edu.
References
- 1.May BP, Dennis PP. Evolution and regulation of the gene encoding superoxide dismutase from the archaebacterium Halobacterium cutirubrum. J Biol Chem. 1989;264:12253–12258. [PubMed] [Google Scholar]
- 2.Aschner M, Dorman DC. Health Canada, Air Quality and Health Division. Ottawa, ON, Canada: Aug 1, 2002. Review of Manganese Toxicokinetics. [Google Scholar]
- 3.Chen JY, Tsao GC, Zhao Q, Zheng W. Differential cytotoxicity of Mn(II) and Mn(III): special reference to mitochondrial [Fe-S] containing enzymes. Toxicol Appl Pharmacol. 2001;175:160–168. doi: 10.1006/taap.2001.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali SF, Duhart HM, Newport GD, Lipe GW, Slikker W., Jr Manganese-induced reactive oxygen species: comparison between Mn+2 and Mn+3. Neurodegeneration. 1995;4:329–334. doi: 10.1016/1055-8330(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 5.Aschner M, Gannon M. Manganese (Mn) transport across the rat blood-brain barrier: saturable and transferrin-dependent transport mechanisms. Brain Res Bull. 1994;33:345–349. doi: 10.1016/0361-9230(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 6.Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology. 2008;29:569–576. doi: 10.1016/j.neuro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erikson K, Aschner M. Manganese causes differential regulation of glutamate transporter (GLAST) taurine transporter and metallothionein in cultured rat astrocytes. Neurotoxicology. 2002;23:595–602. doi: 10.1016/s0161-813x(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 8.Erikson KM, John CE, Jones SR, Aschner M. Manganese accumulation in striatum of mice exposed to toxic doses is dependent upon a functional dopamine transporter. Environ Toxicol Pharmacol. 2005;20:390–394. doi: 10.1016/j.etap.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Lockman PR, Roder KE, Allen DD. Inhibition of the rat blood-brain barrier choline transporter by manganese chloride. J Neurochem. 2001;79:588–594. doi: 10.1046/j.1471-4159.2001.00589.x. [DOI] [PubMed] [Google Scholar]
- 10.Lorkovic H, Feyrer A. Manganese ions inhibit acetylcholine receptor synthesis in cultured mouse soleus muscles. Neurosci Lett. 1984;51:331–335. doi: 10.1016/0304-3940(84)90398-7. [DOI] [PubMed] [Google Scholar]
- 11.Lucaciu CM, Dragu C, Copaescu L, Morariu VV. Manganese transport through human erythrocyte membranes. An EPR study. Biochim Biophys Acta. 1997;1328:90–98. doi: 10.1016/s0005-2736(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 12.Riccio A, Mattei C, Kelsell RE, Medhurst AD, Calver AR, Randall AD, Davis JB, Benham CD, Pangalos MN. Cloning and functional expression of human short TRP7, a candidate protein for store-operated Ca2+ influx. J Biol Chem. 2002;277:12302–12309. doi: 10.1074/jbc.M112313200. [DOI] [PubMed] [Google Scholar]
- 13.Kannurpatti SS, Joshi PG, Joshi NB. Calcium sequestering ability of mitochondria modulates influx of calcium through glutamate receptor channel. Neurochem Res. 2000;25:1527–1536. doi: 10.1023/a:1026602100160. [DOI] [PubMed] [Google Scholar]
- 14.Crossgrove JS, Allen DD, Bukaveckas BL, Rhineheimer SS, Yokel RA. Manganese distribution across the blood-brain barrier. I. Evidence for carrier-mediated influx of managanese citrate as well as manganese and manganese transferrin. Neurotoxicology. 2003;24:3–13. doi: 10.1016/s0161-813x(02)00089-x. [DOI] [PubMed] [Google Scholar]
- 15.Fujishiro H, Doi M, Enomoto S, Himeno S. High sensitivity of RBL-2H3 cells to cadmium and manganese: an implication of the role of ZIP8. Metallomics. 2011;3:710–718. doi: 10.1039/c1mt00020a. [DOI] [PubMed] [Google Scholar]
- 16.He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol. 2006;70:171–180. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- 17.Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, Caldwell KA, Caldwell GA, Cooper AA, Rochet JC, Lindquist S. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Health criteria and other supporting information. Geneva, Switzerland: W. H. O. Guidelines for drinking water quality; pp. 275–278. [Google Scholar]
- 19.Aschner M, Guilarte TR, Schneider JS, Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aschner M, Aschner JL. Manganese transport across the blood-brain barrier: relationship to iron homeostasis. Brain Res Bull. 1990;24:857–860. doi: 10.1016/0361-9230(90)90152-p. [DOI] [PubMed] [Google Scholar]
- 21.Chua AC, Morgan EH. Effects of iron deficiency and iron overload on manganese uptake and deposition in the brain and other organs of the rat. Biol Trace Elem Res. 1996;55:39–54. doi: 10.1007/BF02784167. [DOI] [PubMed] [Google Scholar]
- 22.Erikson KM, Shihabi ZK, Aschner JL, Aschner M. Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol Trace Elem Res. 2002;87:143–156. doi: 10.1385/BTER:87:1-3:143. [DOI] [PubMed] [Google Scholar]
- 23.Miller KB, Caton JS, Schafer DM, Smith DJ, Finley JW. High dietary manganese lowers heart magnesium in pigs fed a low-magnesium diet. J Nutr. 2000;130:2032–2035. doi: 10.1093/jn/130.8.2032. [DOI] [PubMed] [Google Scholar]
