In patients with stage III or IV epithelial ovarian cancer, a moderate-to-large pleural effusion on CT images was independently associated with poorer overall survival after controlling for age, stage, preoperative CA-125 level, and debulking status.
Abstract
Purpose:
To determine the prognostic importance of pleural effusions on preoperative computed tomographic (CT) images in patients with advanced epithelial ovarian cancer.
Materials and Methods:
The institutional review board waived informed consent for this HIPAA-compliant study of 203 patients with International Federation of Obstetrics and Gynecology stage III (n = 172) or IV (n = 31) epithelial ovarian cancer who underwent CT before primary cytoreductive surgery between 1997 and 2004 (mean age, 61 years; range, 37–96 years). Two radiologists retrospectively evaluated chest and/or abdominal CT images for pleural malignancy and the presence, size, and laterality of pleural effusions. To evaluate survival, Kaplan-Meier methods were used, with log-rank P values for comparisons. Multivariate analyses were conducted by using Cox proportional hazards regression. κ Statistics were calculated for interreader agreement.
Results:
Median survival was 50 months (95% confidence interval [CI]: 45, 55 months) for patients with stage III disease and 41 months (95% CI: 27, 58 months) for patients with stage IV disease. Readers 1 and 2 found pleural effusions in 40 and 41 stage III and 20 and 21 stage IV patients, respectively. At multivariate analysis, after controlling for stage, age at surgery, preoperative serum CA-125 level, debulking status, and ascites, moderate-to-large pleural effusion on CT images was significantly associated with worse overall survival (reader 1: hazard ratio = 2.27 [95% CI: 1.31, 3.92], P < .01; reader 2: hazard ratio = 2.25 [95% CI: 1.26, 4.01], P = .02). Preoperative CA-125 level, debulking status, and ascites were also significant survival predictors (P ≤ .03 for all for both readers). Readers agreed substantially in distinguishing small from moderate-to-large effusions (κ = 0.764).
Conclusion:
Moderate-to-large pleural effusion on preoperative CT images in patients with stage III or IV epithelial ovarian cancer was independently associated with poorer overall survival after controlling for age, preoperative CA-125 level, surgical stage, ascites, and cytoreductive status.
© RSNA, 2011
Introduction
Among gynecologic cancers in the United States, ovarian cancer is the second most common and results in the greatest number of deaths (1). The primary management of ovarian cancer involves initial surgical staging for disease apparently confined to the ovary or surgical cytoreduction or debulking in cases of grossly evident metastatic disease. On the basis of the primary surgical findings, patients are staged according to the International Federation of Obstetrics and Gynecology (FIGO) system (2). FIGO stage is one of the most powerful prognostic factors for patients with ovarian cancer: The higher the stage, the lower the rate of survival.
The FIGO staging system can be summarized as follows: Stage I describes any disease confined only to the ovaries, stage II is assigned when the disease spreads to pelvic organs, stage III includes peritoneal spread beyond the pelvis and/or pelvic or paraaortic lymph node involvement, and stage IV involves any distant metastasis (2). Unfortunately, early disease causes minimal, nonspecific, or no symptoms, and more than 75% of all ovarian cancer cases are diagnosed at stage III or IV (3).
The use of computed tomography (CT) to locate tumor deposits before primary debulking has become standard practice. Typically, an attempt is made to identify signs of transdiaphragmatic tumor spread, such as pleural metastases and pleural effusions, on chest CT images because their presence may have a substantial effect on further management. Previous reports in the literature have identified CT findings that are considered to be indicators of whether a pleural effusion is malignant, such as pleural thickening greater than 1 cm and/or pleural nodularity and irregularity (4,5). In patients with ovarian cancer, pleural effusions have been reported to be more likely to be malignant when they are moderate to large in size and when they are associated with enlarged superior diaphragmatic lymph nodes (6).
