Abstract
Asymmetric meiotic divisions in mammalian oocytes are driven by the eccentric positioning of the spindle, along with a dramatic reorganization of the overlying cortex, including a loss of microvilli and formation of a thick actin cap. Actin polarization relies on a Ran-GTP gradient centered on metaphase chromosomes; however, the downstream signaling cascade is not completely understood. In a recent study, we have shown that Ran promotes actin cap formation via the polarized activation of Cdc42. The related GTPase Rac is also activated in a polarized fashion in the oocyte cortex and co-localizes with active Cdc42. In other cells, microvilli collapse can be triggered by inactivation of the ERM (Ezrin/Radixin/Moesin) family of actin-membrane crosslinkers under the control of Rac. Accordingly, we show here that Ran-GTP promotes a substantial loss of phosphorylated ERMs in the cortex overlying the spindle in mouse oocytes. However, this polarized phospho-ERM exclusion zone was unaffected by Rac or Cdc42 inhibition. Therefore, we suggest that Ran activates two distinct pathways to regulate actin cap formation and microvilli disassembly in the polarized cortex of mouse oocytes. The possibility of a crosstalk between Rho GTPase and ERM signaling and a role for ERM inactivation in promoting cortical actin dynamics are also discussed.
Keywords: oocyte, meiosis, polarity, GTPase, actin, chromatin
Introduction
In mammalian female meiosis, successful haploidization of the maternal genome is achieved via two successive asymmetric divisions, resulting in the formation of a large oocyte and two small polar bodies that will eventually degenerate. The small size of the polar bodies ensures that minimal amount of maternal organelles and macromolecules (e.g., mRNAs, proteins, mitochondria) are lost in the process.1,2 Early embryo viability is thus highly dependent on the establishment of oocyte asymmetry.1,2 The key mechanism underlying the asymmetry of oocyte meiotic divisions is the positioning of the meiotic spindle in the vicinity of the cortex.3-5 In meiosis I, the spindle forms in the center of the oocyte then moves slowly toward the cortex. At the time of homologous chromosome segregation (metaphase I-anaphase I transition), the spindle has reached a subcortical location, resulting in an asymmetric division and the formation of a small-sized first polar body. This is promptly followed by the formation of the metaphase II spindle, which remains localized in the vicinity of the cortex (Fig. 1). At fertilization, meiosis II resumes, and the activated oocyte emits a small-sized second polar body containing one set of segregated chromatids.
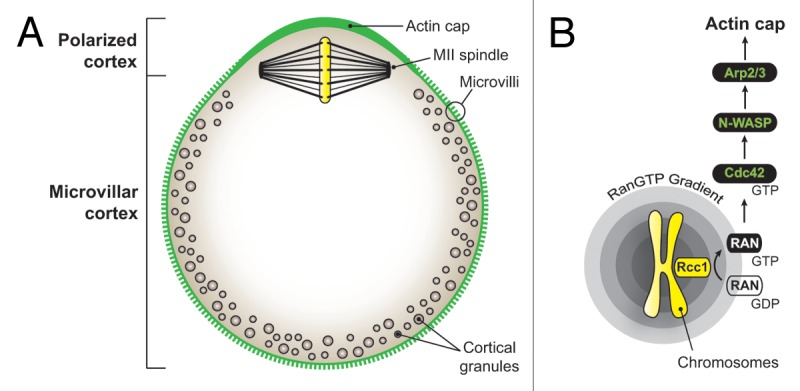
Figure 1. Chromatin-driven polarization in the mammalian oocyte. (A) Schematic of the mouse metaphase II (MII) oocyte and its major polarization features. The polarized cortex is defined as the cortical area overlying the MII spindle, devoided of microvilli and cortical granules, and enriched in actin filaments. (B) Signaling cascade leading to the formation of the polarized actin cap. RCC1, regulator of chromosome condensation 1.