- 24.Thompson K, Molina R, Donaghey T, Brain JD, Wessling-Resnick M. The influence of high iron diet on rat lung manganese absorption. Toxicol Appl Pharmacol. 2006;210:17–23. doi: 10.1016/j.taap.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Erikson KM, Dobson AW, Dorman DC, Aschner M. Manganese exposure and induced oxidative stress in the rat brain. Sci Total Environ. 2004;334–335:409–416. doi: 10.1016/j.scitotenv.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen ME, Gearhart JM, Clewell HJ., 3rd Pharmacokinetic data needs to support risk assessments for inhaled and ingested manganese. Neurotoxicology. 1999;20:161–171. [PubMed] [Google Scholar]
- 27.Roels H, Meiers G, Delos M, Ortega I, Lauwerys R, Buchet JP, Lison D. Influence of the route of administration and the chemical form (MnCl2, MnO2) on the absorption and cerebral distribution of manganese in rats. Arch Toxicol. 1997;71:223–230. doi: 10.1007/s002040050380. [DOI] [PubMed] [Google Scholar]
- 28.Davis CD, Zech L, Greger JL. Manganese metabolism in rats: an improved methodology for assessing gut endogenous losses. Proc Soc Exp Biol Med. 1993;202:103–108. doi: 10.3181/00379727-202-43518. [DOI] [PubMed] [Google Scholar]
- 29.Britton AA, Cotzias GC. Dependence of manganese turnover on intake. Am J Physiol. 1966;211:203–206. doi: 10.1152/ajplegacy.1966.211.1.203. [DOI] [PubMed] [Google Scholar]
- 30.Finley JW. Manganese absorption and retention by young women is associated with serum ferritin concentration. Am J Clin Nutr. 1999;70:37–43. doi: 10.1093/ajcn/70.1.37. [DOI] [PubMed] [Google Scholar]
- 31.Mahoney JP, Small WJ. Studies on manganese. 3. The biological half-life of radiomanganese in man and factors which affect this half-life. J Clin Invest. 1968;47:643–653. doi: 10.1172/JCI105760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malecki EA, Radzanowski GM, Radzanowski TJ, Gallaher DD, Greger JL. Biliary manganese excretion in conscious rats is affected by acute and chronic manganese intake but not by dietary fat. J Nutr. 1996;126:489–498. doi: 10.1093/jn/126.2.489. [DOI] [PubMed] [Google Scholar]
- 33.Papavasiliou PS, Miller ST, Cotzias GC. Role of liver in regulating distribution and excretion of manganese. Am J Physiol. 1966;211:211–216. doi: 10.1152/ajplegacy.1966.211.1.211. [DOI] [PubMed] [Google Scholar]
- 34.Hauser RA, Zesiewicz TA, Martinez C, Rosemurgy AS, Olanow CW. Blood manganese correlates with brain magnetic resonance imaging changes in patients with liver disease. Can J Neurol Sci. 1996;23:95–98. doi: 10.1017/s0317167100038786. [DOI] [PubMed] [Google Scholar]
- 35.Suarez N, Walum E, Eriksson H. Cellular neurotoxicity of trivalent manganese bound to transferrin or pyrophosphate studied in human neuroblastoma (SH-SY5Y) cell cultures. Toxicol In Vitro. 1995;9:717–721. doi: 10.1016/0887-2333(95)00062-d. [DOI] [PubMed] [Google Scholar]
- 36.Takeda A, Sawashita J, Okada S. Biological half-lives of zinc and manganese in rat brain. Brain Res. 1995;695:53–58. doi: 10.1016/0006-8993(95)00916-e. [DOI] [PubMed] [Google Scholar]
- 37.Cotzias GC, Horiuchi K, Fuenzalida S, Mena I. Chronic manganese poisoning. Clearance of tissue manganese concentrations with persistance of the neurological picture. Neurology. 1968;18:376–382. doi: 10.1212/wnl.18.4.376. [DOI] [PubMed] [Google Scholar]
- 38.Newland MC, Cox C, Hamada R, Oberdorster G, Weiss B. The clearance of manganese chloride in the primate. Fundam Appl Toxicol. 1987;9:314–328. doi: 10.1016/0272-0590(87)90054-6. [DOI] [PubMed] [Google Scholar]
- 39.Dorman DC, Struve MF, James RA, Marshall MW, Parkinson CU, Wong BA. Influence of particle solubility on the delivery of inhaled manganese to the rat brain: manganese sulfate and manganese tetroxide pharmacokinetics following repeated (14-day) exposure. Toxicol Appl Pharmacol. 2001;170:79–87. doi: 10.1006/taap.2000.9088. [DOI] [PubMed] [Google Scholar]
- 40.Dorman DC, Struve MF, James RA, McManus BE, Marshall MW, Wong BA. Influence of dietary manganese on the pharmacokinetics of inhaled manganese sulfate in male CD rats. Toxicol Sci. 2001;60:242–251. doi: 10.1093/toxsci/60.2.242. [DOI] [PubMed] [Google Scholar]
- 41.Vitarella D, Wong BA, Moss OR, Dorman DC. Pharmacokinetics of inhaled manganese phosphate in male Sprague-Dawley rats following subacute (14-day) exposure. Toxicol Appl Pharmacol. 2000;163:279–285. doi: 10.1006/taap.1999.8874. [DOI] [PubMed] [Google Scholar]
- 42.Registry, A. f. T. S. a. D. Draft Toxicological Profile for Manganese. U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES; 2008. [Google Scholar]
- 43.Aschner M, Erikson KM, Herrero Hernandez E, Tjalkens R. Manganese and its role in Parkinson’s disease: from transport to neuropathology. Neuromolecular Med. 2009;11:252–266. doi: 10.1007/s12017-009-8083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gavin CE, Gunter KK, Gunter TE. Manganese and calcium transport in mitochondria: implications for manganese toxicity. Neurotoxicology. 1999;20:445–453. [PubMed] [Google Scholar]
- 45.Lai JC, Minski MJ, Chan AW, Leung TK, Lim L. Manganese mineral interactions in brain. Neurotoxicology. 1999;20:433–444. [PubMed] [Google Scholar]