The relationship between survival and malignant pleural effusions, as compared with other stage IV–defining findings (eg, parenchymal liver metastases), is uncertain. Some authors have found comparable survival rates in patients with cytologically proved malignant pleural effusion versus patients with stage IV disease on the basis of liver metastases, whereas others have reported a trend toward a more favorable prognosis for those patients with malignant pleural effusions (7–9). Results of a previous study from our institution (10) showed that patients with advanced ovarian cancer who underwent optimal primary cytoreduction but had malignant pleural effusions had a worse prognosis than those who underwent optimal primary cytoreduction and did not have effusions. The presence of pleural effusion does not preclude surgical cytoreduction (8,9). The decision regarding whether or not to perform a thoracentesis to evaluate the cytology of the pleural fluid in patients with effusions is usually based on clinical judgment (11). In a series of studies at our institution (10,12,13), it was found that a considerable percentage of pleural effusions were presumed to be reactive and were therefore not properly evaluated for possible malignancy, leaving viable tumor in the chest even after optimal intraabdominal debulking. So far, to our knowledge, no large studies have been performed to assess whether pleural effusion at initial presentation has a negative effect on survival.
The identification of new prognostic markers to stratify patients with advanced ovarian cancer could improve treatment decision making. The goal of our study was to determine the prognostic importance of pleural effusions identified preoperatively on CT images in patients undergoing primary cytoreduction for stage III or IV epithelial ovarian cancer.
Materials and Methods
Patients
Our retrospective cross-sectional imaging study was compliant with the Health Insurance Portability and Accountability Act. The institutional review board of Memorial Sloan-Kettering Cancer Center approved the study and issued a waiver of informed consent. By means of a computerized review of institutional gynecologic surgery and radiology databases for the period from January 15, 1997, to December 22, 2004, we identified 212 consecutive patients with stage III or IV ovarian cancer who met the following inclusion criteria: primary surgery performed at our institution, CT of the chest and/or abdomen performed up to 45 days before the primary surgery, and no neoadjuvant chemotherapy in the interval between CT examination and surgical staging.
Although all patients had to have good performance status to undergo major surgical cytoreduction, medical records were reviewed for the presence of comorbidities that might cause pleural effusions (eg, heart failure, low protein status, renal failure), and no such comorbidities were found. Age, preoperative CA-125 level, and cancer stage were recorded from the patients’ medical records. Nine patients were excluded from the study because their preoperative CA-125 levels were missing from the medical records. Thus, a total of 203 patients (stage III, n = 172; stage IV, n = 31) were included. We combined stage III and IV disease into the category of advanced ovarian carcinoma on the basis of management in the past several years, during which patients with stage IV disease started undergoing primary cytoreduction similar to patients with stage III disease. If a patient underwent CT imaging more than once within the 45 days before surgery, the most recent CT study was used.
CT Scans
CT examinations were performed with section thicknesses ranging from 5 to 10 mm. For CT examinations not performed at our institution, the type of CT scanner and the amount of contrast material were unknown. At our institution, CT imaging was performed with various scanners (GE Medical Systems, Milwaukee, Wis). Our standard CT protocols were tailored to the individual scanners. Before October 1, 2000, studies were obtained with a conventional nonhelical scanner; afterward, they were obtained with a helical scanner with one to 16 detector rows. Standard collimation was 10 mm for the nonhelical scanners, 7 mm for single–detector row scanners, 3.75 mm for four–detector row scanners, 2.5 mm for eight–detector row scanners, and 1.25 mm for 16–detector row scanners. The pitch used with helical scanners varied during the examination and with different scanners (range, 0.75–1.5). The standard section thickness for image viewing was 10 mm for nonhelical scanners, 7 mm for single–detector row scanners, and 7.5 mm for four–, eight–, and 16–detector row scanners.
For all CT examinations performed at our institution, a dynamic power injection of 150 mL of nonionic intravenous contrast material was administered at a rate of 2.5 mL/sec (or more slowly if mandated by suboptimal venous access). Time delay to scanning varied with the type of scanner used but was determined on the basis of the typical time to portal venous phase imaging. All CT studies that were performed outside our institution were digitized and sent to our enterprise-wide picture archiving and communication system. Of the 203 patients in the study, 202 (99.5%) underwent CT with intravenous contrast material.