In both meiosis I and II, the asymmetric positioning of the meiotic spindle is accompanied by a range of polarization events, ultimately resulting in the differentiation of a polar body-forming region around the spindle. Thus, cortical granules, which are released upon fertilization to induce the zona-block to polyspermy, redistribute away from the spindle area, resulting in the formation of the so-called cortical granule-free domain (Fig. 1).6 Another emblematic feature of oocyte polarization is the formation of the “actin cap,” a thick layer of actin filaments that accumulates in the cortex overlying the spindle (Fig. 1).7-9 Interestingly, actin caps are maintained over the chromatin clusters that remain in close apposition with the cortex, after destruction of spindle microtubules with nocodazole.7-9 These observations have led to the concept that oocyte chromatin generates a signal capable of remodeling, from a distance, the nearby cortex. This signal was identified as a gradient of active Ran GTPase (Ran-GTP), which exchange factor RCC1 (regulator of chromosome condensation 1) binds chromatin.10-12 Ran signaling was shown to promote polarized accumulation of the N-WASP-Arp2/3 machinery in the cortex overlying the spindle, thereby driving the formation of the polarized actin cap.13 Recent studies based on live imaging of mouse oocytes have revealed that actin filaments flow continuously away from the cortical cap. This actin flow generates cytoplasmic streaming, which is suggested to apply a directional pushing force on the spindle, thereby maintaining its off-center positioning.13-15
In a recent study, we have added a new protagonist, the small GTPase Cdc42, in this signaling cascade. Using a fluorescent reporter for GTP-bound Cdc42, we have shown that Cdc42 is activated in a polarized fashion in the cortex overlying the meiotic spindle and drives the cortical accumulation of N-WASP and the formation of the actin cap in mouse oocytes.16 Furthermore, we have shown that this polarized activation of Cdc42 requires the proximity of meiotic chromatin and Ran-GTP signaling.16 We therefore proposed the following signaling cascade for actin polarization in oocytes (Fig. 1):
Chromatin → Ran-GTP → Cdc42-GTP → N-WASP-Arp2/3 → Actin cap
Our data also suggested that the Ran/Cdc42/N-WASP signaling cascade is conserved during anaphase, promoting the formation of a thick actin layer in the cortex of the polar bodies.16 Inhibition of Cdc42 signaling resulted in a complete failure of second polar body protrusion in activated MII oocytes, associated with a relocalization of N-WASP to the cytosol.16 Our current view on these findings is that actin filaments generated via the polarized Cdc42/N-WASP pathway may provide the necessary pushing force to deform the cortex and drive polar body protrusion in a manner similar to actin-driven leading edge extension in motile cells.17,18 In a previous study, we presented evidence for a similar polarized activation of the small GTPase Rac in the cortex of mouse oocytes,19 also under the control of the Ran-GTP gradient (G.Halet, unpublished observations). At present, it is unclear whether Rac and Cdc42 play complementary or redundant roles in oocyte polarization. Our recent investigations suggest, however, that Cdc42 is the major determinant of N-WASP localization in the mouse oocyte cortex.16
Another major polarization feature is the loss of membrane microvilli in the polarized cortex (Fig. 1).7,8 Microvilli provide binding and fusion sites for the fertilizing sperm,20-22 and the rationale for their localized disassembly may be to avoid sperm fusion in the vicinity of the spindle, which could otherwise interfere with spindle function and/or lead to the untimely extrusion of paternal chromatids into the second polar body.23 The assembly and maintenance of membrane microvilli require proteins of the ERM (Ezrin, Radixin, Moesin) family, which act as membrane-cytoskeleton crosslinkers.24,25 ERMs interact with integral membrane proteins via their N-terminal FERM (4.1-Ezrin-Radixin-Moesin) domain and with underlying actin filaments via their C terminus. In their inactive state, ERMs exhibit a “closed” conformation, where the N- and C termini establish an intramolecular or intermolecular association, resulting in mutual inhibition and retention of the protein in the cytosol as a dormant pool. According to the current model, ERM activation requires the initial binding to the phosphoinositide PIP2 via the FERM domain, facilitating the subsequent phosphorylation of a conserved threonine residue at the C terminus (T567 in ezrin, T564 in radixin, T558 in moesin).25,26 This phosphorylation stabilizes the active conformation of ERMs (hereafter collectively referred to as phospho-ERMs) and promotes their crosslinking activity.
Mouse oocytes express all three ERM family members and seem to be particularly enriched in radixin.27,28 Consistent with a role in microvilli formation, phospho-ERMs are enriched in the oocyte cortex, except for the amicrovillar region overlying the MII spindle.28 These observations suggest that ERM dephosphorylation may be the trigger for microvilli disassembly in the polarized cortex of oocytes, as seen in other cells.29-32 Interestingly, Rac or Cdc42 activation were shown to promote ERM dephosphorylation and collapse of microvilli in T cells.32-35 Thus, one attractive hypothesis is that ERM dephosphorylation in oocytes is triggered by the polarized activation of Rac and/or Cdc42, under the control of the Ran-GTP gradient.
Here, we investigated further the mechanism of mouse oocyte polarization by asking two questions: (1) Does the polarized dephosphorylation of ERM rely on the chromatin-centered Ran-GTP gradient? and (2) Is the polarized phospho-ERM exclusion a downstream event of Rac/Cdc42 activation?
