Abstract
Background
During organ transplantation, it is inevitable that tissues undergo cold ischemia during harvest and transport prior to implantation. Polyethylene based polymers have been proposed and tested as preservation agents with promising results. We have previously reported that a high molecular weight polyethylene glycol (15–20,000 MW, PEG15–20) protects the intestinal epithelium against a variety of cellular stresses including radiation injury and microbial invasion by mechanisms that appear to involve lipid rafts. The aim of this study was to determine the preservation effect of PEG 15–20 on the integrity of intestine grafts harvested for subsequent transplantation.
Materials and Methods
Intestinal grafts from mice were harvested using a complete surgical technique for intestinal transplantation and assessed for the effect of PEG on graft tissue integrity. Half of the grafts were preserved in histidine-tryptophan-ketoglutarate solution (HTK) alone and half preserved in HTK-PEG 15–20 solution at 4°C for 24 hours. Gross morphology, wet to dry ratios, histology, TUNEL assay for apoptosis, goblet cell numbers, and bacterial localization studies were performed to evaluate the effect of PEG on tissue integrity.
Results
Results demonstrate that PEG 15–20 had a superior preservation effect over HTK alone in all parameters tested. Notable was the effect of PEG on attenuation of epithelial apoptosis, preservation of mucus producing cells, and bacterial adherence to the epithelium.
Conclusion
Taken together, these studies suggest that use of PEG 15–20 as a potential adjuvant during intestinal transplant may offer significant promise to prolong graft survival during organ harvest.
Introduction
Despite important progress in intestinal transplantation over the past decades, outcomes remain inferior to solid organ transplantation with high morbidity and mortality in the post-transplant period [1, 2]. The success of intestinal transplantation has been hindered in large part by limitations in the duration and quality of organ preservation with cold preservation times stagnant at 6–10 hours and variable degrees of graft injury during the preservation process [3, 4]. Several structural and functional attributes of the intestine present unique challenges for graft preservation. As a result of a three compartment structure including the intravascular and extravascular spaces and a quasi-closed compartment of the intestinal lumen, the intestinal graft is susceptible to marked tissue edema. The mucosal layer of the intestine is exquisitely sensitive to hypoperfusion and ischemia with rapid onset of severe epithelial barrier dysfunction [5, 6]. Perhaps most significantly, the intestine constitutes an extensive and complex interface with endogenous gut flora with sophisticated mechanisms of adaptation, immunity and host-microbe cross-talk.
Given the unique properties of intestinal grafts, standard preservation techniques well established for solid organ transplantation will require modification for intestinal transplantation. Much attention has been paid to the role of luminal flushing and perfusate composition including nutrients, energy substrates and osmotically active molecules with the goal of preserving barrier function and epithelial cell viability and reducing graft edema [7]. While an optimal solution will incorporate many of these components, there is substantial evidence to support the protective properties of polyethylene glycol compounds (PEG). PEGs are already widely used in clinical medicine as osmotic bowel preparations (GoLytely®) and as adjuvants for drug delivery (e.g. pegylated interferon). Transplant preservation solutions containing PEG have been shown to attenuate damage from cold perfusion in animal models of kidney [8], pancreas [9], liver [10–12] and small bowel [7, 13] transplantation and are currently being investigated in clinical trials for kidney and liver transplantation.
PEGs are multi-functional molecules that simultaneously target several pathologic processes associated with graft injury during preservation, including tissue edema, cell membrane dysfunction, immune activation, tight junction function and the integrity of the mucosal barrier. As a consequence of their large molecular size and hydrophilic properties, PEGs generate a oncotic “sink” to sequester water molecules and reduce tissue edema. During periods of reperfusion they act as free-radical scavengers to attenuate lipid peroxidation and cell membrane injury. In addition to preventing oxidative membrane injury, PEGs can temporarily “patch” damaged cell membranes by forming reversible complexes with membrane lipids, maintaining cell integrity until more favorable conditions are present. Perhaps an underappreciated property of PEGs is their unique behavior on biological surfaces. PEGs can anchor to innate surfaces and exert major changes in the physico-chemical properties that govern protein and surface interactions, making them good candidates as surrogate mucins to preserve and restore the epithelial mucin layer.