Image Analysis
Two radiologists separately read all of the CT scans, evaluating the presence, size, and laterality of pleural effusions and assessing the pleura for evidence of malignancy, such as nodularity, pleural thickness greater than 1 cm, and regions of enhancement. When a scan showed bilateral pleural effusion, the maximum effusion size was recorded as the size. Reader 1 (S.M.) was fellowship trained in body imaging and had 9 years of clinical experience in oncologic imaging at our institution. Reader 2 (H.V.A.) was a body imaging fellow. Both radiologists were aware that the patients had stage III or IV ovarian cancer but were blinded to the patients’ clinical data, prospective CT reports, and pathologic findings. The radiologists were not involved with the patients’ clinical care or the interpretation of the patients’ original CT studies. Both radiologists reviewed the CT studies during five to six sessions. Pleural effusion size was categorized on the basis of visual estimation as small (occupying less than one-third of the visualized hemithorax), moderate (occupying one-third to two-thirds of the hemithorax), or large (occupying more than two-thirds of the hemithorax). Previous work has suggested visual approximation to be about 85% accurate compared with three-dimensional CT reconstruction (14). The laterality of the pleural effusions was recorded as right, left, or bilateral.
Standard of Reference
Intraoperative notes and pathology reports were retrospectively reviewed by a research study assistant to determine whether the effusions were tapped preoperatively and, if so, what their cytopathologic results were. All patients had cytoreduction and staging performed at our institution by a gynecologic oncology surgical team with experience that ranged from 3 to 30 years. The definitive intervention was comprehensive staging laparotomy, which included total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, extensive tumor debulking, peritoneal washing, multiple biopsies from peritoneal sites, and pelvic and retroperitoneal lymphadenectomy.
Statistical Analyses
Survival was defined as time from surgery until death. Patients who did not die were censored at the last follow-up. Overall survival estimates were computed by using the Kaplan-Meier method, and comparisons in survival were made by using the log-rank test. To determine independent predictors of survival, Cox proportional hazards regression models were used, and the resulting hazard ratios were reported with their 95% confidence intervals (CIs). Differences in features between subgroups were evaluated by using a χ2 or Fisher exact test. For multivariate analysis, patients were grouped on the basis of whether or not they had any pleural effusion on CT images. All P values less than .05 were considered to indicate a significant difference.
κ Statistics with 95% CIs were calculated to assess interreader agreement on the presence (yes vs no), size (small, moderate, or large), and laterality (right, left, or bilateral) of pleural effusion and the diagnosis of pleural metastasis. The κ statistics were interpreted as follows: a κ statistic of zero was considered to indicate no agreement; 0–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–1, almost perfect agreement. All statistical analyses were performed by using commercially available software (Intercooled Stata, version 8.0 for Windows, Stata, College Station, Tex; SAS, version 9.0 for Windows, SAS Institute, Cary, NC).
Results
Patient and Scan Characteristics
Patient age ranged from 37 to 96 years, with a mean of 61 years. The time from CT scanning to surgery ranged from 1 to 43 days, with a mean of 15 days. Fifty-nine patients (29%) had their CT scanning performed at our institution, and 144 (71%) had their studies performed elsewhere. The distributions of CT scan types and pathologic disease characteristics are displayed in Table 1.
Table 1.
Distribution of CT Scan Types and Pathologic Disease Characteristics
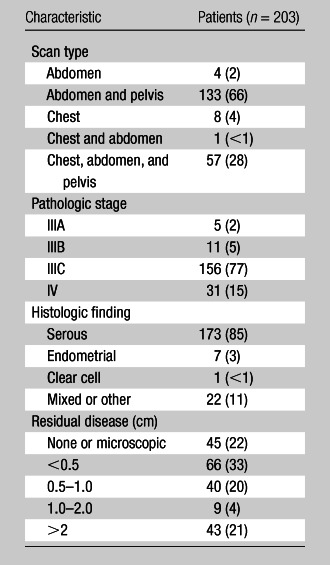
Note.—Data are numbers of patients, with percentages in parentheses.