Results and Discussion
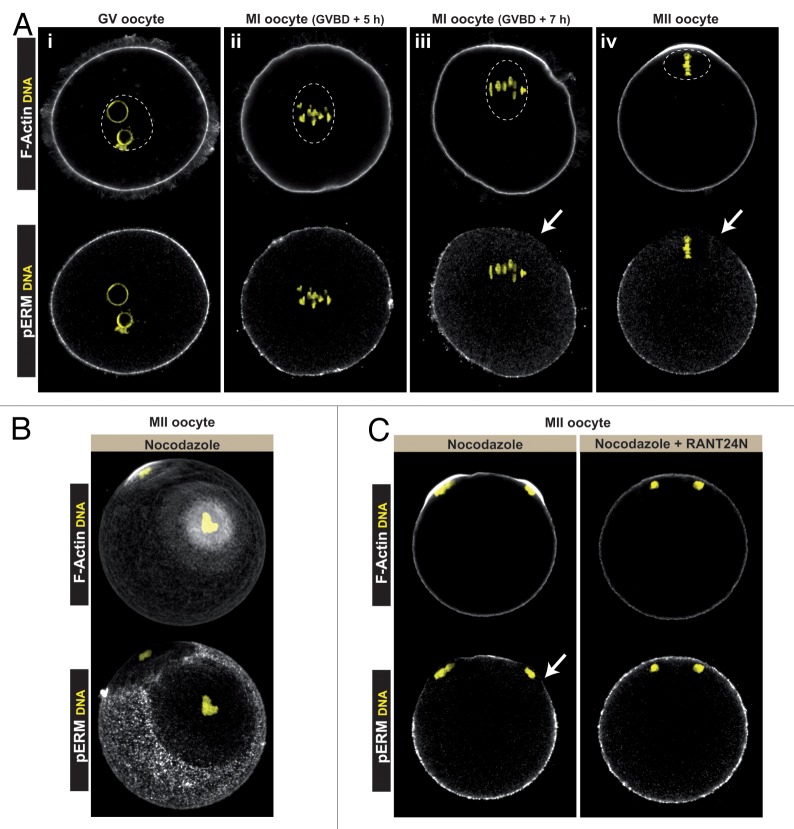
Polarization of the mouse oocyte cortex is induced by the proximity of meiotic chromatin, resulting in a graded polarizing response as the distance between the spindle and the cortex shortens.10,16 To test whether this is also true for ERM activation, we examined the pattern of cortical ERM activation at successive stages of oocyte meiosis, using immunolabeling against phospho-ERMs.28 In agreement with a previous report,28 phospho-ERMs and actin filament staining showed a homogenous distribution over the cortex of prophase I-arrested GV oocytes (Fig. 2A, panel i), consistent with the presence of numerous actin-rich microvilli. Metaphase-I oocytes with a centrally located spindle (5 h post-germinal vesicle breakdown/GVBD) exhibited a similar distribution of activated ERMs and actin filaments all over the cortex (Fig. 2A, panel ii). In contrast, at a later stage of metaphase I (GVBD + 7 h), when the spindle apparatus exhibits an eccentric position, the phospho-ERM signal was depleted in the cortical area overlying the spindle (Fig. 2A, panel iii, arrow), along with the appearance of a cortical actin cap. In MII oocytes, phospho-ERMs were detected in the microvillar cortex but but were virtually absent from the actin-rich polarized cortex overlying the MII spindle (Fig. 2A, panel iv, arrow). These observations suggest that the meiotic apparatus generates a signal capable of inducing, from a distance, ERM dephosphorylation in the nearby cortex, coincident with the formation of the actin cap. To investigate a possible contribution of spindle microtubules in cortical ERM inactivation, MII oocytes were treated with the microtubule poison nocodazole, which results in the formation of chromatin clusters closely opposed to the cortex.7-9 As shown in Figure 2B, each of the nocodazole-induced chromatin clusters was associated with a localized depletion of phospho-ERM labeling and a reciprocal increase in actin filament polymerization. Thus, similar to the polarization of actin filaments, N-WASP and activated Rac and Cdc42,7-10,16,19 ERM dephosphorylation is driven by the proximity of meiotic chromatin in a microtubule-independent manner.
Figure 2. Ran-dependent dephosphorylation of cortical ERMs in the vicinity of oocyte chromatin. (A) Actin filaments (F-actin, upper images) and phospho-ERM (pERM, lower images) labeling at successive stages of oocyte meiosis. Note the phospho-ERM exclusion zone (arrows in panels iii and iv) in the cortical regions overlying the late MI (GVBD + 7 h) and MII spindles. The dashed circles indicate the outline of the germinal vesicle (GV, panel i) and meiotic spindles (panels ii–iv). (B) MII oocytes were treated with nocodazole (2 µM) to destroy spindle microtubules. In the oocyte shown, two chromatin clusters have formed, closely apposed to the cortex. Note the polarized actin caps and phospho-ERM exclusion zones overlying each of the clusters. The images are compression of confocal Z-stacks (45 µm in depth). (C) The left panel shows an MII oocyte treated with nocodazole, where the arrow points to the phospho-ERM exclusion zone over a chromatin cluster. The right panel shows a nocodazole-treated MII oocyte overexpressing dominant-negative RanT24N. Note the absence of phospho-ERM exclusion zones in the cortical regions overlying the chromatin clusters. Note also the effective inhibition of actin cap formation over the clusters. Chromatin (labeled with To Pro-3) is shown in yellow.
