Abstract
Following microbial pathogen invasion, the human immune system of activated phagocytes generates and releases the potent oxidant hypochlorous acid (HOCl), which contributes to the killing of menacing microorganisms. Though tightly controlled, HOCl generation by the myeloperoxidase-hydrogen peroxide-chloride system of neutrophils/monocytes may occur in excess and lead to tissue damage. It is thus of marked importance to delineate the molecular pathways underlying HOCl cytotoxicity in both microbial and human cells. Here, we show that HOCl induces the generation of reactive oxygen species (ROS), apoptotic cell death and the formation of specific HOCl-modified epitopes in the budding yeast Saccharomyces cerevisiae. Interestingly, HOCl cytotoxicity can be prevented by treatment with ROS scavengers, suggesting oxidative stress to mediate the lethal effect. The executing pathway involves the pro-apoptotic protease Kex1p, since its absence diminishes HOCl-induced production of ROS, apoptosis and protein modification. By characterizing HOCl-induced cell death in yeast and identifying a corresponding central executor, these results pave the way for the use of Saccharomyces cerevisiae in HOCl research, not least given that it combines both being a microorganism as well as a model for programmed cell death in higher eukaryotes.
Keywords: hypochlorous acid, HOCl, apoptosis, reactive oxygen species, yeast, Saccharomyces cerevisiae, mitochondria
Introduction
During inflammation, the activation of phagocytes both in vivo and in vitro can result in the generation and release of superoxide anion radical and hydrogen peroxide (respiratory burst) as well as in the secretion of myeloperoxidase. Myeloperoxidase, which is highly expressed in neutrophils, reacts with hydrogen peroxide in the presence of physiological chloride concentrations, catalyzing the formation of hypochlorous acid/hypochlorite (HOCl/OCl−). This short-lived and diffusible oxidant plays a major role as a potent microbicidal agent in the immune defense against invading microbial pathogens.1 Upon phagocyte activation, HOCl concentrations can increase at least up to 340 µM 2. However, excessive or permanent HOCl production may cause tissue damage, since HOCl can participate in secondary non-enzymatic reactions, including oxidation and/or chlorination. HOCl can react with a variety of target compounds, for instance with unsaturated fatty acids to form chlorohydrins or with amines resulting in reactive chloramines, which, in turn, are powerful oxidants.3 Of note, lysine-derived chloramines can generate nitrogen- and, subsequently, carbon-centered radicals, which may amplify the formation of oxygen radicals.4 HOCl-modified target compounds are present during early and acute stages of inflammation.1,5,6 This suggests an implication of the myeloperoxidase-HOCl pathway in the pathogenesis of various inflammatory diseases, such as atherosclerosis or coronary heart disease.7 Thereby, a number of studies have reported that HOCl may activate the apoptotic machinery of cells involved in the initiation and progression of disease.8-10
Establishing the mode(s) of action underlying HOCl-mediated cytotoxicity is thus important for two distinct reasons: (1) the mechanistic disclosure of HOCl’s microbicidal effects and (2) the understanding of deleterious HOCl-modifications in human proteins. In order to examine the lethal mechanism(s) triggered by HOCl, we used the budding yeast Saccharomyces cerevisiae. On the one hand, HOCl has been previously reported to elicit cytotoxic effects against S. cerevisiae,11-13 thus allowing to investigate antifungal HOCl activities. On the other hand, S. cerevisiae also represents a well-established model organism to explore the core machinery executing programmed cell death (PCD), which, over the past decade, has been demonstrated to be phylogenetically conserved.14-16 In fact, the yeast genome encodes orthologs of crucial mammalian apoptosis and necrosis regulators, including one caspase (Yca1p), the serine protease OMI (Nma111p), cathepsin D (Pep4p), endonuclease G (Nuc1p) or BH3-only proteins (Ybh3p).17-22 Additionally, yeast PCD is connected to complex PCD features reminiscent of mammalian cells, such as mitochondrial fragmentation, mitochondrial outer membrane permeabilization or histone H2B phosphorylation.23-25 Furthermore, S. cerevisiae has been successfully used to explore cell death connections to, e.g., lipids or the cell cycle, as well as to investigate lethal pathways underlying pharmacologically relevant agents such as the anticancer drug cisplatin.26-30 Of note, two physiological scenarios deriving in yeast PCD, chronological and replicative aging, have been effectively applied to investigate phylogenetically conserved aging pathways of postmitotic mammalian cells and stem cells, respectively.31-33 For instance, the naturally occurring polyamine spermidine has been shown to exert anti-aging effects that share a common mechanism in yeast, flies and nematodes.34,35 Interestingly, recent evidence suggests that also the broader functional realm of spermidine (e.g., which includes, e.g., roles in reproduction) can be modeled in budding yeast.36,37 Altogether, this functional correspondence allows using S. cerevisiae to analyze lethal pathways and scenarios of higher eukaryotes including those elicited in mammalian cells.
In this study, we aimed at characterizing the cytotoxic effects of HOCl in S. cerevisiae and at identifying corresponding molecular executors. We show that HOCl-mediated cell death in S. cerevisiae is mainly of apoptotic origin and results in elevated levels of reactive oxygen species (ROS) and formation of HOCl-modified proteins. Thereby, the protease Kex1p appears to mediate HOCl-induced lethality, since its absence enhances cell viability and decreases levels of ROS as well as formation of HOCl-modified epitopes upon challenge with lethal doses of HOCl.