- 46.Liccione JJ, Maines MD. Selective vulnerability of glutathione metabolism and cellular defense mechanisms in rat striatum to manganese. J Pharmacol Exp Ther. 1988;247:156–161. [PubMed] [Google Scholar]
- 47.Morello M, Canini A, Mattioli P, Sorge RP, Alimonti A, Bocca B, Forte G, Martorana A, Bernardi G, Sancesario G. Sub-cellular localization of manganese in the basal ganglia of normal and manganese-treated rats An electron spectroscopy imaging and electron energy-loss spectroscopy study. Neurotoxicology. 2008;29:60–72. doi: 10.1016/j.neuro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H. Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol. 1986;70:273–278. doi: 10.1007/BF00686083. [DOI] [PubMed] [Google Scholar]
- 49.Olanow CW, Good PF, Shinotoh H, Hewitt KA, Vingerhoets F, Snow BJ, Beal MF, Calne DB, Perl DP. Manganese intoxication in the rhesus monkey: a clinical, imaging, pathologic, and biochemical study. Neurology. 1996;46:492–498. doi: 10.1212/wnl.46.2.492. [DOI] [PubMed] [Google Scholar]
- 50.Pentschew A, Ebner FF, Kovatch RM. Experimental Manganese Encephalopathy in Monkeys. A Preliminary Report. J Neuropathol Exp Neurol. 1963;22:488–499. doi: 10.1097/00005072-196307000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Klockgether T, Turski L. Toward an understanding of the role of glutamate in experimental parkinsonism: agonist-sensitive sites in the basal ganglia. Ann Neurol. 1993;34:585–593. doi: 10.1002/ana.410340413. [DOI] [PubMed] [Google Scholar]
- 52.Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias) Neurotoxicology. 1984;5:13–35. [PubMed] [Google Scholar]
- 53.Canavan MCS, Drinker CK. Chronic manganese poisoning-report of a case with autopsy. Arch Neurol Psychiat. 1934;32:501–512. [Google Scholar]
- 54.Mella H. The experimental production of bassal ganglia symptomatology in macacus rhesus. Arch Neurol Psychiat. 1924;11:405–417. [Google Scholar]
- 55.Stadler LJ, Sprague GF. Genetic Effects of Ultra-Violet Radiation in Maize: III. Effects of Nearly Monochromatic lambda 2537, and Comparison of Effects of X-Ray and UltraViolet Treatment. Proc Natl Acad Sci U S A. 1936;22:584–591. doi: 10.1073/pnas.22.10.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bird ED, Anton AH, Bullock B. The effect of manganese inhalation on basal ganglia dopamine concentrations in rhesus monkey. Neurotoxicology. 1984;5:59–65. [PubMed] [Google Scholar]
- 57.Dorman DC, Struve MF, Marshall MW, Parkinson CU, James RA, Wong BA. Tissue manganese concentrations in young male rhesus monkeys following subchronic manganese sulfate inhalation. Toxicol Sci. 2006;92:201–210. doi: 10.1093/toxsci/kfj206. [DOI] [PubMed] [Google Scholar]
- 58.Guilarte TR, McGlothan JL, Degaonkar M, Chen MK, Barker PB, Syversen T, Schneider JS. Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: a 1H-MRS and MRI study. Toxicol Sci. 2006;94:351–358. doi: 10.1093/toxsci/kfl106. [DOI] [PubMed] [Google Scholar]
- 59.Criswell SR, Perlmutter JS, Videen TO, Moerlein SM, Flores HP, Birke AM, Racette BA. Reduced uptake of [(1)(8)F]FDOPA PET in asymptomatic welders with occupational manganese exposure. Neurology. 2011;76:1296–1301. doi: 10.1212/WNL.0b013e3182152830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gavin CE, Gunter KK, Gunter TE. Mn2+ sequestration by mitochondria and inhibition of oxidative phosphorylation. Toxicol Appl Pharmacol. 1992;115:1–5. doi: 10.1016/0041-008x(92)90360-5. [DOI] [PubMed] [Google Scholar]
- 61.Stredrick DL, Stokes AH, Worst TJ, Freeman WM, Johnson EA, Lash LH, Aschner M, Vrana KE. Manganese-induced cytotoxicity in dopamine-producing cells. Neurotoxicology. 2004;25:543–553. doi: 10.1016/j.neuro.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Cohen M. Manganese balance studies in infants after operations on the heart. Pediatr Res. 1984;18:300–301. doi: 10.1203/00006450-198403000-00023. [DOI] [PubMed] [Google Scholar]
- 63.Donaldson J, LaBella FS, Gesser D. Enhanced autoxidation of dopamine as a possible basis of manganese neurotoxicity. Neurotoxicology. 1981;2:53–64. [PubMed] [Google Scholar]
- 64.Graham DG. Catecholamine toxicity: a proposal for the molecular pathogenesis of manganese neurotoxicity and Parkinson’s disease. Neurotoxicology. 1984;5:83–95. [PubMed] [Google Scholar]
- 65.Archibald FS, Tyree C. Manganese poisoning and the attack of trivalent manganese upon catecholamines. Arch Biochem Biophys. 1987;256:638–650. doi: 10.1016/0003-9861(87)90621-7. [DOI] [PubMed] [Google Scholar]
- 66.Cawte J, Kilburn C, Florence M. Motor neurone disease of the western Pacific: do the foci extend to Australia? Neurotoxicology. 1989;10:263–270. [PubMed] [Google Scholar]
- 67.Segura-Aguilar J, Lind C. On the mechanism of the Mn3(+)-induced neurotoxicity of dopamine:prevention of quinone-derived oxygen toxicity by DT diaphorase and superoxide dismutase. Chem Biol Interact. 1989;72:309–324. doi: 10.1016/0009-2797(89)90006-9. [DOI] [PubMed] [Google Scholar]