In previous studies we demonstrated the efficacy of PEG 15–20 to protect intestinal epithelial cells against various stresses including radiation injury [14] and bile acid exposure [15] PEG 15–20 has also been shown by our group to protect cardiomyocytes from hypoxia via its action on lipid rafts [16]
Here we investigate the effect of a high-molecular weight polyethylene glycol on static cold storage preservation of intestinal grafts in a murine model. In addition to corroborating the recently published observations of Oltean et al [17], we focus on the action of polyethylene glycol as a potential surrogate mucin to sustain mucosal microbial barrier function and preserve host microbe homeostasis.
Materials and Methods
Animals
Animal experiments were approved by the Animal Care and Use Committee at the University of Chicago (IACUC protocol 71870). Fifty eight week-old C57BL/6 mice (mean weight 20g) were purchased from Charles River Laboratories (Wilmington, MA) and acclimated at the University of Chicago Animal Resource Center. Animals were maintained with controlled light-dark cycles, ambient temperature control and water ad lib.
Surgical Procedures
Mice underwent total enterectomy in preparation for the small intestine transplantation with the preservation of vascular inflow and outflow pedicles. Briefly, mice were anesthetized with ketamine/xylazine and the abdomen entered via a midline incision. Manipulation of the intestine was minimized and a cotton swab used when necessary to handle the graft. The total colon, including the cecum, was separated from the graft. The portal vein was carefully separated from the pancreas. Following the exposure of the aorta, the renal, colic, celiac and splenic vessels were suture ligated with 8-0 silk sutures. The supra-celiac aorta was ligated and the infra-renal aorta cannulated with a 30 gauge needle for retrograde in situ perfusion of the intestinal graft with 0.5 ml ice cold, heparinized isotonic saline solution. After in situ perfusion, the portal vein was ligated, the intestinal graft separated from the colon and removed with the Carrel patch of aorta. Grafts were stored at 4°C in either Histidine-Tryptophan-Ketoglutarate (Essential pharmaceuticals, Newtown, PA) solution alone (HTK) or in HTK with 5% high molecular weight polyethylene glycol (PEG 15–20, Sigma, St Louis, MO) (HTK-PEG). A direct luminal flush was attempted as a trial in an initial cohort of animals, but was not technically successful due to the friability and size of murine intestine.
Wet to Dry Ratio
Intestinal grafts from 50 mice were used to assess the effects of PEG on graft tissue edema. Half of the grafts (N=25) were preserved in HTK alone and half (N=25) preserved in HTK-PEG solution at 4°C for 24 hours. At the conclusion of the preservation period, the intestinal grafts were removed from the preservation bath, immediately weighed (wet weight), then lyophilized using FreeZone Freeze Dry System (Labconco, MO) and weighed again (dry weight). The wet-to-dry ratio was then calculated as a representation of tissue water content.
Histology
Full thickness samples of terminal ileum were fixed, embedded in paraffin and cut to 5-μm sections for hematoxylin-eosin staining. Ischemic injury was scored using an epithelial injury index scoring system: a composite of five histopathologic parameters of intestinal injury: tissue damage, erosion, ulceration, architectural changes and fibrosis. All evaluations were performed by a pathologist in a blinded fashion.
TUNEL assay
Characterization of apoptosis was achieved using a commercially available in situ cell death detection kit using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) reagent according to the manufacturer’s protocol (Chemicon International, Temecula, CA). Intestinal grafts from 10 mice were used with 5 grafts preserved in HTK alone and 5 in HTK-PEG for comparison. The extent of apoptosis was quantified by measurement of positive stained area per 10 μm2 using ACIS software (Automated Cellular Imaging System) (DAKO, Carpenteria, CA).
Assessment of Mucous-producing Goblet
To assess the intestinal mucus-gel layer, sections were preserved in carnoy solution (6:3:1 of ethanol, chloroform, acetic acid) and stained with Alcian blue (3%). Random image fields of the intestinal epithelial cell layer were selected to quantify the presence of mucus producing goblet cells. Goblet cells were identified based on mucopolysaccharide binding of the Alcian blue stain and quantified using the ACIS image analysis software (DAKO, Carpenteria, CA). All evaluations were performed by a pathologist in a blinded fashion.