Results
HOCl induces ROS-mediated cell death
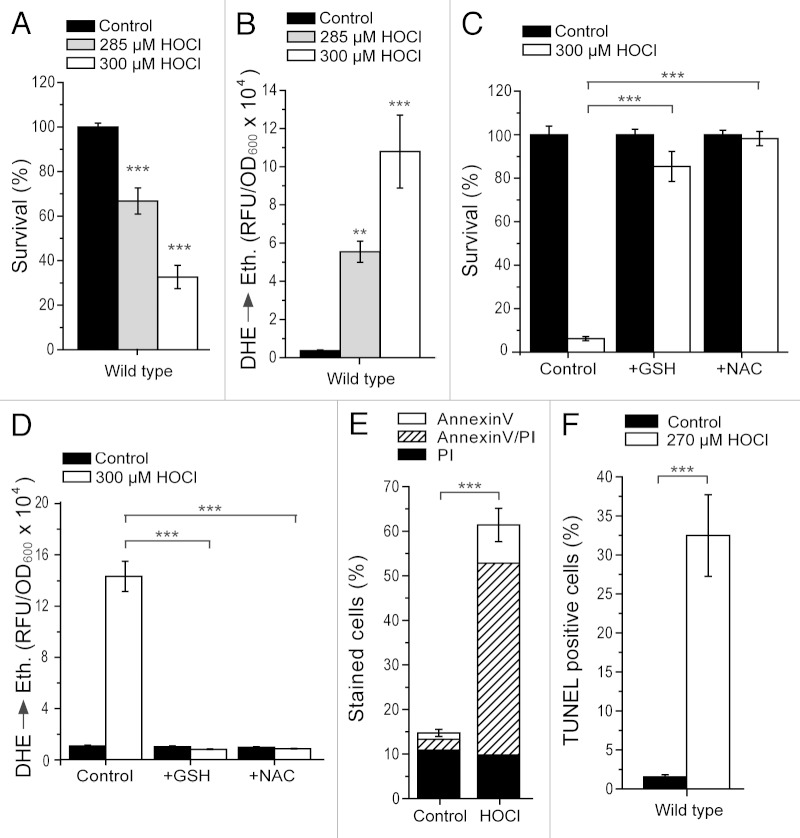
HOCl can exert cytotoxicity on both microbial and human cells. In order to explore both scenarios, we assessed the impact of HOCl on the budding yeast S. cerevisiae, which combines both being a microorganism and an established eukaryotic cell death model. Application of submillimolar concentrations of HOCl to exponentially growing yeast cells resulted in a significant and dose-dependent decrease of clonogenic survival (Fig. 1A). At 285 and 300 µM HOCl, survival rates were 30 and 60% lower compared with the untreated controls (Fig. 1A). This concentration range is similar to that calculated at sites of acute inflammation, where HOCl levels can increase up to 340 µM or even higher.2 The determined loss of survival was paralleled by an increased production of ROS (Fig. 1B), as indicated by the enhanced superoxide-driven conversion of non-fluorescent dihydroethidium (DHE) into fluorescent ethidium (Eth). Conversely, the pre-treatment of yeast cells with the ROS-scavengers glutathione and N-acetyl-cysteine maintained cell viability (Fig. 1C) and prevented ROS accumulation (Fig. 1D) upon HOCl challenge. Altogether, these results suggest that HOCl-triggered yeast cell death is mediated by ROS. In fact, it is likely that the sources of these ROS are mitochondria, since cells devoid of mitochondrial DNA (ρ0) are protected against HOCl-inflicted ROS production and cell death (Fig. S2A and B).
Figure 1. HOCl induces apoptotic cell death and specific protein modifications in S. cerevisiae. (A) Survival determined by clonogenicity of wild type cells after treatment with or without different concentrations of HOCl for 16 h. Data represent means ± s.e.m. (n = 12; ***, p < 0.001). Survival was normalized to the untreated control. (B) ROS production of wild type cells shown in (A) as determined via DHE→Ethidium conversion using a fluorescence plate reader. Data represent means ± s.e.m. (n = 12; **, p < 0.01; ***, p < 0.001). RFU, relative fluorescence unit. (C and D) Survival normalized to the untreated control (C) and ROS production (D) of wild type cells grown to exponential phase in the absence (control) or presence of 5 mM L-glutathione (GSH) or N-acetyl-l-cysteine (NAC) and subsequent treatment with or without 300 µM HOCl for 16 h as determined via clonogenicity (C) and DHE→Ethidium conversion using a fluorescence plate reader (D). Data represent means ± s.e.m. (n = 9; ***, p < 0.001). RFU, relative fluorescence unit. (E) Phosphatidylserine externalization and loss of plasma membrane integrity of wild type cells after treatment with or without 270 µM HOCl for 16 h as determined using Annexin V/PI costaining and quantified via flow cytometry. Data indicate means ± s.e.m. (n = 6; ***, p < 0.001). In each experiment 30,000 cells were evaluated. (F) DNA fragmentation of wild type cells after treatment with or without 270 µM HOCl for 16 h as determined via TUNEL staining and quantified via flow cytometry. Data indicate means ± s.e.m. (n = 6; ***, p < 0.001). In each experiment 30,000 cells were evaluated.
It should be noted that there exists a quantitative difference between the survival levels upon HOCl treatment (300 µM) displayed in Figure 1A vs. Figure 1C (compare also Fig. 2A–C). In fact, the degree of HOCl cytotoxicity at a given concentration differed depending on the batch of SMD growth medium used, though a priori all batches should have had exactly the same composition. Conversely, the range of concentrations in which HOCl killed wild type yeast cells at a comparable quantitative level varied between 260–300 µM, depending on the specific medium batch (note the use of different HOCl concentrations in Fig. 1E and F vs. Fig. 2E and F). Within a batch and at a determined concentration, the qualitative and quantitative results remained stable. For this reason, we assume that some compound(s) in the medium cross-reacting with HOCl and slightly modifying its potency to kill yeast cells are sensitive to external factors that are difficult to control and might slightly differ from medium preparation to preparation. One of these compounds might be photosensitive, since the medium needed to be kept in the dark to maintain constant HOCl cytotoxic performance within one medium batch over time. Interestingly, it has been reported that ammonium ions, which are a component in the medium used in this study, influence HOCl cytotoxicity.38 Also, moderate modifications in extracellular pH interfere with HOCl apoptotic induction.39 These examples suggest that, indeed, slight changes in media composition may have an impact on the quantitative extension of HOCl-induced cytotoxicity, and that the corresponding concentration range must be assessed prior to the use of a specific medium batch. However, the actual nature of the described medium batch-dependent variance remains undetermined.