Size, Laterality, and Tapping Status of Pleural Effusions
On CT images, reader 1 detected pleural effusions in a total of 60 patients (stage III, n = 40; stage IV, n = 20), while reader 2 detected pleural effusions in a total of 62 patients (stage III, n = 41; stage IV, n = 21). Out of the 62 patients with pleural effusion identified on CT images by at least one reader, only one patient had undergone CT imaging without intravenous contrast material, and thus, the rate of intravenous contrast material usage in this group was 98%. The two readers displayed nearly perfect agreement in detecting pleural effusion (κ = 0.977; 95% CI: 0.944, 1). For patients identified by both readers as having pleural effusion, the two readers displayed substantial agreement in distinguishing between small, moderate, and large pleural effusions (κ = 0.748; 95% CI: 0.612, 0.885) and slightly higher agreement in distinguishing between small and moderate-to-large pleural effusions (κ = 0.764; 95% CI: 0.599, 0.928). They also agreed substantially on the laterality of pleural effusion (κ = 0.615; 95% CI: 0.436, 0.795). Right-sided pleural effusion was found to be more common (a well-known fact); it was identified by reader 1 in 44 patients and by reader 2 in 50 patients.
Interreader agreement regarding the diagnosis of pleural metastasis (yes vs no) was fair (κ = 0.232; 95% CI: 0.024, 0.440) (Fig 1). Of the 41 patients with stage III disease in whom pleural effusion was detected by at least one reader, 40 (98%; 95% CI: 88%, 100%) had not had the effusion tapped. Of the 21 patients with stage IV disease in whom pleural effusion was detected by at least one reader, three (14%; 95% CI: 3%, 36%) had not had it tapped. Patients with stage III disease were significantly less likely to have had their effusions tapped (P < .001) (Table 2).
Figure 1a:
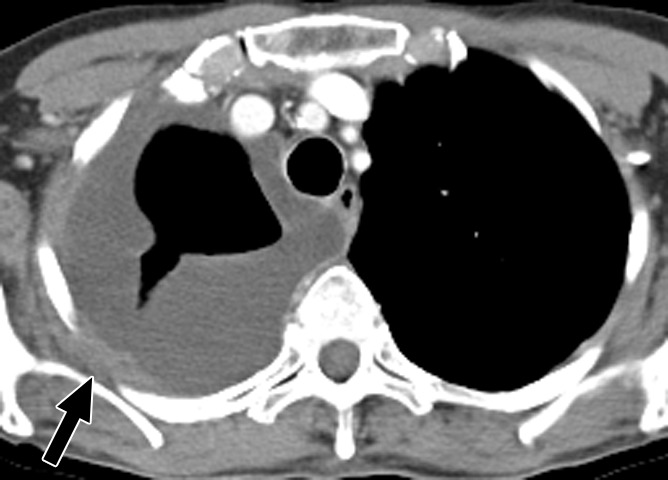
Contrast material–enhanced CT images of the thorax in a 58-year-old woman with high-grade papillary-serous ovarian carcinoma. (a) Large partially loculated right-sided pleural effusion with posterior pleural thickening in the upper thorax (arrow) at initial presentation. (b) Mild right pleural enhancement in the lower thorax suggestive of malignant pleural involvement (arrow), which was confirmed with video-assisted thoracic surgery. (c) Image obtained 6 months after optimal debulking in the abdomen and pelvis and a few cycles of platinum-based chemotherapy shows an increase in circumferential pleural metastasis (arrowheads), consistent with persistent thoracic disease.
Table 2.
Tapping Status and Cytology Results for Patients with Pleural Effusion by Stage

Note.—Data are given for patients in whom pleural effusion was detected by at least one reader. Data are numbers of patients, with percentages in parentheses and 95% CIs in brackets.