Bacterial Localization
Fluorescent In Situ Hybridization (FISH) was used to quantify and localize bacteria within the mucous and epithelial layers. Using a probe for general bacterial domain (EUB338: 5′-GCTGCCTCCCGTAGGAGT-3′), the samples were mounted in SlowFade Gold antifade reagent with Dapi (Invitrogen, Carlsbad, CA) for laser scanning confocal microscopy (Leica, model TCS SP2 AOS, Allendale, PA).
Statistical Analysis
Statistical differences between groups were calculated for comparison of wet-to-dry ratios and epithelial injury scores using the Mann-Whitney U test (GraphPad Prism). A P value <0.05 was considered significant.
Results
Tissue Water Content
Organ preservation with HTK-PEG yielded significantly less graft edema compared to HTK alone. Macroscopic inspection of the grafts after a 24 hour preservation period demonstrated grossly less tissue edema and consistent preservation of the vascular architecture in the HTK-PEG grafts compared with a pale and ill-defined appearance of the HTK grafts (Figure 1A,C). Calculated wet-to-dry ratios after lyophilization were significantly lower in grafts preserved with HTK-PEG compared with HTK alone.
Figure 1.
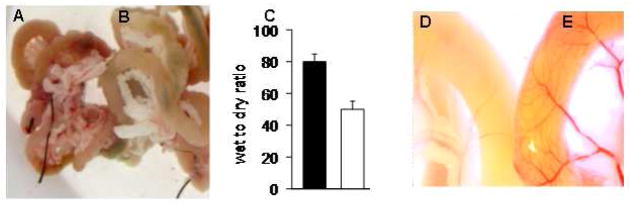
HTK plus high-molecular-weight polyethylene glycol (PEG 15–20) significantly reduces intestinal graft edema after 24 hours of cold storage compared with HTK preservation solution alone. Images of mice donor intestinal grafts preserved in HTK-PEG (A) and HTK alone (B). The mean wet-to-dry ratio of intestinal grafts preserved in HTK [■] was significantly higher than those preserved in HTK-PEG 15–20 [□] (C). (D,E) Images of donor grafts demonstrating the gross attenuation of intestinal vasculature in grafts preserved in HTK (D) compared with a grossly well-preserved in grafts preserved in HTK-PEG (E).
Intestinal Graft Histology
Severe and diffuse architectural distortion was evident after 24 hours of preservation in HTK alone, while crypt architecture was generally well-preserved after 24 hours in the HTK-PEG group (Figure 2A,B respectively). Intestinal grafts were systematically assessed after 9 and 24 hours of cold preservation in either HTK or HTK-PEG solution and the extent of injury scored based on the presence and extent of focal ulcerations, erosions, crypt distortion, and denuded epithelium (Fig. 2C). The mean injury score for grafts preserved in HTK-PEG was significantly less than grafts preserved in HTK alone at 9 hours and 24 hours of cold storage (Fig. 2D).
Figure 2.
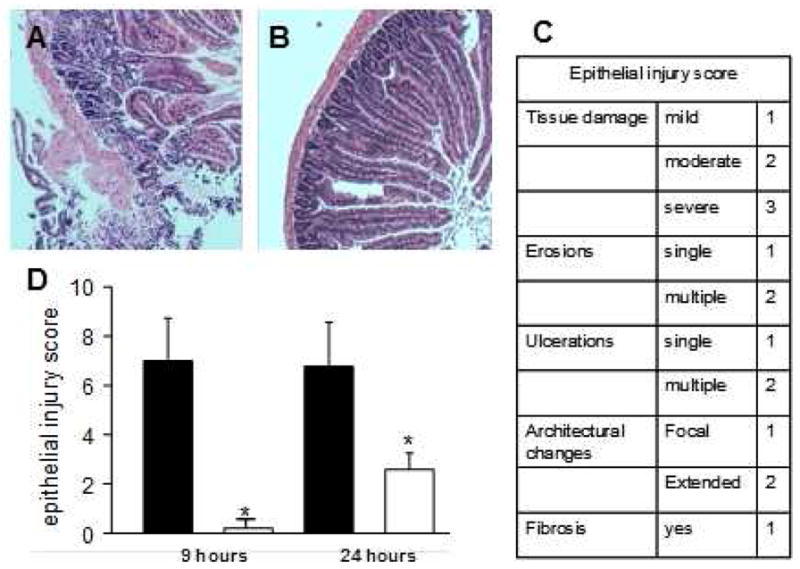
Histological analysis of the donor intestinal grafts. H&E staining of mice donor intestinal grafts preserved in (A) histidine-tryptophan-ketoglutarate (HTK) or in (B) HTK containing 5% PEG 15–20 (HTK/PEG). (C). Epithelial injury score. (D) Evaluation of severity of structural injury of donor intestinal grafts preserved in either HTK (■) or HTK containing 5% PEG 15–20 (□). n=50 donor grafts, *p<0.001.