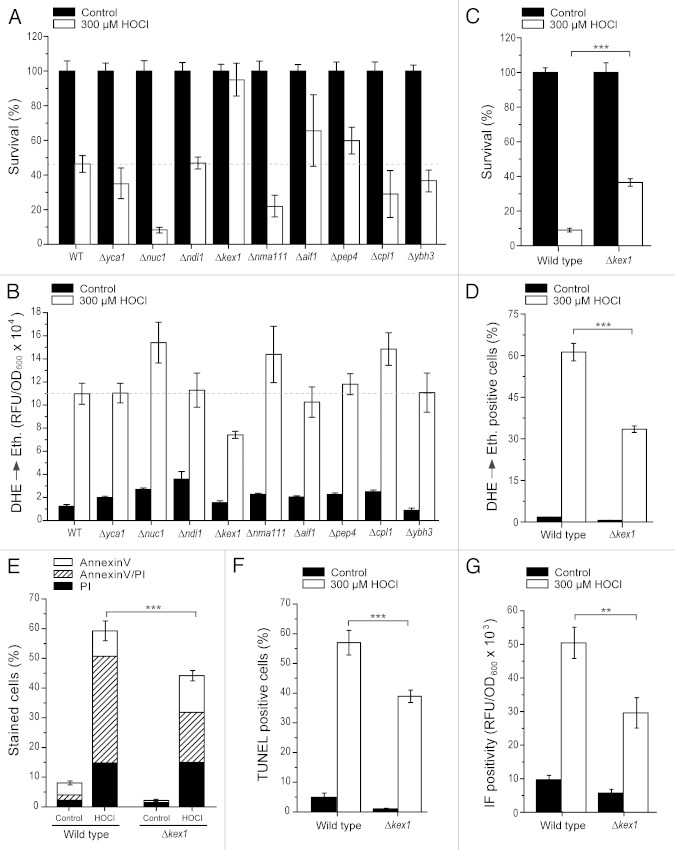
Figure 2. The protease Kex1p is involved in HOCl-induced cytotoxicity. (A and B) Survival (A) and ROS production (B) of wild type, Δyca1, Δnuc1, Δndi1, Δkex1, Δnma111, Δaif1, Δpep4, Δcpl1 and Δybh3 cells after treatment with or without 300 µM HOCl for 16 h as determined via clonogenicity (A) and DHE→Ethidium conversion (B), respectively. Survival (A) was normalized to the untreated control. Experiments in (B) were quantified using a fluorescence plate reader. Data represent means ± s.e.m. (n = 8 – 16). The dashed line draws the survival (A) and ethidium fluorescence (B) levels of the HOCl-treated wild type, respectively. RFU, relative fluorescence unit. (C–F) Survival (C), ROS production (D), phosphatidylserine externalization (E), loss of membrane integrity (E) and apoptotic DNA fragmentation (F) of wild type and Δkex1 cells after treatment with or without 300 µM HOCl for 16 h as determined via clonogenicity (C), DHE→Ethidium conversion (D), Annexin V/PI co-staining (E) and TUNEL staining (F). Data represent means ± s.e.m. (n = 8; ***, p < 0.001). Survival (A) was normalized to the untreated control. In each experiment for (D–F), 30,000 cells were evaluated using flow cytometry. (G) Fluorescence intensity of wild type and Δkex1 cells after treatment with or without 300 µM HOCl for 16 h and subsequent immunofluorescence (IF) staining using mAb 2D10G9 as a primary antibody and quantified using a fluorescence plate reader. Data represent means ± s.e.m. (n = 9; **, p < 0.01). RFU, relative fluorescence unit.
HOCl treatment results in specific protein modifications
In mammalian cells, HOCl cytotoxicity is accompanied by distinct protein oxidation products. Using a specific antibody that selectively recognizes HOCl-modified epitopes,40 immunocytochemistry revealed increased staining in HOCl-treated wild type cells as compared with the untreated control (Fig. 2G). Omission of the primary antibody or its replacement with an IgG isotype control eliminated all staining, demonstrating that the observed staining was not due to a non-specific effect (data not shown). Also, competition with authentic HOCl-modified albumin (30 µg/ml) prevented antibody binding, demonstrating that the staining observed was specific for HOCl-modified proteins (data not shown). Thus, our data suggest that, similarly to mammalian cells, HOCl-mediated cell death and oxidative stress in S. cerevisiae is associated with oxidation-dependent specific modifications.
HOCl elicits yeast cell death mainly through apoptosis
In order to characterize HOCl-induced cell death in S. cerevisiae, we examined a typical phenotypic feature of apoptotic cells: externalized phosphatidylserine at the outer leaflet of the plasma membrane, which binds to FITC-conjugated Annexin V and thus can be quantified. In parallel, we monitored a characteristic marker of necrosis, loss of membrane integrity, by assessing membrane permeability with propidium iodide (PI). By co-staining yeast cells with FITC-Annexin V and PI (Fig. 1E), we were able to discriminate between early apoptosis (only Annexin V-stained cells), late apoptosis leading to secondary necrosis (double stained cells) and primary necrosis (only PI-stained cells).15,16 Compared with the untreated wild type cells, yeast cells exposed to a physiologically relevant HOCl concentration (here 270 µM; see above) showed an increase of primarily apoptotic and more specifically late apoptotic cells, though a small rise in the primary necrotic population was also detectable (Fig. 1E). This mainly apoptotic phenotype was further confirmed by TdT-mediated dUTP-biotin nick end labeling (TUNEL) staining, which revealed exacerbated apoptotic DNA fragmentation upon HOCl treatment (Fig. 1F). In conclusion, HOCl cytotoxicity in yeast is mainly of apoptotic origin and only marginally involves primary necrosis.
Absence of the protease Kex1p but not of other lethal hydrolases partly reverts HOCl-induced cytotoxicity
The knowledge about the mechanistic details accounting for HOCl-triggered cell death in yeast is only limited, even though it seems clear that oxidative damage represents a key component in the lethal process.13 To gain further insights into the molecular regulators accounting for the execution of HOCl-induced cytotoxicity, we decided to determine loss of viability and ROS levels in the deletion background of genes encoding known apoptotic effectors, including the yeast metacaspase Yca1p,17 the pro-apoptotic proteases Nma111p and Kex1p,18,41 the yeast homologs of the human death regulators cathepsin D and calpain (Pep4p and Cpl1p),22 the mitochondrial nuclease Nuc1p,20 the yeast BH3-only protein Ybh3p21 and the pro-apoptotic oxidoreductases Ndi1p and Aif1p.42,43 Upon HOCl treatment, all disruptants except Δkex1 showed either no significant changes or even reduced cell viability in comparison to wild type cells (Fig. 2A). Accordingly, ROS levels in these strains, except Δkex1, remained either unchanged or were even increased as compared with the wild type control (Fig. 2B). Thus, Kex1p (but not other hydrolases tested in this study) is required to execute the full cytotoxic activity of HOCl.
KEX1-disruption diminishes HOCl-mediated apoptosis and the formation of HOCl-modified epitopes
Kex1p has been previously involved in yeast apoptotic scenarios.41 To further characterize the rescuing effect of Δkex1 upon HOCl-stress, we analyzed if its improvement in cell viability and reduction in ROS production would correlate with a possible diminishment of apoptosis. Indeed, reduction of cell death (Fig. 2C) and ROS generation (Fig. 2D; Fig. S1A) was accompanied by a specific and quantitatively similar decrease of the late apoptotic population as determined via Annexin V/PI co-staining (Fig. 2E; Fig. S1B). This was confirmed by TUNEL staining, which showed a corresponding decline of apoptotic DNA fragmentation in Δkex1 compared with the wild type control when stressed with HOCl (Fig. 2F; Fig. S1C). In addition, the formation of HOCl-modified epitopes, as detected via immunochemical techniques using the above mentioned specific antibody, was significantly reduced compared with wild type cells (Fig. 2G, Fig. S1D). Thus, Kex1p seems to promote HOCl-induced apoptosis apparently by acting upstream of ROS production and specific protein modification(s).