We next examined whether chromatin-induced ERM dephosphorylation was mediated by the Ran-GTP gradient emanating from meiotic chromosomes. MII oocytes treated with nocodazole were injected with cRNA encoding RanT24N, which acts as a dominant-negative version of the GTPase.10,11 Previous work from our and other groups has shown that the Ran-GTP gradient promotes activation of the Cdc42/N-WASP/Arp2/3 cascade to assemble the polarized actin cap.13,16 Therefore, disappearance of the polarized actin cap can be used as a validation of effective inhibition of Ran signaling. Interestingly, in addition to the loss of the polarized actin cap, oocytes expressing RanT24N exhibited a uniform distribution of phospho-ERMs at the cortex, including the regions overlying the chromatin clusters (Fig. 2C, right panel). These findings suggest that Ran signaling is necessary for oocyte chromatin to induce ERM dephosphorylation in the nearby cortex.
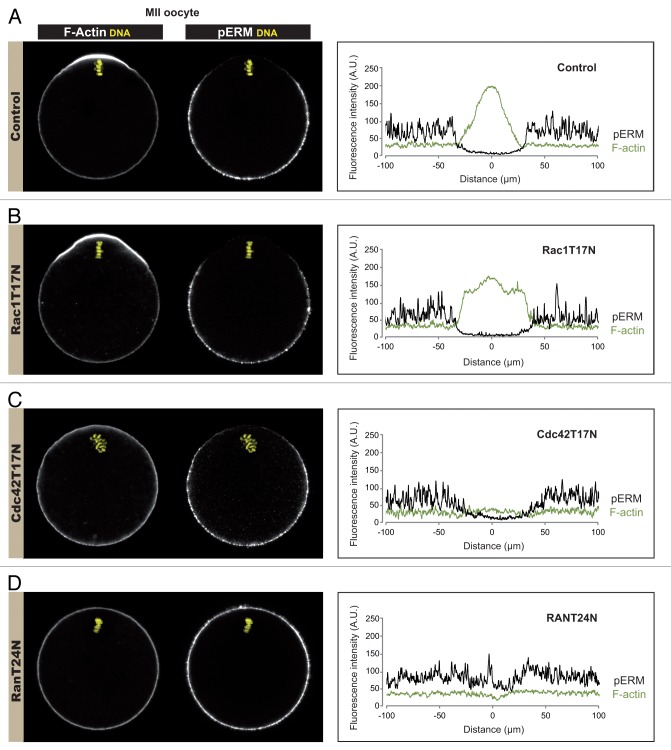
There is increasing evidence linking Rac activation to ERM dephosphorylation and microvilli collapse in other cells.32-35 Since our previous findings indicate that Rac, and the related GTPase Cdc42, are activated in the cortex overlying oocyte chromatin,16,19 we tested if the signaling cascade culminating in ERM dephosphorylation in the polarized cortex involved Rac or Cdc42 activation. MII oocytes were injected with mRNA encoding dominant-negative RacT17N19 or Cdc42T17N16 and examined for phospho-ERM and actin filament distribution. Fluorescence profiles were generated along the perimeter of the oocytes to better visualize the changes in fluorescence intensity corresponding to the actin cap and the phospho-ERM exclusion zone. MII oocytes injected with water were used as a control for the polarized phospho-ERM exclusion (Fig. 3A), while oocytes expressing RanT24N were used as a control for effective inhibition of the phospho-ERM exclusion (Fig. 3D). In oocytes expressing RacT17N, the actin cap overlying the MII spindle was preserved, and the polarized inactivation of ERMs remained also unaffected (Fig. 3B). Consistent with our recently published work,16 Cdc42T17N prevented the assembly of the polarized actin cap in the cortex overlying the MII chromosomes; however, the polarized exclusion of phospho-ERMs was still observed (Fig. 3C). In contrast, overexpression of RanT24N induced a substantial recovery of phospho-ERM staining in the cortex overlying the MII chromosomes, in conjunction with the expected loss of the actin cap (Fig. 3D). We also tested a constitutively active Rac mutant, Rac1Q61L, which was reported to trigger substantial ERM dephosphorylation and microvilli collapse in other cells.32-35 However, MII oocytes expressing EGFP-Rac1Q61L exhibited a phospho-ERM exclusion zone similar to control oocytes (data not shown).