Epithelial Cell Apoptosis
Intestinal epithelium is known to be extremely sensitive to ischemic injury and activation of apoptotic pathways may not be evident with standard histologic analysis. TUNEL staining and automated image analysis was used to assess the extent of DNA fragmentation and activation of apoptotic signaling cascades that would manifest in epithelial cell death in a delayed fashion. In addition to the architectural distortion observed in standard histologic staining, grafts preserved in HTK alone demonstrated a greater magnitude of TUNEL staining compared with grafts preserved in HTK-PEG (Figure 3A,B). Standardized image analysis used to quantify apoptotic activity corroborated this observation with a significantly lower mean uptake of dUTP marker in the HTK-PEG group compared with HTK alone (Figure 3C).
Figure 3.
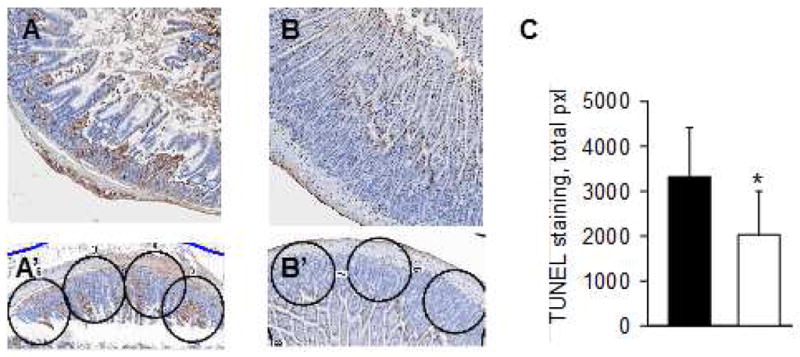
TUNEL assay was performed to assess intestinal epithelial cell apoptosis in intestinal grafts after 24 hours of cold storage. A higher degree of TUNEL staining was evident in grafts preserved with histidine-tryptophan-ketoglutarate (HTK) alone (A, A′) compared with those preserved in HTK containing 5% PEG 15–20 (B, B′). (C) Apoptosis score of donor intestinal grafts preserved in either HTK (■) or HTK containing 5% PEG 15–20 (□). n=10 donor grafts, *p<0.05. The examples of apoptosis score analysis are displayed in A′ and B′ panels.
Assessment of Mucous-Producing Goblet Cells
Mucous-producing goblet cells were identified and quantified using Alcian blue staining and image analysis software. Goblet cells were more abundant in HTK-PEG preserved grafts compared with HTK alone (Figure 4A,B). Quantification of Alcian blue staining within the epithelial cell layer of randomly selected fields confirmed this observation. The mean field staining was significantly higher in grafts preserved with HTK-PEG compared with HTK alone (Figure 4C).
Figure 4.