Discussion
The present study aimed at establishing a cell death model for HOCl cytotoxicity, both to investigate its microbicidal effects and to model its cytotoxicity in mammalian cells. To this end, we used the eukaryotic unicellular fungus S. cerevisiae (budding yeast), which combines being (1) a microorganism and (2) an established model system to study PCD.15,16 While the antimicrobial properties of phagocyte-generated HOCl have been known for some decades,44,45 initial evidence for the cytotoxicity of HOCl in mammalian cells was provided by studies performed with activated neutrophils as well as the cell-free myeloperoxidase-hydrogen peroxide-chloride system.8 Both inhibition of myeloperoxidase as well as scavenging of HOCl, added as reagent or generated by the myeloperoxidase-hydrogen peroxide-chloride system, confirmed that HOCl is responsible for impaired cell viability and cell death in various mammalian cell types.8-10,46-49
The results presented here characterize the cell death process occurring in S. cerevisiae upon exposure to lethal doses of HOCl. Previous reports have shown that budding yeast succumbs to cell death in response to chlorinative stress,11,50 and that HOCl elicits cyto- and/or genotoxic effects against S. cerevisiae.11-13 Further work has demonstrated that toxicity of HOCl in wild type yeast strains is abolished by hypoxic and anoxic conditions, and that antioxidant-deficient S. cerevisiae strains are more sensitive to treatment with oxychloro compounds, including HOCl.13 In contrast to this earlier study, we more specifically monitored actual survival rates (via clonogenic tests), establishing a concentration-dependent deadly effect of HOCl on yeast. This cytotoxicity is accompanied by increased levels of ROS and can be counteracted by treatment with antioxidants, including glutathione. This confirms previous data showing elevated intracellular oxidative stress to be causative and the endogenous antioxidant system to be preventive during HOCl toxicity.13
Moreover, the present study defines the type of PCD induced by HOCl, which, according to our Annexin V/PI and TUNEL staining data, is mainly of apoptotic origin. However, a previous study13 suggested necrosis to be the death type preferentially occurring in HOCl-treated yeast cells based on the absence of TUNEL- and the prominent presence of PI-positive cells. This discrepancy might be due to various reasons. First, different culture conditions were used; while our study was performed in minimal (SMD) medium, Kwolek-Mirek et al.13 conducted theirs in complete (YEPD) medium, which differs in composition. This may have consequences for the extent and consequently type of toxicity (see below) at a particular concentration, especially given the high sensitivity of HOCl toxicity on slight media variance (see above). Second, the experiments performed with PI do not include a co-staining with an apoptotic stain like Annexin V that would allow discrimination between primary necrotic (PI+/Annexin V−) and late apoptotic (PI+/Annexin V+) cells, which both stain positive for PI. Thus, the authors13 cannot exclude that part of the displayed PI positivity may actually correspond to a possible late apoptotic cell population. In fact, in our experiments, the main part of the stained population is late apoptotic and thus both PI- and Annexin V-positive. Third, the HOCl concentrations used in Kwolek-Mirek et al.13 triggered PI staining of almost the complete yeast cell population, which raises the question whether the chosen toxic HOCl doses were too high to observe a putative apoptotic phenotype.
Indeed, HOCl may induce necrosis and/or apoptosis in a time- and concentration-dependent manner,51,52 apparently via formation and decay of chloramines and generation of radical intermediates.53,54 For instance, exposure to increasing doses of HOCl in endothelial cells induces cell death that progressively shifts from apoptosis to necrosis.46 Similarly, many yeast cell death inducers known to cause apoptosis at moderate concentrations promote a necrotic phenotype when applied at higher doses.16 The mere concentration increase of typical apoptotic triggers may, in most but not all cases, promote a non-regulated type of necrosis that likely arises from uncontrolled damage of cellular integrity. Thus, to test whether the necrotic phenotype displayed upon the high toxic HOCl dosage used in Kwolek-Mirek et al.13 represents such accidental or alternatively programmed necrosis, experiments assaying for typical markers of programmed necrosis would need to be performed under their conditions. Irrespective of this discrepancy, which may have multifactorial causes, HOCl cytotoxicity in S. cerevisiae as induced in our hands is mainly linked to an apoptotic phenotype. This is in line with several processes that have been involved in HOCl-mediated cell death, including upregulation of pro-apoptotic genes,50 downregulation of anti-apoptotic proteins9 or DNA strand breakage.55
Intriguingly, HOCl-induced yeast apoptosis is accompanied by formation of HOCl-modified proteins. HOCl and/or chlorinated proteins/chloramines have been shown to trigger the apoptotic machinery in different human cell lines and seem to preferentially activate the mitochondrial apoptotic pathway.49,56,57 Since the antibody used in this study to detect HOCl-modified epitopes in yeast also cross-reacts with HOCl-modified proteins occurring under inflammatory conditions in humans,58 it is likely that similar epitopes are formed. We thus speculate that yeast and human cells share, at least in part, some molecular features of HOCl cytotoxicity. In this respect, follow-up studies will aim at identifying these modified proteins and elucidate their function in HOCl-triggered cell death execution.
While the induction of cell death by HOCl has been established in diverse cell types (human as well as microbial), the molecular players involved in each case remain elusive. Here, we aimed at identifying lethal hydrolases involved in HOCl-mediated yeast apoptosis. Interestingly, the single known yeast metacaspase Yca1p does not seem to represent the molecular executioner in this process. In contrast, HOCl cytotoxicity in mammalian cells has been linked to caspase activation.46 Our data suggest, that—at least under conditions of fast proliferation and high glycolytic activity (represented by the model presented in this work)—HOCl exhibits its cytotoxicity through a caspase-independent mechanism. However, we also show that well-known caspase-independent effectors, such as the yeast homologs of endonuclease G (Nuc1p), BH3-only proteins (Ybh3p), the nuclear serine protease HtrA-like protein (Nma111p) or the apoptosis-inducing factor (Aif1p) are dispensable for HOCl-induced cytotoxicity. Instead, we identify the pro-apoptotic serine protease Kex1p as a pivotal factor in this process. Disruption of KEX1 causes a decrease in clonogenic cell death, ROS production and the apoptotic population upon challenge with HOCl, while at the same time it diminishes the extent of HOCl-modified proteins.
Kex1p has been previously described to be a major player in Yca1p-independent yeast apoptotic cell death induced by protein N-glycosylation defects, where the production of ROS is a determinant factor for lethality.41 Our results showing Kex1p-dependent increase of ROS production and formation of HOCl-modified proteins upon challenge with HOCl further suggest Kex1p as an executor of oxidation-mediated cytotoxicity. Thus, Kex1p-induced apoptosis seems to generally involve oxidative stress and possibly thereof resulting oxidized products, including modified proteins. HOCl-derived apoptosis could further comprise mechanistic elements present in cell death derived from defective protein N-glycosylation. At the same time, deletion of KEX1 has also been shown to promote survival under different PCD-inducing conditions, namely acetic acid stress and chronological aging.41 Thus, Kex1p may exert a more general role in yeast PCD. In that case, HOCl-mediated toxicity would not necessarily need to recapitulate upstream lethal pathways involved in N-glycosylation defects but would converge, possibly with other inducers, at the stage of Kex1p execution. Future work will have to clarify whether HOCl-induced cell death shares an upstream pathway with any of the above-mentioned lethal scenarios involving Kex1p.