- 68.Desole MS, Miele M, Esposito G, Migheli R, Fresu L, De Natale G, Miele E. Dopaminergic system activity and cellular defense mechanisms in the striatum and striatal synaptosomes of the rat subchronically exposed to manganese. Arch Toxicol. 1994;68:566–570. doi: 10.1007/s002040050115. [DOI] [PubMed] [Google Scholar]
- 69.Benedetto A, Au C, Avila DS, Milatovic D, Aschner M. Extracellular dopamine potentiates mn-induced oxidative stress, lifespan reduction, and dopaminergic neurodegeneration in a BLI-3-dependent manner in Caenorhabditis elegans. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Erikson KM, Dorman DC, Lash LH, Aschner M. Manganese inhalation by rhesus monkeys is associated with brain regional changes in biomarkers of neurotoxicity. Toxicol Sci. 2007;97:459–466. doi: 10.1093/toxsci/kfm044. [DOI] [PubMed] [Google Scholar]
- 71.Gwiazda RH, Lee D, Sheridan J, Smith DR. Low cumulative manganese exposure affects striatal GABA but not dopamine. Neurotoxicology. 2002;23:69–76. doi: 10.1016/s0161-813x(02)00002-5. [DOI] [PubMed] [Google Scholar]
- 72.Lipe GW, Duhart H, Newport GD, Slikker W, Jr, Ali SF. Effect of manganese on the concentration of amino acids in different regions of the rat brain. J Environ Sci Health B. 1999;34:119–132. doi: 10.1080/03601239909373187. [DOI] [PubMed] [Google Scholar]
- 73.Reaney SH, Bench G, Smith DR. Brain accumulation and toxicity of Mn(II) and Mn(III) exposures. Toxicol Sci. 2006;93:114–124. doi: 10.1093/toxsci/kfl028. [DOI] [PubMed] [Google Scholar]
- 74.Zwingmann C, Leibfritz D, Hazell AS. Nmr spectroscopic analysis of regional brain energy metabolism in manganese neurotoxicity. Glia. 2007;55:1610–1617. doi: 10.1002/glia.20575. [DOI] [PubMed] [Google Scholar]
- 75.Bonilla E, Arrieta A, Castro F, Davila JO, Quiroz I. Manganese toxicity: free amino acids in the striatum and olfactory bulb of the mouse. Invest Clin. 1994;35:175–181. [PubMed] [Google Scholar]
- 76.Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp Neurol. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- 77.Gianutsos G, Murray MT. Alterations in brain dopamine and GABA following inorganic or organic manganese administration. Neurotoxicology. 1982;3:75–81. [PubMed] [Google Scholar]
- 78.Lai JC, Leung TK, Lim L. Brain regional distribution of glutamic acid decarboxylase, choline acetyltransferase, and acetylcholinesterase in the rat: effects of chronic manganese chloride administration after two years. J Neurochem. 1981;36:1443–1448. doi: 10.1111/j.1471-4159.1981.tb00585.x. [DOI] [PubMed] [Google Scholar]
- 79.Bonilla E. Increased GABA content in caudate nucleus of rats after chronic manganese chloride administration. J Neurochem. 1978;31:551–552. doi: 10.1111/j.1471-4159.1978.tb02672.x. [DOI] [PubMed] [Google Scholar]
- 80.Fordahl SC, Anderson JG, Cooney PT, Weaver TL, Colyer CL, Erikson KM. Manganese exposure inhibits the clearance of extracellular GABA and influences taurine homeostasis in the striatum of developing rats. Neurotoxicology. 2010;31:639–646. doi: 10.1016/j.neuro.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seth PK, Hong JS, Kilts CD, Bondy SC. Alteration of cerebral neurotransmitter receptor function by exposure of rats to manganese. Toxicol Lett. 1981;9:247–254. doi: 10.1016/0378-4274(81)90157-0. [DOI] [PubMed] [Google Scholar]
- 82.Struve MF, McManus BE, Wong BA, Dorman DC. Basal ganglia neurotransmitter concentrations in rhesus monkeys following subchronic manganese sulfate inhalation. Am J Ind Med. 2007;50:772–778. doi: 10.1002/ajim.20489. [DOI] [PubMed] [Google Scholar]
- 83.Antonini JM, Sriram K, Benkovic SA, Roberts JR, Stone S, Chen BT, Schwegler-Berry D, Jefferson AM, Billig BK, Felton CM, Hammer MA, Ma F, Frazer DG, O’Callaghan JP, Miller DB. Mild steel welding fume causes manganese accumulation and subtle neuroinflammatory changes but not overt neuronal damage in discrete brain regions of rats after short-term inhalation exposure. Neurotoxicology. 2009;30:915–925. doi: 10.1016/j.neuro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Dydak U, Jiang YM, Long LL, Zhu H, Chen J, Li WM, Edden RA, Hu S, Fu X, Long Z, Mo XA, Meier D, Harezlak J, Aschner M, Murdoch JB, Zheng W. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ Health Perspect. 2011;119:219–224. doi: 10.1289/ehp.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bae YS, Oh H, Rhee SG, Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol Cells. 2011;32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lambert AJ, Brand MD. Reactive oxygen species production by mitochondria. Methods Mol Biol. 2009;554:165–181. doi: 10.1007/978-1-59745-521-3_11. [DOI] [PubMed] [Google Scholar]
- 87.Puddu P, Puddu GM, Cravero E, Rosati M, Muscari A. The molecular sources of reactive oxygen species in hypertension. Blood Press. 2008;17:70–77. doi: 10.1080/08037050802029954. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol. 2010;174:307–316. doi: 10.1016/j.resp.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goldstein S, Meyerstein D, Czapski G. The Fenton reagents. Free Radic Biol Med. 1993;15:435–445. doi: 10.1016/0891-5849(93)90043-t. [DOI] [PubMed] [Google Scholar]