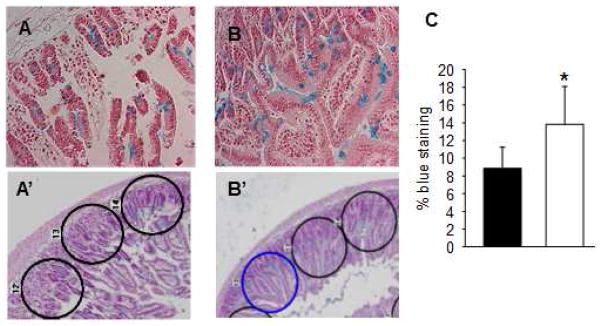
Alcian blue staining of intestinal grafts was used to assess the integrity of the intestinal mucus layer of the donor intestinal grafts preserved in (A, A′) histidine-tryptophan-ketoglutarate (HTK) or in (B, B′) HTK containing 5% PEG 15–20 (HTK/PEG). The integrity of the mucus barrier in grafts preserved in HTK alone (A, A′) was attenuated relative to grafts preserved in HTK-PEG (B, B′). (C) Alcian blue staining of the mucus barrier was quantified using automated image analysis software.
Blue staining score of donor intestinal grafts preserved in either HTK (■) or HTK containing 5% PEG 15–20 (□). n=10 donor grafts, *p<0.01.
Microbial Barrier Function
The integrity of the mucous layer overlying epithelial cell layer of the small intestine plays a crucial role as a physical barrier separating intestinal microbes from epithelial cells. In a state of health, intestinal flora is excluded from the densely packed mucin molecules that directly oppose the epithelial cell layer. The integrity of this sterile mucin layer during conditions of preservation was assessed by localizing intestinal bacteria with fluorescent in situ hybridization with a common bacterial domain probe. In the grafts preserved with HTK alone, bacteria were frequently localized deep within the mucous layer in contact with the epithelial cells and often translocating across the epithelial barrier. In contrast, intestinal bacteria were largely excluded from the dense mucin barrier and bacterial translocation was rare in grafts preserved with HTK-PEG.
Discussion
The future success of intestinal transplantation as a definitive therapy for intestinal failure will depend on marked improvements in graft quality and preservation techniques. Polyethylene glycol compounds represent an important class of versatile polymers that can target several of the pathologic processes associated with organ preservation. In this study we corroborate finding by others that PEG as an additive to intestinal preservation solution is effective at reducing graft edema, preserving architectural integrity and maintaining barrier function. Furthermore, we demonstrate that PEG preserves the dense mucin barrier at the epithelial cell layer and sustains microbial barrier function during the preservation process.
The utility of PEG compounds in organ preservation are well established and have already translated into early clinical trials for renal [8] and liver transplantation [10–12]. In several pre-clinical studies, PEG has been shown to enhance organ preservation through several mechanisms of action. PEG compounds exert oncotic pressure to reduce tissue edema, adsorb into cell membranes to prevent and repair oxidative injury, and interact at the level of the immunologic synapse to provide immunologic camouflage to the graft. Our findings and those of others support these actions of PEG in the preservation of small intestine grafts. The most potent of these effects in small bowel preservation appears to be the prevention of tissue edema that represents a major barrier to small intestine preservation.
A continued challenge in intestinal transplantation is rejection, bacterial overgrowth in the graft, loss of barrier function, and sepsis from gut-derived bacteria. There is increasing evidence that all of these events are interrelated. The human host maintains a dynamic equilibrium with its endogenous microbiota that becomes highly disrupted in the process of organ transplantation both from the procedure itself and immunsuppressive therapy. A major factor regulating these complex interactions is the dynamic function of the intestinal mucus layer which shields bacteria away from the epithelial surface preventing microbe-mediated disruptions in epithelial barrier function. During periods of host stress and ischemia, the mucus layer becomes depleted [18], resulting in disruption of barrier function and bacterial invasion. In the present study, we demonstrated that PEG 15–20 can preserve mucus function and prevent bacterial adherence. This effect may well play a role by which epithelial barrier function is maintained. The finding that apoptosis was prevented is suggestive of this mechanisms since apoptosis can be accelerated by microbial invasion. These results are limited however by confirmation of apoptosis versus necrosis which would require more in depth analysis such as by measuring caspase 3. Further work is in progress to elucidate these mechanistic details.