By establishing the PCD mode in yeast upon challenge with HOCl and unveiling Kex1p as a molecular executor in this process, this study provides insight into the mechanisms underlying the microbicidal effects of HOCl toward fungal infections. However, these mechanisms might not correspond to those elicited during HOCl-induced killing of prokaryotes. In bacteria exposed to HOCl, the observed patterns of metabolic dysfunction suggest mechanisms where death occurs by interruption of the energy-transducing capabilities of the cell via inhibition of plasma membrane-localized proteins.12 These patterns thus involve processes (like energy transduction or glucose transport) that differ from eukaryotic cells. This has previously put in doubt whether the microbicidal mechanisms of HOCl toxicity in S. cerevisiae mirror those observed in bacteria.12 Interestingly, our results show that mitochondria seem to play a significant role in ROS-dependent HOCl lethality. Considering an evolutionary point of view, it would be interesting to explore whether mitochondrial membrane-localized proteins (similar to the situation in bacteria) are a target of HOCl. In addition, it has been recently shown that under certain circumstances, bacteria can succumb to an apoptotic-like cell death.59-61 To which extent this or other forms of PCD might be involved in the bactericidal action of HOCl and how or if mitochondrial targeting in eukaryotes might recapitulate cytotoxic characteristics of HOCl in prokaryotes remains to be investigated.
In humans, activated phagocytic cells generate and release HOCl, a process, which (though firmly controlled) represents a major contributor to inflammatory tissue damage. Interestingly, several characteristics of HOCl-mediated yeast cell death described in this study, including ROS generation, mitochondrial involvement, an apoptotic phenotype and increased levels of HOCl-modified proteins, parallel already known features of HOCl-mediated cytotoxicity in humans.8,10 Thus, our results also support the idea of employing S. cerevisiae as a model of HOCl-mediated cellular demise in human cells. In that respect, it will be interesting to test whether a similar protease, such as the herein identified yeast Kex1p, is involved in chlorinated protein-mediated apoptosis of inflamed human tissue. While there is no clear homolog of Kex1p in humans, the mammalian cathepsin A (like Kex1p a member of the serine protease group of hydrolases) shares about 20% overall similarity with Kex1p and represents a putative candidate for a factor involved in HOCl-induced apoptosis in human cells.
Summing up, this study establishes that the cytotoxic effect of HOCl on S. cerevisiae follows a Kex1p-dependent apoptotic pathway that possibly involves specific protein modifications reminiscent of those present in mammalian proteins upon HOCl stress. Whether or not these results compare with the situation in other eukaryotic and/or prokaryotic microorganisms needs further investigation. Similarly, future work will have to further address if budding yeast, in fact, represents a valid means to explore human HOCl-associated cellular demise, as the present study suggests.
Materials and Methods
Reagents
The following reagents were purchased from the indicated suppliers: FITC-labeled Annexin V (Roche Applied Science, 11828681001), reagents for “terminal deoxynucleotidyl transferase dUTP nick end labeling” (TUNEL, Roche Applied Science, 11684795910), diyhdroethidium (DHE, Sigma-Aldrich, D7008), propidium iodide (PI, Sigma-Aldrich, P4170), L-glutathione (Sigma-Aldrich, G4251), N-acetyl- L-cysteine (Sigma-Aldrich, A9165) and sodium hypochlorite (NaOCl, Sigma-Aldrich, A7191). HOCl solutions were prepared immediately before use and the concentration was determined spectrophotometrically (ε292 350 M−1 cm−1).
Yeast strains and growth conditions
Experiments were performed in wild type BY4741 and the respective aif1-, nuc1-, yca1-, pep4-, nma111-, ndi1-, cpl1- and kex1-null mutants, all obtained from Euroscarf. The strain BY4741 ρ0, previously described,62,63 was used to analyze mitochondrial dependency. All experiments were performed in synthetic minimal medium (SMD) medium containing 0.17% yeast nitrogen base (Difco), 0.5% (NH4)2SO4 and 30 mg/l of all amino acids (except 80 mg/l histidine, 200 mg/l leucine), 30 mg/l adenine and 320 mg/l uracil with 2% glucose. Incubation of liquid cultures was performed with 145 rpm at 28°C. Cell densities were measured using an automated cell counter (CASY1, Roche Innovatis). As described in the results section, the quantitative degree of the cytotoxic effect elicited by HOCl seemed to be slightly dependent on the batch of SMD growth medium used. Thus, before using a new batch of medium, a concentration series was performed to determine the concentration range at which to carry out the subsequent experiments.
For all assays, overnight cultures of corresponding strains were inoculated in 45 ml SMD medium using 250 ml flasks (or—for the mini-screen as well as for experiments with L-glutathione and N-acetyl-L-cysteine— inoculated in 15 ml using 100 ml flasks) at a density of 1.5 × 106 cells/ml. Next, cells were grown to a cell density of 7–9 × 106 cells/ml, subsequently distributed into 100 ml quadruple-indented flasks at 10 ml cell culture each (or— for the mini-screen as well as for experiments with L-glutathione and N-acetyl-L-cysteine—into 96 deep-well plates at 250 µl cell culture medium each) and then either challenged with HOCl stock solution to reach the indicated final concentration or left untreated (control). Cells were incubated for 16 h after HOCl treatment and used for diverse assays. For experiments with antioxidants, cells were incubated with L-glutathione or N-acetyl-L-cysteine at indicated concentrations starting at the time of inoculation and thus prior to HOCl treatment.
Survival plating and tests for cell death markers
Clonogenic survival plating was performed as previously described.17 Briefly, cell counts of HOCl-treated cultures and controls were measured; 500 cells were plated on YEPD agar plates and incubated for two days at 28°C to allow colony formation. The colony-forming units (CFUs) were analyzed using an automated colony counter (LemnaTech). For each strain, the CFUs determined for the control cultures were set to 100%, and the survival of the respective HOCl-treated cultures calculated relative to the corresponding control culture.
DHE staining (indicative for ROS production) was performed as described.20,63 Briefly, 5 × 106 cells were harvested by centrifugation, resuspended in 250 µl of 2.5 µg/ml DHE in PBS and incubated in the dark for 10 min. Relative fluorescence units (RFU) were determined by using a fluorescence reader (Tecan, GeniusPRO) and normalized to the OD600 of each sample. Alternatively, positive cells were counted using flow cytometry (BD FACSAria); 30,000 cells were used per experiment. Annexin V/PI costaining (apoptosis/necrosis marker) and TUNEL staining (apoptosis marker) were performed and quantified by flow cytometry as described.20,22 30,000 cells per sample were evaluated using BD FACSDiva software. Representative samples were subjected to qualitative analysis by fluorescence microscopy.