- 90.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta. 2006;1757:553–561. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 92.Hoffman DL, Brookes PS. Oxygen sensitivity of mitochondrial reactive oxygen species generation depends on metabolic conditions. J Biol Chem. 2009;284:16236–16245. doi: 10.1074/jbc.M809512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoffman DL, Salter JD, Brookes PS. Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am J Physiol Heart Circ Physiol. 2007;292:H101–108. doi: 10.1152/ajpheart.00699.2006. [DOI] [PubMed] [Google Scholar]
- 94.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Skulachev VP. Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis. 2006;11:473–485. doi: 10.1007/s10495-006-5881-9. [DOI] [PubMed] [Google Scholar]
- 96.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 97.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- 99.Chen CJ, Liao SL. Oxidative stress involves in astrocytic alterations induced by manganese. Exp Neurol. 2002;175:216–225. doi: 10.1006/exnr.2002.7894. [DOI] [PubMed] [Google Scholar]
- 100.Kowaltowski AJ, Castilho RF, Vercesi AE. Ca(2+)-induced mitochondrial membrane permeabilization: role of coenzyme Q redox state. Am J Physiol. 1995;269:C141–147. doi: 10.1152/ajpcell.1995.269.1.C141. [DOI] [PubMed] [Google Scholar]
- 101.Prabhakaran K, Ghosh D, Chapman GD, Gunasekar PG. Molecular mechanism of manganese exposure-induced dopaminergic toxicity. Brain Res Bull. 2008;76:361–367. doi: 10.1016/j.brainresbull.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 102.Tamm C, Sabri F, Ceccatelli S. Mitochondrial-mediated apoptosis in neural stem cells exposed to manganese. Toxicol Sci. 2008;101:310–320. doi: 10.1093/toxsci/kfm267. [DOI] [PubMed] [Google Scholar]
- 103.Kowaltowski AJ, Naia-da-Silva ES, Castilho RF, Vercesi AE. Ca2+-stimulated mitochondrial reactive oxygen species generation and permeability transition are inhibited by dibucaine or Mg2+ Arch Biochem Biophys. 1998;359:77–81. doi: 10.1006/abbi.1998.0870. [DOI] [PubMed] [Google Scholar]
- 104.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fridovich I. Mitochondria: are they the seat of senescence? Aging Cell. 2004;3:13–16. doi: 10.1046/j.1474-9728.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 107.Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med. 1999;26:463–471. doi: 10.1016/s0891-5849(98)00216-0. [DOI] [PubMed] [Google Scholar]
- 108.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 109.Bernardi P, Rasola A. Calcium and cell death: the mitochondrial connection. Subcell Biochem. 2007;45:481–506. doi: 10.1007/978-1-4020-6191-2_18. [DOI] [PubMed] [Google Scholar]
- 110.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341(Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- 111.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 112.Halestrap AP, Woodfield KY, Connern CP. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J Biol Chem. 1997;272:3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- 113.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 114.Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol. 2002;34:1259–1271. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- 115.Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 116.Gunter TE, Gerstner B, Lester T, Wojtovich AP, Malecki J, Swarts SG, Brookes PS, Gavin CE, Gunter KK. An analysis of the effects of Mn2+ on oxidative phosphorylation in liver, brain, and heart mitochondria using state 3 oxidation rate assays. Toxicol Appl Pharmacol. 2010;249:65–75. doi: 10.1016/j.taap.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zwingmann C, Leibfritz D, Hazell AS. Energy metabolism in astrocytes and neurons treated with manganese: relation among cell-specific energy failure, glucose metabolism, and intercellular trafficking using multinuclear NMR-spectroscopic analysis. J Cereb Blood Flow Metab. 2003;23:756–771. doi: 10.1097/01.WCB.0000056062.25434.4D. [DOI] [PubMed] [Google Scholar]
- 118.Hoffman DL. Biochemistry and Biophysics. Rochester, NY: University of Rochester; 2009. The production of mitochondrial reactive oxygen species at steady-state oxygen concentrations: Implications for oxygen sensing; p. 166. [Google Scholar]
- 119.Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 121.Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 122.Hongpaisan J, Winters CA, Andrews SB. Calcium-dependent mitochondrial superoxide modulates nuclear CREB phosphorylation in hippocampal neurons. Mol Cell Neurosci. 2003;24:1103–1115. doi: 10.1016/j.mcn.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 123.Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 124.Lee ES, Yin Z, Milatovic D, Jiang H, Aschner M. Estrogen and tamoxifen protect against Mn-induced toxicity in rat cortical primary cultures of neurons and astrocytes. Toxicol Sci. 2009;110:156–167. doi: 10.1093/toxsci/kfp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim KH, Rodriguez AM, Carrico PM, Melendez JA. Potential mechanisms for the inhibition of tumor cell growth by manganese superoxide dismutase. Antioxid Redox Signal. 2001;3:361–373. doi: 10.1089/15230860152409013. [DOI] [PubMed] [Google Scholar]