Figure 3. Ran-induced ERM inactivation is independent of Rac and Cdc42 signaling. MII oocytes were injected with water (control, A) or with cRNA encoding dominant-negative RacT17N (B), Cdc42T17N (C) or RanT24N (D). Confocal images were obtained for actin filament (F-actin) and phospho-ERM (pERM) distribution (left panel), and corresponding fluorescence intensity profiles were generated along the oocyte perimeter (right panel: green line, actin; black line, phospho-ERMs). All fluorescence profiles were centered on the nearest cortical point overlying the metaphase chromosomes (indicated as 0 on the x axis).
From the above data, we conclude that the signaling pathway leading to chromatin-induced ERM inactivation in the oocyte cortex is independent of Rac or Cdc42 activation and therefore may represent a separate Ran-dependent signaling cascade (Fig. 4, scenario 1). The mechanism and signaling intermediates leading to polarized ERM inactivation remain to be identified. One possibility is that localized ERM inactivation occurs consecutively to a depletion of the phosphoinositide PIP2, as shown in chemokine-stimulated lymphocytes.36 However, we do not favor this hypothesis, since we have shown previously that PIP2 could still be detected in the membrane region overlying the MII spindle.37 In other cells, ERM phosphorylation is typically counteracted by calyculin A-sensitive phosphatases of the PP1/PP2a families.29,31-33,38 Likewise, treatment of oocytes with calyculin A (50 nM for 15 min) prevented the development of the phospho-ERM exclusion zone in the polarized cortex (unpublished observations). This finding suggests that ERM activation in oocytes is dynamically regulated by a balance of kinase and phosphatase activities. Further investigations are needed to find out whether Ran-GTP regulates the kinase activity, the phosphatase activity, or both.
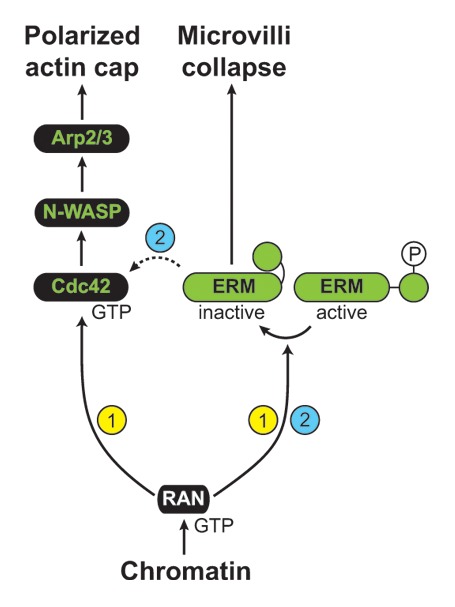
Figure 4. Ran-induced signaling cascades leading to oocyte polarization. Two scenarios are depicted, based on previously published work, and evidence obtained in the present study. In scenario 1, Ran-GTP activates two separate signaling cascades, to promote actin cap formation and the collapse of microvilli in the polarized cortex. The signaling intermediates leading to polarized Cdc42 activation and ERM dephosphorylation are still unknown. In scenario 2, it is suggested that ERM inactivation promotes the polarized activation of Cdc42, in addition to the collapse of microvilli.
As an alternative to the dual cascade hypothesis, we can also envisage that ERM dephosphorylation lies upstream of the polarized activation of Rac/Cdc42 and actin cap formation (Fig. 4, scenario 2). For instance, through their direct interaction with Rho guanine nucleotide dissociation inhibitor/RhoGDI, phospho-ERMs have been shown to promote the activation of Rho,39,40 which is notoriously antagonistic to Rac/Cdc42.41-43 According to this model, a localized dephosphorylation of ERMs would downregulate Rho activity at the cortex, thus promoting polarized Rac/Cdc42 activation. Consistent with this idea, a recent study has shown that radixin depletion promotes Rac activation and cell spreading in cancer cells.44
Beyond the formation of an amicrovillar region unfavorable for sperm fusion, ERM inactivation could play additional roles in cortical reorganization and polar body protrusion. Thus, ERM dephosphorylation, by releasing actin filaments from their anchoring to the overlying membrane, could enable the continuous cortical actin flow that powers cytoplasmic streaming.13 In addition, the polarized inactivation of ERMs may facilitate rapid actin-driven membrane deformation during polar body emission by locally reducing the cortical tension, which would otherwise oppose protrusion.45 Consistent with this idea, increasing membrane tension by expression of constitutively active ERMs prevents membrane protrusion in polarizing T cells.29,46 In the same way, the overactivation of moesin, by increasing cortical rigidity, interferes with polar relaxation and anaphase elongation in mitotic Drosophila cells, resulting in cytokinesis defects.38,47 Further investigations to test these possibilities will provide a better picture of the mammalian oocyte polarization mechanism.