Immunofluorescence staining
For immunofluorescence studies, 1.5 × 107 cells were fixed with 4% formaldehyde solution and their cell wall digested for 30 min with glucuronidase/arylsulfatase and lyticase as described.20 After washing with sorbitol-containing buffer B [35 mM potassium phosphate buffer (pH 6.8), 0.5 mM MgCl2 and 1.2 M Sorbitol], cells were blocked with 100 µl blocking solution (Ultra V block, Thermo Fisher Scientific) for 20 min at 20°C. Cells were then spun down and the resulting pellets resuspended in 100 µl mAb antibody solution (clone 2D10G9, dilution 1:2, v/v in antibody diluent, Dako; 25°C for 30 min); the mAb recognizes HOCl-oxidized proteins, but does not cross-react with epitopes generated by oxidative reactions involving nitrating species, transition metals or lipid peroxidation reactions.40 After washing once with sorbitol-containing buffer B, cells were incubated with 100 µl cyanine 3-conjugated goat anti-mouse antibody (Jackson Dianova, 1:300 in antibody diluent) for 20 min at 25°C. Fluorescence was microscopically evaluated and quantified using a fluorescence reader (Tecan, GeniusPRO). The obtained intensity was normalized to the OD600 of each sample.
Microscopy
Microscopy was performed with a Zeiss Axioskop microscope using a Zeiss Plan-Neofluar objective lense with 100× magnification and 1.30 numerical aperture in oil (using Zeiss Immersol®) at room temperature. Imaging medium was AnnexinV/PI incubation buffer (10 mM HEPES, 140 mM NaCl, 5 mM CaCl2 pH 7.4) + 0.6 M sorbitol for pictures shown in Figure S1B and water for pictures shown in Figures 1F and Figure. S1A, C and D. Fluorochromes were fluorescein (used as fluorescein isothiocyanate, FITC), propidium iodide (PI) and ethidium (after conversion from dihydroethidium, DHE) for pictures shown in Figure S1A–C. Fluorescence microscopic sample images were taken with a Diagnostic Instruments camera (Model: SPOT 9.0 Monochrome-6), acquired using the Metamorph software (version 6.2r4, Universal Imaging Corp.) and processed with Adobe Photoshop (version CS2) software. Specifically, picture processing involved creation of AnnexinV and PI overlays in Figure S1B and coloring (Figs. 1F; Fig. S1A–D) with Metamorph. In addition, contrast and brightness was adjusted with Adobe Photoshop (applying equal adjustment parameters to all pictures of an experiment).
Statistical analyses
Error bars (± s.e.m.) are shown for independent experiments. When experiments were performed in parallel, a common overnight culture and common pre-cultures were used for each strain. In these cases, pre-cultures were divided into separate flasks (or wells in the mini-screen) prior to HOCl stress. The number of independent data points (n) is indicated in the figure legends of the corresponding graphs. Significances were calculated using an ANOVA approach and were corrected by the Bonferroni post hoc test.
Supplementary Material
Acknowledgments
This work was supported by funding provided by the Austrian Science Fund FWF (SFB-LIPOTOX F3007, F3012, P23490-B12, P24381-B20, V235-B09) and the European Commission (APOSYS). A.A.G. and L.H. are funded by the Medical University of Graz/University of Graz within the “Doctoral College Metabolic and Cardiovascular Disease” (FWF W1226-B18).
Glossary
Abbreviations:
- CFU
colony-forming units
- DHE
dihydroethidium
- Eth
ethidium
- FITC
fluorescein isothiocyanate
- HOCl/OCl-
hypochlorous acid/hypochlorite
- PCD
programmed cell death
- PI
propidium iodide
- ROS
reactive oxygen species S. cerevisiae, Saccharomyces cerevisiae
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/cc/article/24801
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24801
References
- 1.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 2.Katrantzis M, Baker MS, Handley CJ, Lowther DA. The oxidant hypochlorite (OCl-), a product of the myeloperoxidase system, degrades articular cartilage proteoglycan aggregate. Free Radic Biol Med. 1991;10:101–9. doi: 10.1016/0891-5849(91)90003-L. [DOI] [PubMed] [Google Scholar]
- 3.Thomas EL, Grisham MB, Jefferson MM. Cytotoxicity of chloramines. Methods Enzymol. 1986;132:585–93. doi: 10.1016/S0076-6879(86)32043-3. [DOI] [PubMed] [Google Scholar]
- 4.Malle E, Marsche G, Arnhold J, Davies MJ. Modification of low-density lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Biochim Biophys Acta. 2006;1761:392–415. doi: 10.1016/j.bbalip.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Malle E, Buch T, Grone H-J. Myeloperoxidase in kidney disease. Kidney Int. 2003;64:1956–67. doi: 10.1046/j.1523-1755.2003.00336.x. [DOI] [PubMed] [Google Scholar]
- 6.Pattison DI, Davies MJ. Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases. Curr Med Chem. 2006;13:3271–90. doi: 10.2174/092986706778773095. [DOI] [PubMed] [Google Scholar]
- 7.Zheng L, Nukuna B, Brennan M-L, Sun M, Goormastic M, Settle M, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–41. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pullar JM, Vissers MC, Winterbourn CC. Living with a killer: the effects of hypochlorous acid on mammalian cells. IUBMB Life. 2000;50:259–66. doi: 10.1080/15216540051080958. [DOI] [PubMed] [Google Scholar]
- 9.Pullar JM, Winterbourn CC, Vissers MCM. The effect of hypochlorous acid on the expression of adhesion molecules and activation of NF-kappaB in cultured human endothelial cells. Antioxid Redox Signal. 2002;4:5–15. doi: 10.1089/152308602753625807. [DOI] [PubMed] [Google Scholar]
- 10.Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol. 2004;24:1309–14. doi: 10.1161/01.ATV.0000131784.50633.4f. [DOI] [PubMed] [Google Scholar]
- 11.Buschini A, Carboni P, Furlini M, Poli P, Rossi C. Sodium hypochlorite-, chlorine dioxide- and peracetic acid-induced genotoxicity detected by the Comet assay and Saccharomyces cerevisiae D7 tests. Mutagenesis. 2004;19:157–62. doi: 10.1093/mutage/geh012. [DOI] [PubMed] [Google Scholar]