- 126.Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- 127.Cai J, Jones DP. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem. 1998;273:11401–11404. doi: 10.1074/jbc.273.19.11401. [DOI] [PubMed] [Google Scholar]
- 128.Ranganathan AC, Nelson KK, Rodriguez AM, Kim KH, Tower GB, Rutter JL, Brinckerhoff CE, Huang TT, Epstein CJ, Jeffrey JJ, Melendez JA. Manganese superoxide dismutase signals matrix metalloproteinase expression via H2O2-dependent ERK1/2 activation. J Biol Chem. 2001;276:14264–14270. doi: 10.1074/jbc.M100199200. [DOI] [PubMed] [Google Scholar]
- 129.Pomytkin IA, Kolesova OE. Key role of succinate dehydrogenase in insulin-induced inactivation of protein tyrosine phosphatases. Bull Exp Biol Med. 2002;133:568–570. doi: 10.1023/a:1020229724717. [DOI] [PubMed] [Google Scholar]
- 130.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 131.Mailer K. Superoxide radical as electron donor for oxidative phosphorylation of ADP. Biochem Biophys Res Commun. 1990;170:59–64. doi: 10.1016/0006-291x(90)91240-s. [DOI] [PubMed] [Google Scholar]
- 132.Maroz A, Anderson RF, Smith RA, Murphy MP. Reactivity of ubiquinone and ubiquinol with superoxide and the hydroperoxyl radical: implications for in vivo antioxidant activity. Free Radic Biol Med. 2009;46:105–109. doi: 10.1016/j.freeradbiomed.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 133.Chacko BK, Reily C, Srivastava A, Johnson MS, Ye Y, Ulasova E, Agarwal A, Zinn KR, Murphy MP, Kalyanaraman B, Darley-Usmar V. Prevention of diabetic nephropathy in Ins2(+/)(−)(AkitaJ) mice by the mitochondria-targeted therapy MitoQ. Biochem J. 2010;432:9–19. doi: 10.1042/BJ20100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chacko BK, Srivastava A, Johnson MS, Benavides GA, Chang MJ, Ye Y, Jhala N, Murphy MP, Kalyanaraman B, Darley-Usmar VM. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011;54:153–163. doi: 10.1002/hep.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 136.Skulachev MV, Antonenko YN, Anisimov VN, Chernyak BV, Cherepanov DA, Chistyakov VA, Egorov MV, Kolosova NG, Korshunova GA, Lyamzaev KG, Plotnikov EY, Roginsky VA, Savchenko AY, Severina II, Severin FF, Shkurat TP, Tashlitsky VN, Shidlovsky KM, Vyssokikh MY, Zamyatnin AA, Jr, Zorov DB, Skulachev VP. Mitochondrial-targeted plastoquinone derivatives. Effect on senescence and acute age-related pathologies. Curr Drug Targets. 2011;12:800–826. doi: 10.2174/138945011795528859. [DOI] [PubMed] [Google Scholar]
- 137.Skulachev VP. Mitochondria-targeted antioxidants as promising drugs for treatment of age-related brain diseases. J Alzheimers Dis. 2012;28:283–289. doi: 10.3233/JAD-2011-111391. [DOI] [PubMed] [Google Scholar]
- 138.Smith RA, Adlam VJ, Blaikie FH, Manas AR, Porteous CM, James AM, Ross MF, Logan A, Cocheme HM, Trnka J, Prime TA, Abakumova I, Jones BA, Filipovska A, Murphy MP. Mitochondria-targeted antioxidants in the treatment of disease. Ann N Y Acad Sci. 2008;1147:105–111. doi: 10.1196/annals.1427.003. [DOI] [PubMed] [Google Scholar]
- 139.Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 140.Smith RA, Murphy MP. Mitochondria-targeted antioxidants as therapies. Discov Med. 2011;11:106–114. [PubMed] [Google Scholar]
- 141.Szeto HH. Development of mitochondria-targeted aromatic-cationic peptides for neurodegenerative diseases. Ann N Y Acad Sci. 2008;1147:112–121. doi: 10.1196/annals.1427.013. [DOI] [PubMed] [Google Scholar]
- 142.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:601–619. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- 143.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22:1041–1052. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rodriguez SA, Nazareno MA, Baumgartner MT. Effect of different C3-aryl substituents on the antioxidant activity of 4-hydroxycoumarin derivatives. Bioorg Med Chem. 2011;19:6233–6238. doi: 10.1016/j.bmc.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 145.Underwood BR, Imarisio S, Fleming A, Rose C, Krishna G, Heard P, Quick M, Korolchuk VI, Renna M, Sarkar S, Garcia-Arencibia M, O’Kane CJ, Murphy MP, Rubinsztein DC. Antioxidants can inhibit basal autophagy and enhance neurodegeneration in models of polyglutamine disease. Hum Mol Genet. 2010;19:3413–3429. doi: 10.1093/hmg/ddq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chance B, Williams GR. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- 147.Gunter TE, Sheu SS. Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms. Biochim Biophys Acta. 2009;1787:1291–1308. doi: 10.1016/j.bbabio.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kroemer G. Mitochondrial control of apoptosis: an overview. Biochem Soc Symp. 1999;66:1–15. doi: 10.1042/bss0660001. [DOI] [PubMed] [Google Scholar]
- 149.Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 150.Zamzami N, Susin SA, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lehninger AL. Biochemistry. New York: Worth Publishers Inc; 1970. [Google Scholar]