Materials and Methods
Oocyte recovery and treatments
All experimental procedures were approved by the local ethics committee (CREEA). OF-1 mice (8–10-w-old; Charles River) were injected with 7–10 IU PMSG (Sigma) for priming, followed 44–48 h later by 5–7.5 IU hCG (Sigma) to induce ovulation. Germinal vesicle (GV)-stage oocytes were collected from antral follicles and maintained in prophase arrest by supplementing the culture medium (M2 or M16, Sigma) with 250 µM dibutyryl-cAMP (Sigma). To observe metaphase I progression, oocytes were released from the GV arrest by washing off the dibutyryl-cAMP and cultured in M16 medium in a 5% CO2 incubator. Metaphase II (MII) oocytes were recovered from the oviducts and denuded of the surrounding cumulus cells by incubation with 300 µg/ml hyaluronidase (Sigma) in M2 medium, followed by wash. For nocodazole treatment, MII oocytes were incubated in M2 medium containing 2 µM nocodazole (Calbiochem) for 1.5 h.
Expression of RacT17N, Cdc42T17N, Rac1Q61L and RanT24N
Plasmids encoding Cdc42T17N in pRK5-myc (subsequently subcloned into pcDNA3.1) and EGFP-Rac1Q61L in pcDNA3 were obtained from Gary Bokoch via Addgene (plasmids 12973, 12981). RacT17N in pcDNA3.1 was described previously.19 RanT24N in pcDNA3.1 was a generous gift from Ben Margolis. After plasmid linearization, cRNAs were prepared in vitro using the mMessage mMachine T7 kit (Life Technologies) and pressure-injected in MII oocytes. Oocytes were allowed to express the mutant GTPase constructs for 3 h before fixation and processing for immunostaining. Control oocytes were injected with an equivalent amount of water.
Immunolabeling
Oocytes were fixed in paraformaldehyde (3.7% in PBS) for 30 min and permeabilized with Triton X100 (0.25% in PBS) for 20 min. After a 3 h incubation in a block solution consisting of 3% BSA in PBS, oocytes were incubated overnight at 4°C with a rabbit monoclonal antibody that recognizes ERMs phosphorylated on the critical C-terminal threonine residue (#3149, Cell Signaling Technology). After three washes, oocytes were incubated with a secondary antibody (Alexa Fluor 488 Goat anti-rabbit IgG, Life Technologies) for 1 h at 37°C.
Staining of actin filaments and chromatin
To label actin filaments, permeabilized oocytes were incubated for 5 min in PBS containing Alexa Fluor 546 Phalloidin (5 units/ml; Life Technologies), followed by wash. Chromatin was labeled with To Pro-3 (5 µM; Life Technologies).
Confocal imaging and image processing
Oocytes were placed on glass-bottom dishes (MatTek) and imaged using a Leica SP5 confocal microscope equipped with 488, 561 and 633-nm laser lines. Images were processed with ImageJ. All confocal images depicted are representative of at least 20 similar observations. Fluorescence intensity profiles along the oocyte perimeter were generated using the segmented line and plot profile functions in ImageJ. The thickness of the line was 3.5 µm.
Acknowledgments
This work was funded by a CNRS ATIP fellowship to G.H. B.D. is the recipient of a doctoral fellowship from the French Ministry of Research. We are grateful to Ben Margolis for the RanT24N plasmid. We appreciate the technical support from the UMS 3480 BIOSIT animal and microscopy facilities.