Our results suggest that PEG 15–20 could exert its protective action via 3 different protective mechanisms: 1) an increased oncotic pressure that limits the deleterious effects of edema, 2) stabilization of membrane lipids that enhance the immunoprotection of donor cells, tissues, and organs [19] and 3) behaving as an artificial mucin. Work is in progress to independently evaluate the causal role of each of these factors on the preservative effects of PEG on intestinal grafts.
The choice of the murine model herein described allowed for a simplified system of organ preservation in static solution, but prohibited more dynamic preservation techniques such as directed luminal flushing. A larger scale model would be required as a next step to investigate the effects of PEG with systemic and luminal flushes. Others have demonstrated the specific effects of PEG on the expression and function of epithelial barrier tight junction proteins and were thus excluded from our experimental design. Our study establishes a proof-of-concept for PEG as a mucus-barrier preservative and further larger scale studies are required to elucidate mechanisms of action and applicability to human intestinal transplantation.
Conclusion
Taken together, these studies suggest that use of PEG 15–20 as a potential adjuvant during intestinal transplant may offer significant promise to prolong graft survival during organ harvest. As the intestinal tract is unique given the presence of both microbial and host cells, PEG 15–20 may function by inhibiting processes by which microbes and host cells interact during graft harvest. Future studies are aimed at testing this compound in a mouse model of intestinal transplantation.
Figure 5.
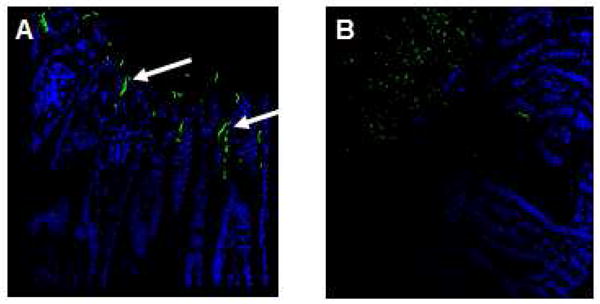
Fluorescent in situ hybridization (FISH) for a general bacterial domain was used to map bacteria location within the mucus layer and intestinal epithelial barrier. Among grafts preserved in HTK alone, bacteria were localized in the deep layers of the mucus layer in contact with and translocating through the intestinal epithelial layer, as represented by image (A) and demonstrated by white arrows.. In contrast, a sterile mucus barrier was conserved in intestinal grafts preserved in HTK-PEG, preserving the microbial barrier function of the mucus layer (B).
Footnotes
The authors (JCA, OZ) have a financial interest in Midway Pharmaceuticals which owns patents on PEG 15–20.
Author contribution: VV performed the intestinal transplantation model in mice, performed all of the experiments, drafted the manuscript, MT helped with the pathology analysis, JS, GT, OZ, JA participated in the design of the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fryer JP. The current status of intestinal transplantation. Current opinion in organ transplantation. 2008;13(3):266–272. doi: 10.1097/MOT.0b013e3282fd6901. [DOI] [PubMed] [Google Scholar]
- 2.Pascher A, Kohler S, Neuhaus P, Pratschke J. Present status and future perspectives of intestinal transplantation. Transplant international: official journal of the European Society for Organ Transplantation. 2008;21(5):401–414. doi: 10.1111/j.1432-2277.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 3.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45(4):673–676. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Maathuis MH, Leuvenink HG, Ploeg RJ. Perspectives in organ preservation. Transplantation. 2007;83(10):1289–1298. doi: 10.1097/01.tp.0000265586.66475.cc. [DOI] [PubMed] [Google Scholar]
- 5.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101(4):478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 6.Park PO, Haglund U, Bulkley GB, Falt K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery. 1990;107(5):574–580. [PubMed] [Google Scholar]
- 7.Roskott AM, Nieuwenhuijs VB, Dijkstra G, Koudstaal LG, Leuvenink HG, Ploeg RJ. Small bowel preservation for intestinal transplantation: a review. Transplant international: official journal of the European Society for Organ Transplantation. 2011;24(2):107–131. doi: 10.1111/j.1432-2277.2010.01187.x. [DOI] [PubMed] [Google Scholar]