- 152.Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE, Fleming MD. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sheftel AD, Zhang AS, Brown C, Shirihai OS, Ponka P. Direct interorganellar transfer of iron from endosome to mitochondrion. Blood. 2007;110:125–132. doi: 10.1182/blood-2007-01-068148. [DOI] [PubMed] [Google Scholar]
- 154.Territo PR, French SA, Dunleavy MC, Evans FJ, Balaban RS. Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of mVO2, NADH, AND light scattering. J Biol Chem. 2001;276:2586–2599. doi: 10.1074/jbc.M002923200. [DOI] [PubMed] [Google Scholar]
- 155.Altschafl BA, Beutner G, Sharma VK, Sheu SS, Valdivia HH. The mitochondrial ryanodine receptor in rat heart: a pharmaco-kinetic profile. Biochim Biophys Acta. 2007;1768:1784–1795. doi: 10.1016/j.bbamem.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 156.Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem. 2001;276:21482–21488. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- 157.Beutner G, Sharma VK, Lin L, Ryu SY, Dirksen RT, Sheu SS. Type 1 ryanodine receptor in cardiac mitochondria: transducer of excitation-metabolism coupling. Biochim Biophys Acta. 2005;1717:1–10. doi: 10.1016/j.bbamem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 158.Chappell JB, Greville GD, Bicknell KE. Stimulation of respiration of isolated mitochondria by manganese ion. Biochem J. 1962;84:61. [Google Scholar]
- 159.Drahota Z, Gazzotti P, Carafoli E, Rossi CS. A comparison of the effects of different divalent cations on a number of mitochondrial reactions linked to ion translocation. Arch Biochem Biophys. 1969;130:267–273. doi: 10.1016/0003-9861(69)90033-2. [DOI] [PubMed] [Google Scholar]
- 160.Gunter RE, Puskin JS, Russell PR. Quantitative magnetic resonance studies of manganese uptake by mitochondria. Biophys J. 1975;15:319–333. doi: 10.1016/S0006-3495(75)85822-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gunter TE, Puskin JS. Manganous ion as a spin label in studies of mitochondrial uptake of manganese. Biophys J. 1972;12:625–635. doi: 10.1016/S0006-3495(72)86108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Du P, Buerstatte CR, Chan AWK, Minski MJ, Bennett L, Lai JCK. Accumulation of manganese in liver can result in decreases in energy metabolism in mitochondria. Proceedings of 1997 Conference of Hazardous Wastes and Materials; 1997. pp. 1–14. [Google Scholar]
- 163.Galvani P, Fumagalli P, Santagostino A. Vulnerability of mitochondrial complex I in PC12 cells exposed to manganese. Eur J Pharmacol. 1995;293:377–383. doi: 10.1016/0926-6917(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 164.Malecki EA. Manganese toxicity is associated with mitochondrial dysfunction and DNA fragmentation in rat primary striatal neurons. Brain Res Bull. 2001;55:225–228. doi: 10.1016/s0361-9230(01)00456-7. [DOI] [PubMed] [Google Scholar]
- 165.Malthankar GV, White BK, Bhushan A, Daniels CK, Rodnick KJ, Lai JC. Differential lowering by manganese treatment of activities of glycolytic and tricarboxylic acid (TCA) cycle enzymes investigated in neuroblastoma and astrocytoma cells is associated with manganese-induced cell death. Neurochem Res. 2004;29:709–717. doi: 10.1023/b:nere.0000018841.98399.ce. [DOI] [PubMed] [Google Scholar]
- 166.Roth JA, Feng L, Walowitz J, Browne RW. Manganese-induced rat pheochromocytoma (PC12) cell death is independent of caspase activation. J Neurosci Res. 2000;61:162–171. doi: 10.1002/1097-4547(20000715)61:2<162::AID-JNR7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 167.Roth JA, Horbinski C, Higgins D, Lein P, Garrick MD. Mechanisms of manganese-induced rat pheochromocytoma (PC12) cell death and cell differentiation. Neurotoxicology. 2002;23:147–157. doi: 10.1016/s0161-813x(01)00077-8. [DOI] [PubMed] [Google Scholar]
- 168.Gavin CE, Gunter KK, Gunter TE. Manganese and calcium efflux kinetics in brain mitochondria. Relevance to manganese toxicity. Biochem J. 1990;266:329–334. doi: 10.1042/bj2660329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Halestrap AP, Doran E, Gillespie JP, O’Toole A. Mitochondria and cell death. Biochem Soc Trans. 2000;28:170–177. doi: 10.1042/bst0280170. [DOI] [PubMed] [Google Scholar]
- 170.Pfeiffer DR, Gunter TE, Eliseev R, Broekemeier KM, Gunter KK. Release of Ca2+ from mitochondria via the saturable mechanisms and the permeability transition. IUBMB Life. 2001;52:205–212. doi: 10.1080/15216540152846019. [DOI] [PubMed] [Google Scholar]
- 171.Pfeiffer DR, Kuo TH, Tchen TT. Some effects of Ca2+, Mg2+, and Mn2+ on the ultrastructure, light-scattering properties, and malic enzyme activity of adrenal cortex mitochondria. Arch Biochem Biophys. 1976;176:556–563. doi: 10.1016/0003-9861(76)90199-5. [DOI] [PubMed] [Google Scholar]
- 172.Pfeiffer DR, Tchen TT. The role of Ca 2+ in control of malic enzyme activity in bovine adrenal cortex mitochondria. Biochem Biophys Res Commun. 1973;50:807–813. doi: 10.1016/0006-291x(73)91316-8. [DOI] [PubMed] [Google Scholar]