Glossary
Abbreviations:
- GV
germinal vesicle
- GVBD
germinal vesicle breakdown
- MI
metaphase I
- MII
metaphase II
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24901
References
- 1.Brunet S, Verlhac MH. Positioning to get out of meiosis: the asymmetry of division. Hum Reprod Update. 2011;17:68–75. doi: 10.1093/humupd/dmq044. [DOI] [PubMed] [Google Scholar]
- 2.Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol. 2013;14:141–52. doi: 10.1038/nrm3531. [DOI] [PubMed] [Google Scholar]
- 3.Azoury J, Lee KW, Georget V, Rassinier P, Leader B, Verlhac MH. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol. 2008;18:1514–9. doi: 10.1016/j.cub.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 4.Schuh M, Ellenberg J. A new model for asymmetric spindle positioning in mouse oocytes. Curr Biol. 2008;18:1986–92. doi: 10.1016/j.cub.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Chaigne A, Verlhac MH, Terret ME. Spindle positioning in mammalian oocytes. Exp Cell Res. 2012;318:1442–7. doi: 10.1016/j.yexcr.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Connors SA, Kanatsu-Shinohara M, Schultz RM, Kopf GS. Involvement of the cytoskeleton in the movement of cortical granules during oocyte maturation, and cortical granule anchoring in mouse eggs. Dev Biol. 1998;200:103–15. doi: 10.1006/dbio.1998.8945. [DOI] [PubMed] [Google Scholar]
- 7.Longo FJ, Chen DY. Development of cortical polarity in mouse eggs: involvement of the meiotic apparatus. Dev Biol. 1985;107:382–94. doi: 10.1016/0012-1606(85)90320-3. [DOI] [PubMed] [Google Scholar]
- 8.Maro B, Johnson MH, Webb M, Flach G. Mechanism of polar body formation in the mouse oocyte: an interaction between the chromosomes, the cytoskeleton and the plasma membrane. J Embryol Exp Morphol. 1986;92:11–32. [PubMed] [Google Scholar]
- 9.Van Blerkom J, Bell H. Regulation of development in the fully grown mouse oocyte: chromosome-mediated temporal and spatial differentiation of the cytoplasm and plasma membrane. J Embryol Exp Morphol. 1986;93:213–38. [PubMed] [Google Scholar]
- 10.Deng M, Suraneni P, Schultz RM, Li R. The Ran GTPase mediates chromatin signaling to control cortical polarity during polar body extrusion in mouse oocytes. Dev Cell. 2007;12:301–8. doi: 10.1016/j.devcel.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Dumont J, Petri S, Pellegrin F, Terret ME, Bohnsack MT, Rassinier P, et al. A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J Cell Biol. 2007;176:295–305. doi: 10.1083/jcb.200605199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao Y, Macara IG. Regulation of chromatin binding by a conformational switch in the tail of the Ran exchange factor RCC1. J Cell Biol. 2008;182:827–36. doi: 10.1083/jcb.200803110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi K, Unruh JR, Deng M, Slaughter BD, Rubinstein B, Li R. Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat Cell Biol. 2011;13:1252–8. doi: 10.1038/ncb2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi K, Li R. Actin cytoskeleton in cell polarity and asymmetric division during mouse oocyte maturation. Cytoskeleton (Hoboken) 2012;69:727–37. doi: 10.1002/cm.21048. [DOI] [PubMed] [Google Scholar]
- 15.Yi K, Rubinstein B, Unruh JR, Guo F, Slaughter BD, Li R. Sequential actin-based pushing forces drive meiosis I chromosome migration and symmetry breaking in oocytes. J Cell Biol. 2013;200:567–76. doi: 10.1083/jcb.201211068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dehapiot B, Carrière V, Carroll J, Halet G. Polarized Cdc42 activation promotes polar body protrusion and asymmetric division in mouse oocytes. Dev Biol. 2013;377:202–12. doi: 10.1016/j.ydbio.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Condeelis J. Life at the leading edge: the formation of cell protrusions. Annu Rev Cell Biol. 1993;9:411–44. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- 18.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 19.Halet G, Carroll J. Rac activity is polarized and regulates meiotic spindle stability and anchoring in mammalian oocytes. Dev Cell. 2007;12:309–17. doi: 10.1016/j.devcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Yanagimachi R. Sperm-egg association in animals. Curr Top Dev Biol. 1978;12:83–105. doi: 10.1016/S0070-2153(08)60594-3. [DOI] [PubMed] [Google Scholar]
- 21.Runge KE, Evans JE, He ZY, Gupta S, McDonald KL, Stahlberg H, et al. Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol. 2007;304:317–25. doi: 10.1016/j.ydbio.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 22.Jégou A, Ziyyat A, Barraud-Lange V, Perez E, Wolf JP, Pincet F, et al. CD9 tetraspanin generates fusion competent sites on the egg membrane for mammalian fertilization. Proc Natl Acad Sci USA. 2011;108:10946–51. doi: 10.1073/pnas.1017400108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo J, McGinnis LK, Kinsey WH. Fyn kinase activity is required for normal organization and functional polarity of the mouse oocyte cortex. Mol Reprod Dev. 2009;76:819–31. doi: 10.1002/mrd.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yonemura S, Tsukita S, Tsukita S. Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins. J Cell Biol. 1999;145:1497–509. doi: 10.1083/jcb.145.7.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–87. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, et al. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol. 2004;164:653–9. doi: 10.1083/jcb.200307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louvet S, Aghion J, Santa-Maria A, Mangeat P, Maro B. Ezrin becomes restricted to outer cells following asymmetrical division in the preimplantation mouse embryo. Dev Biol. 1996;177:568–79. doi: 10.1006/dbio.1996.0186. [DOI] [PubMed] [Google Scholar]