- 12.King DA, Hannum DM, Qi J-S, Hurst JK. HOCl-mediated cell death and metabolic dysfunction in the yeast Saccharomyces cerevisiae. Arch Biochem Biophys. 2004;423:170–81. doi: 10.1016/j.abb.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Kwolek-Mirek M, Bartosz G, Spickett CM. Sensitivity of antioxidant-deficient yeast to hypochlorite and chlorite. Yeast. 2011;28:595–609. doi: 10.1002/yea.1889. [DOI] [PubMed] [Google Scholar]
- 14.Madeo F, Fröhlich E, Fröhlich KU. A yeast mutant showing diagnostic markers of early and late apoptosis. J Cell Biol. 1997;139:729–34. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmona-Gutierrez D, Eisenberg T, Büttner S, Meisinger C, Kroemer G, Madeo F. Apoptosis in yeast: triggers, pathways, subroutines. Cell Death Differ. 2010;17:763–73. doi: 10.1038/cdd.2009.219. [DOI] [PubMed] [Google Scholar]
- 16.Eisenberg T, Carmona-Gutierrez D, Büttner S, Tavernarakis N, Madeo F. Necrosis in yeast. Apoptosis. 2010;15:257–68. doi: 10.1007/s10495-009-0453-4. [DOI] [PubMed] [Google Scholar]
- 17.Madeo F, Herker E, Maldener C, Wissing S, Lächelt S, Herlan M, et al. A caspase-related protease regulates apoptosis in yeast. Mol Cell. 2002;9:911–7. doi: 10.1016/S1097-2765(02)00501-4. [DOI] [PubMed] [Google Scholar]
- 18.Fahrenkrog B, Sauder U, Aebi U. The S. cerevisiae HtrA-like protein Nma111p is a nuclear serine protease that mediates yeast apoptosis. J Cell Sci. 2004;117:115–26. doi: 10.1242/jcs.00848. [DOI] [PubMed] [Google Scholar]
- 19.Büttner S, Carmona-Gutierrez D, Vitale I, Castedo M, Ruli D, Eisenberg T, et al. Depletion of endonuclease G selectively kills polyploid cells. Cell Cycle. 2007;6:1072–6. doi: 10.4161/cc.6.9.4218. [DOI] [PubMed] [Google Scholar]
- 20.Büttner S, Eisenberg T, Carmona-Gutierrez D, Ruli D, Knauer H, Ruckenstuhl C, et al. Endonuclease G regulates budding yeast life and death. Mol Cell. 2007;25:233–46. doi: 10.1016/j.molcel.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 21.Büttner S, Ruli D, Vögtle F-N, Galluzzi L, Moitzi B, Eisenberg T, et al. A yeast BH3-only protein mediates the mitochondrial pathway of apoptosis. EMBO J. 2011;30:2779–92. doi: 10.1038/emboj.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmona-Gutiérrez D, Bauer MA, Ring J, Knauer H, Eisenberg T, Büttner S, et al. The propeptide of yeast cathepsin D inhibits programmed necrosis. Cell Death Dis. 2011;2:e161. doi: 10.1038/cddis.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludovico P, Rodrigues F, Almeida A, Silva MT, Barrientos A, Côrte-Real M. Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:2598–606. doi: 10.1091/mbc.E01-12-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn S-H, Cheung WL, Hsu J-Y, Diaz RL, Smith MM, Allis CD. Sterile 20 kinase phosphorylates histone H2B at serine 10 during hydrogen peroxide-induced apoptosis in S. cerevisiae. Cell. 2005;120:25–36. doi: 10.1016/j.cell.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Ahn S-H, Diaz RL, Grunstein M, Allis CD. Histone H2B deacetylation at lysine 11 is required for yeast apoptosis induced by phosphorylation of H2B at serine 10. Mol Cell. 2006;24:211–20. doi: 10.1016/j.molcel.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Carmona-Gutierrez D, Jungwirth H, Eisenberg T, Madeo F. Cell cycle control of cell death in yeast. Cell Cycle. 2010;9:4046. doi: 10.4161/cc.9.20.13523. [DOI] [PubMed] [Google Scholar]
- 27.Palermo V, Cundari E, Mangiapelo E, Falcone C, Mazzoni C. Yeast lsm pro-apoptotic mutants show defects in S-phase entry and progression. Cell Cycle. 2010;9:3991–6. doi: 10.4161/cc.9.19.13210. [DOI] [PubMed] [Google Scholar]
- 28.Rockenfeller P, Ring J, Muschett V, Beranek A, Buettner S, Carmona-Gutierrez D, et al. Fatty acids trigger mitochondrion-dependent necrosis. Cell Cycle. 2010;9:2836–42. doi: 10.4161/cc.9.14.12346. [DOI] [PubMed] [Google Scholar]
- 29.Galluzzi L, Vitale I, Senovilla L, Eisenberg T, Carmona-Gutierrez D, Vacchelli E, et al. Independent transcriptional reprogramming and apoptosis induction by cisplatin. Cell Cycle. 2012;11:3472–80. doi: 10.4161/cc.21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galluzzi L, Vitale I, Senovilla L, Olaussen KA, Pinna G, Eisenberg T, et al. Prognostic impact of vitamin B6 metabolism in lung cancer. Cell Rep. 2012;2:257–69. doi: 10.1016/j.celrep.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–90. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 32.Laun P, Pichova A, Madeo F, Fuchs J, Ellinger A, Kohlwein S, et al. Aged mother cells of Saccharomyces cerevisiae show markers of oxidative stress and apoptosis. Mol Microbiol. 2001;39:1166–73. doi: 10.1111/j.1365-2958.2001.02317.x. [DOI] [PubMed] [Google Scholar]
- 33.Herker E, Jungwirth H, Lehmann KA, Maldener C, Fröhlich K-U, Wissing S, et al. Chronological aging leads to apoptosis in yeast. J Cell Biol. 2004;164:501–7. doi: 10.1083/jcb.200310014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–14. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 35.Morselli E, Galluzzi L, Kepp O, Criollo A, Maiuri MC, Tavernarakis N, et al. Autophagy mediates pharmacological lifespan extension by spermidine and resveratrol. Aging. 2009;1:961–70. doi: 10.18632/aging.100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging. 2011;3:716–32. doi: 10.18632/aging.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer MA, Carmona-Gutiérrez D, Ruckenstuhl C, Reisenbichler A, Megalou EV, Eisenberg T, et al. Spermidine promotes mating and fertilization efficiency in model organisms. Cell Cycle. 2013;12:346–52. doi: 10.4161/cc.23199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flemmig J, Arnhold J. Interaction of hypochlorous acid and myeloperoxidase with phosphatidylserine in the presence of ammonium ions. J Inorg Biochem. 2010;104:759–64. doi: 10.1016/j.jinorgbio.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Gerry AB, Leake DS. A moderate reduction in extracellular pH protects macrophages against apoptosis induced by oxidized low density lipoprotein. J Lipid Res. 2008;49:782–9. doi: 10.1194/jlr.M700349-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Malle E, Hazell L, Stocker R, Sattler W, Esterbauer H, Waeg G. Immunologic detection and measurement of hypochlorite-modified LDL with specific monoclonal antibodies. Arterioscler Thromb Vasc Biol. 1995;15:982–9. doi: 10.1161/01.ATV.15.7.982. [DOI] [PubMed] [Google Scholar]