- 173.Pfeiffer DR, Tchen TT. The activation of adrenal cortex mitochondrial malic enzyme by Ca2+ and Mg2+ Biochemistry. 1975;14:89–96. doi: 10.1021/bi00672a015. [DOI] [PubMed] [Google Scholar]
- 174.Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- 175.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90373-4. [DOI] [PubMed] [Google Scholar]
- 176.Igbavboa U, Zwizinski CW, Pfeiffer DR. Release of mitochondrial matrix proteins through a Ca2+-requiring, cyclosporin-sensitive pathway. Biochem Biophys Res Commun. 1989;161:619–625. doi: 10.1016/0006-291x(89)92644-2. [DOI] [PubMed] [Google Scholar]
- 177.Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. J Biol Chem. 1992;267:8834–8839. [PubMed] [Google Scholar]
- 178.Bernardi P, Veronese P, Petronilli V. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore. I. Evidence for two separate Me2+ binding sites with opposing effects on the pore open probability. J Biol Chem. 1993;268:1005–1010. [PubMed] [Google Scholar]
- 179.Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem. 1989;264:7826–7830. [PubMed] [Google Scholar]
- 180.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- 181.Kantrow SP, Tatro LG, Piantadosi CA. Oxidative stress and adenine nucleotide control of mitochondrial permeability transition. Free Radic Biol Med. 2000;28:251–260. doi: 10.1016/s0891-5849(99)00238-5. [DOI] [PubMed] [Google Scholar]
- 182.Lenartowicz E, Bernardi P, Azzone GF. Phenylarsine oxide induces the cyclosporin A-sensitive membrane permeability transition in rat liver mitochondria. J Bioenerg Biomembr. 1991;23:679–688. doi: 10.1007/BF00785817. [DOI] [PubMed] [Google Scholar]
- 183.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- 185.Hazell AS, Normandin L, Norenberg MD, Kennedy G, Yi JH. Alzheimer type II astrocytic changes following sub-acute exposure to manganese and its prevention by antioxidant treatment. Neurosci Lett. 2006;396:167–171. doi: 10.1016/j.neulet.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 186.Brenneman KA, Cattley RC, Ali SF, Dorman DC. Manganese-induced developmental neurotoxicity in the CD rat: is oxidative damage a mechanism of action? Neurotoxicology. 1999;20:477–487. [PubMed] [Google Scholar]
- 187.Burton NC, Guilarte TR. Manganese neurotoxicity: lessons learned from longitudinal studies in nonhuman primates. Environ Health Perspect. 2009;117:325–332. doi: 10.1289/ehp.0800035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J Pharmacol Exp Ther. 2005;313:46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- 189.Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J Neurochem. 2008;105:1948–1959. doi: 10.1111/j.1471-4159.2008.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Migheli R, Godani C, Sciola L, Delogu MR, Serra PA, Zangani D, De Natale G, Miele E, Desole MS. Enhancing effect of manganese on L-DOPA-induced apoptosis in PC12 cells: role of oxidative stress. J Neurochem. 1999;73:1155–1163. doi: 10.1046/j.1471-4159.1999.0731155.x. [DOI] [PubMed] [Google Scholar]
- 191.Ramesh GT, Ghosh D, Gunasekar PG. Activation of early signaling transcription factor, NF-kappaB following low-level manganese exposure. Toxicol Lett. 2002;136:151–158. doi: 10.1016/s0378-4274(02)00332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kitazawa M, Anantharam V, Yang Y, Hirata Y, Kanthasamy A, Kanthasamy AG. Activation of protein kinase C delta by proteolytic cleavage contributes to manganese-induced apoptosis in dopaminergic cells: protective role of Bcl-2. Biochem Pharmacol. 2005;69:133–146. doi: 10.1016/j.bcp.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 193.Milatovic D, Gupta RC, Yu Y, Zaja-Milatovic S, Aschner M. Protective effects of antioxidants and anti-inflammatory agents against manganese-induced oxidative damage and neuronal injury. Toxicol Appl Pharmacol. 2011;256:219–226. doi: 10.1016/j.taap.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 195.Dawson TL, Gores GJ, Nieminen AL, Herman B, Lemasters JJ. Mitochondria as a source of reactive oxygen species during reductive stress in rat hepatocytes. Am J Physiol. 1993;264:C961–967. doi: 10.1152/ajpcell.1993.264.4.C961. [DOI] [PubMed] [Google Scholar]
- 196.Lesnefsky EJ, Chen Q, Slabe TJ, Stoll MS, Minkler PE, Hassan MO, Tandler B, Hoppel CL. Ischemia, rather than reperfusion, inhibits respiration through cytochrome oxidase in the isolated, perfused rabbit heart: role of cardiolipin. Am J Physiol Heart Circ Physiol. 2004;287:H258–267. doi: 10.1152/ajpheart.00348.2003. [DOI] [PubMed] [Google Scholar]
- 197.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 198.Gunter TE, Gavin CA, Gunter KK. The role of mitochondrial oxidative stress and ATP depletion in the pathology of manganese toxicity. In: Zhang YLaJ., editor. Metal Ions in Stroke. New York, New York: Springer Verlag; 2012. in press. [Google Scholar]
- 199.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 200.Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- 201.Erikson A. Remaining problems in the management of patients with Gaucher disease. J Inherit Metab Dis. 2001;24(Suppl 2):122–126. doi: 10.1023/a:1012452715079. discussion 187–128. [DOI] [PubMed] [Google Scholar]