- 28.Larson SM, Lee HJ, Hung PH, Matthews LM, Robinson DN, Evans JP. Cortical mechanics and meiosis II completion in mammalian oocytes are mediated by myosin-II and Ezrin-Radixin-Moesin (ERM) proteins. Mol Biol Cell. 2010;21:3182–92. doi: 10.1091/mbc.E10-01-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown MJ, Nijhara R, Hallam JA, Gignac M, Yamada KM, Erlandsen SL, et al. Chemokine stimulation of human peripheral blood T lymphocytes induces rapid dephosphorylation of ERM proteins, which facilitates loss of microvilli and polarization. Blood. 2003;102:3890–9. doi: 10.1182/blood-2002-12-3807. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Cohn JA, Mandel LJ. Dephosphorylation of ezrin as an early event in renal microvillar breakdown and anoxic injury. Proc Natl Acad Sci USA. 1995;92:7495–9. doi: 10.1073/pnas.92.16.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondo T, Takeuchi K, Doi Y, Yonemura S, Nagata S, Tsukita S. ERM (ezrin/radixin/moesin)-based molecular mechanism of microvillar breakdown at an early stage of apoptosis. J Cell Biol. 1997;139:749–58. doi: 10.1083/jcb.139.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nijhara R, van Hennik PB, Gignac ML, Kruhlak MJ, Hordijk PL, Delon J, et al. Rac1 mediates collapse of microvilli on chemokine-activated T lymphocytes. J Immunol. 2004;173:4985–93. doi: 10.4049/jimmunol.173.8.4985. [DOI] [PubMed] [Google Scholar]
- 33.Faure S, Salazar-Fontana LI, Semichon M, Tybulewicz VLJ, Bismuth G, Trautmann A, et al. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat Immunol. 2004;5:272–9. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- 34.Rossy J, Gutjahr MC, Blaser N, Schlicht D, Niggli V. Ezrin/moesin in motile Walker 256 carcinosarcoma cells: signal-dependent relocalization and role in migration. Exp Cell Res. 2007;313:1106–20. doi: 10.1016/j.yexcr.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Cernuda-Morollón E, Millán J, Shipman M, Marelli-Berg FM, Ridley AJ. Rac activation by the T-cell receptor inhibits T cell migration. PLoS ONE. 2010;5:e12393. doi: 10.1371/journal.pone.0012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao JJ, Liu Y, Kruhlak M, Debell KE, Rellahan BL, Shaw S. Phospholipase C-mediated hydrolysis of PIP2 releases ERM proteins from lymphocyte membrane. J Cell Biol. 2009;184:451–62. doi: 10.1083/jcb.200807047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halet G, Tunwell R, Balla T, Swann K, Carroll J. The dynamics of plasma membrane PtdIns(4,5)P(2) at fertilization of mouse eggs. J Cell Sci. 2002;115:2139–49. doi: 10.1242/jcs.115.10.2139. [DOI] [PubMed] [Google Scholar]
- 38.Roubinet C, Decelle B, Chicanne G, Dorn JF, Payrastre B, Payre F, et al. Molecular networks linked by Moesin drive remodeling of the cell cortex during mitosis. J Cell Biol. 2011;195:99–112. doi: 10.1083/jcb.201106048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, et al. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997;272:23371–5. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- 40.Hamada K, Seto A, Shimizu T, Matsui T, Takai Y, Tsukita S, et al. Crystallization and preliminary crystallographic studies of RhoGDI in complex with the radixin FERM domain. Acta Crystallogr D Biol Crystallogr. 2001;57:889–90. doi: 10.1107/S090744490100556X. [DOI] [PubMed] [Google Scholar]
- 41.Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–22. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 43.Vaughan EM, Miller AL, Yu HY, Bement WM. Control of local Rho GTPase crosstalk by Abr. Curr Biol. 2011;21:270–7. doi: 10.1016/j.cub.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valderrama F, Thevapala S, Ridley AJ. Radixin regulates cell migration and cell-cell adhesion through Rac1. J Cell Sci. 2012;125:3310–9. doi: 10.1242/jcs.094383. [DOI] [PubMed] [Google Scholar]
- 45.Raucher D, Sheetz MP. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J Cell Biol. 2000;148:127–36. doi: 10.1083/jcb.148.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Belkina NV, Park C, Nambiar R, Loughhead SM, Patino-Lopez G, et al. Constitutively active ezrin increases membrane tension, slows migration, and impedes endothelial transmigration of lymphocytes in vivo in mice. Blood. 2012;119:445–53. doi: 10.1182/blood-2011-07-368860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kunda P, Rodrigues NTL, Moeendarbary E, Liu T, Ivetic A, Charras G, et al. PP1-mediated moesin dephosphorylation couples polar relaxation to mitotic exit. Curr Biol. 2012;22:231–6. doi: 10.1016/j.cub.2011.12.016. [DOI] [PubMed] [Google Scholar]