- 41.Hauptmann P, Lehle L. Kex1 protease is involved in yeast cell death induced by defective N-glycosylation, acetic acid, and chronological aging. J Biol Chem. 2008;283:19151–63. doi: 10.1074/jbc.M801303200. [DOI] [PubMed] [Google Scholar]
- 42.Wissing S, Ludovico P, Herker E, Büttner S, Engelhardt SM, Decker T, et al. An AIF orthologue regulates apoptosis in yeast. J Cell Biol. 2004;166:969–74. doi: 10.1083/jcb.200404138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li W, Sun L, Liang Q, Wang J, Mo W, Zhou B. Yeast AMID homologue Ndi1p displays respiration-restricted apoptotic activity and is involved in chronological aging. Mol Biol Cell. 2006;17:1802–11. doi: 10.1091/mbc.E05-04-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clifford DP, Repine JE. Hydrogen peroxide mediated killing of bacteria. Mol Cell Biochem. 1982;49:143–9. doi: 10.1007/BF00231175. [DOI] [PubMed] [Google Scholar]
- 45.Wagner DK, Collins-Lech C, Sohnle PG. Inhibition of neutrophil killing of Candida albicans pseudohyphae by substances which quench hypochlorous acid and chloramines. Infect Immun. 1986;51:731–5. doi: 10.1128/iai.51.3.731-735.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vissers MC, Pullar JM, Hampton MB. Hypochlorous acid causes caspase activation and apoptosis or growth arrest in human endothelial cells. Biochem J. 1999;344:443–9. doi: 10.1042/0264-6021:3440443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C, Patel R, Eiserich JP, Zhou F, Kelpke S, Ma W, et al. Endothelial dysfunction is induced by proinflammatory oxidant hypochlorous acid. Am J Physiol Heart Circ Physiol. 2001;281:H1469–75. doi: 10.1152/ajpheart.2001.281.4.H1469. [DOI] [PubMed] [Google Scholar]
- 48.Whiteman M, Rose P, Siau JL, Cheung NS, Tan GS, Halliwell B, et al. Hypochlorous acid-mediated mitochondrial dysfunction and apoptosis in human hepatoma HepG2 and human fetal liver cells: role of mitochondrial permeability transition. Free Radic Biol Med. 2005;38:1571–84. doi: 10.1016/j.freeradbiomed.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 49.Whiteman M, Chu SH, Siau JL, Rose P, Sabapathy K, Schantz J-T, et al. The pro-inflammatory oxidant hypochlorous acid induces Bax-dependent mitochondrial permeabilisation and cell death through AIF-/EndoG-dependent pathways. Cell Signal. 2007;19:705–14. doi: 10.1016/j.cellsig.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 50.Yap YW, Whiteman M, Cheung NS. Chlorinative stress: an under appreciated mediator of neurodegeneration? Cell Signal. 2007;19:219–28. doi: 10.1016/j.cellsig.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Englert RP, Shacter E. Distinct modes of cell death induced by different reactive oxygen species: amino acyl chloramines mediate hypochlorous acid-induced apoptosis. J Biol Chem. 2002;277:20518–26. doi: 10.1074/jbc.M200212200. [DOI] [PubMed] [Google Scholar]
- 52.Yang YT, Whiteman M, Gieseg SP. HOCl causes necrotic cell death in human monocyte derived macrophages through calcium dependent calpain activation. Biochim Biophys Acta. 2012;1823:420–9. doi: 10.1016/j.bbamcr.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 53.Hawkins CL, Davies MJ. Hypochlorite-induced oxidation of proteins in plasma: formation of chloramines and nitrogen-centred radicals and their role in protein fragmentation. Biochem J. 1999;340:539–48. doi: 10.1042/0264-6021:3400539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Summers FA, Forsman Quigley A, Hawkins CL. Identification of proteins susceptible to thiol oxidation in endothelial cells exposed to hypochlorous acid and N-chloramines. Biochem Biophys Res Commun. 2012;425:157–61. doi: 10.1016/j.bbrc.2012.07.057. [DOI] [PubMed] [Google Scholar]
- 55.Choi J-M, Yoon B-S, Lee S-K, Hwang J-K, Ryang R. Antioxidant properties of neohesperidin dihydrochalcone: inhibition of hypochlorous acid-induced DNA strand breakage, protein degradation, and cell death. Biol Pharm Bull. 2007;30:324–30. doi: 10.1248/bpb.30.324. [DOI] [PubMed] [Google Scholar]
- 56.Robaszkiewicz A, Bartosz G, Soszyński M. N-Chloroamino acids mediate the action of hypochlorite on A549 lung cancer cells in culture. Toxicology. 2010;270:112–20. doi: 10.1016/j.tox.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Resch U, Semlitsch M, Hammer A, Susani-Etzerodt H, Walczak H, Sattler W, et al. Hypochlorite-modified low-density lipoprotein induces the apoptotic machinery in Jurkat T-cell lines. Biochem Biophys Res Commun. 2011;410:895–900. doi: 10.1016/j.bbrc.2011.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malle E, Waeg G, Schreiber R, Gröne EF, Sattler W, Gröne HJ. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions: colocalization of myeloperoxidase and hypochlorite-modified proteins. Eur J Biochem. 2000;267:4495–503. doi: 10.1046/j.1432-1327.2000.01498.x. [DOI] [PubMed] [Google Scholar]
- 59.Carmona-Gutierrez D, Kroemer G, Madeo F. When death was young: an ancestral apoptotic network in bacteria. Mol Cell. 2012;46:552–4. doi: 10.1016/j.molcel.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 60.Dwyer DJ, Camacho DM, Kohanski MA, Callura JM, Collins JJ. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Mol Cell. 2012;46:561–72. doi: 10.1016/j.molcel.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erental A, Sharon I, Engelberg-Kulka H. Two programmed cell death systems in Escherichia coli: an apoptotic-like death is inhibited by the mazEF-mediated death pathway. PLoS Biol. 2012;10:e1001281. doi: 10.1371/journal.pbio.1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Büttner S, Bitto A, Ring J, Augsten M, Zabrocki P, Eisenberg T, et al. Functional mitochondria are required for alpha-synuclein toxicity in aging yeast. J Biol Chem. 2008;283:7554–60. doi: 10.1074/jbc.M708477200. [DOI] [PubMed] [Google Scholar]
- 63.Carmona-Gutierrez D, Reisenbichler A, Heimbucher P, Bauer MA, Braun RJ, Ruckenstuhl C, et al. Ceramide triggers metacaspase-independent mitochondrial cell death in yeast. Cell Cycle. 2011;10:3973–8. doi: 10.4161/cc.10.22.18212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.