- 8.Hauet T, Goujon JM, Baumert H, Petit I, Carretier M, Eugene M, Vandewalle A. Polyethylene glycol reduces the inflammatory injury due to cold ischemia/reperfusion in autotransplanted pig kidneys. Kidney international. 2002;62(2):654–667. doi: 10.1046/j.1523-1755.2002.00473.x. [DOI] [PubMed] [Google Scholar]
- 9.Neuzillet Y, Giraud S, Lagorce L, Eugene M, Debre P, Richard F, Barrou B. Effects of the molecular weight of peg molecules (8, 20 and 35 KDA) on cell function and allograft survival prolongation in pancreatic islets transplantation. Transplantation proceedings. 2006;38(7):2354–2355. doi: 10.1016/j.transproceed.2006.06.117. [DOI] [PubMed] [Google Scholar]
- 10.Franco-Gou R, Mosbah IB, Serafin A, Abdennebi HB, Rosello-Catafau J, Peralta C. New preservation strategies for preventing liver grafts against cold ischemia reperfusion injury. Journal of gastroenterology and hepatology. 2007;22(7):1120–1126. doi: 10.1111/j.1440-1746.2006.04495.x. [DOI] [PubMed] [Google Scholar]
- 11.Ben Abdennebi H, Elrassi Z, Scoazec JY, Steghens JP, Ramella-Virieux S, Boillot O. Evaluation of IGL-1 preservation solution using an orthotopic liver transplantation model. World journal of gastroenterology: WJG. 2006;12(33):5326–5330. doi: 10.3748/wjg.v12.i33.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbas R, Kombu RS, Dignam D, Gunning W, Stulberg JJ, Brunengraber H, Sanabria JR. Polyethylene glycol modified-albumin enhances the cold preservation properties of University of Wisconsin solution in rat liver and a hepatocyte cell line. The Journal of surgical research. 2010;164(1):95–104. doi: 10.1016/j.jss.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Itasaka H, Burns W, Wicomb WN, Egawa H, Collins G, Esquivel CO. Modification of rejection by polyethylene glycol in small bowel transplantation. Transplantation. 1994;57(5):645–648. doi: 10.1097/00007890-199403150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Valuckaite V, Zaborina O, Long J, Hauer-Jensen M, Wang J, Holbrook C, Zaborin A, Drabik K, Katdare M, Mauceri H, et al. Oral PEG 15–20 protects the intestine against radiation: role of lipid rafts. American journal of physiology Gastrointestinal and liver physiology. 2009;297(6):G1041–1052. doi: 10.1152/ajpgi.00328.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edelstein A, Fink D, Musch M, Valuckaite V, Zaborina O, Grubjesic S, Firestone MA, Matthews JB, Alverdy JC. Protective effects of nonionic triblock copolymers on bile acid-mediated epithelial barrier disruption. Shock. 2011;36(5):451–457. doi: 10.1097/SHK.0b013e31822d8de1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malhotra R, Valuckaite V, Staron ML, Theccanat T, D’Souza KM, Alverdy JC, Akhter SA. High-molecular-weight polyethylene glycol protects cardiac myocytes from hypoxia- and reoxygenation-induced cell death and preserves ventricular function. American journal of physiology Heart and circulatory physiology. 2011;300(5):H1733–1742. doi: 10.1152/ajpheart.01054.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oltean M, Joshi M, Herlenius G, Olausson M. Improved intestinal preservation using an intraluminal macromolecular solution: evidence from a rat model. Transplantation. 89(3):285–290. doi: 10.1097/TP.0b013e3181c9905a. [DOI] [PubMed] [Google Scholar]
- 18.Qin X, Sheth SU, Sharpe SM, Dong W, Lu Q, Xu D, Deitch EA. The mucus layer is critical in protecting against ischemia-reperfusion-mediated gut injury and in the restitution of gut barrier function. Shock. 2011;35(3):275–281. doi: 10.1097/SHK.0b013e3181f6aaf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yandza T, Tauc M, Canioni D, Rogel-Gaillard C, Bernard G, Bernard A, Gugenheim J. Effect of polyethylene glycol in pig intestinal allotransplantation without immunosuppression. The Journal of surgical research. 2012;176(2):621–628. doi: 10.1016/j.jss.2011.10.012. [DOI] [PubMed] [Google Scholar]