Figure 1b:
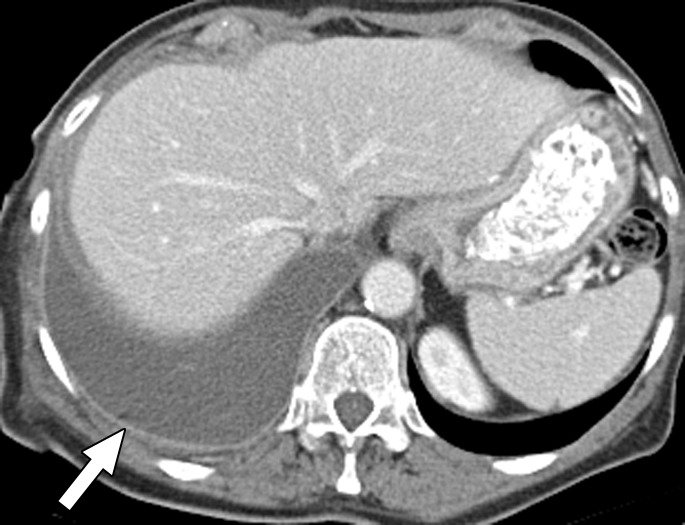
Contrast material–enhanced CT images of the thorax in a 58-year-old woman with high-grade papillary-serous ovarian carcinoma. (a) Large partially loculated right-sided pleural effusion with posterior pleural thickening in the upper thorax (arrow) at initial presentation. (b) Mild right pleural enhancement in the lower thorax suggestive of malignant pleural involvement (arrow), which was confirmed with video-assisted thoracic surgery. (c) Image obtained 6 months after optimal debulking in the abdomen and pelvis and a few cycles of platinum-based chemotherapy shows an increase in circumferential pleural metastasis (arrowheads), consistent with persistent thoracic disease.
Figure 1c:
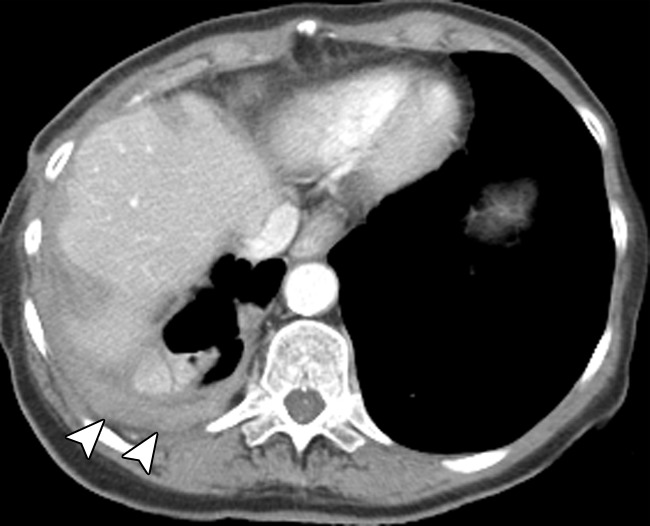
Contrast material–enhanced CT images of the thorax in a 58-year-old woman with high-grade papillary-serous ovarian carcinoma. (a) Large partially loculated right-sided pleural effusion with posterior pleural thickening in the upper thorax (arrow) at initial presentation. (b) Mild right pleural enhancement in the lower thorax suggestive of malignant pleural involvement (arrow), which was confirmed with video-assisted thoracic surgery. (c) Image obtained 6 months after optimal debulking in the abdomen and pelvis and a few cycles of platinum-based chemotherapy shows an increase in circumferential pleural metastasis (arrowheads), consistent with persistent thoracic disease.
Relationship between Pleural Effusion and Survival
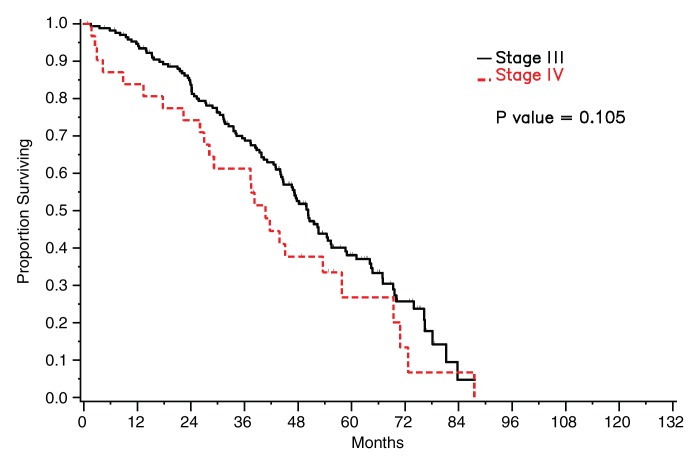
For the entire set of 203 patients, the median survival was 48 months (95% CI: 44, 54 months), with a total of 132 deaths. Median follow-up for survivors was 52 months. Among the 172 patients with stage III disease, 107 deaths occurred during the study period, and median survival was 50 months (95% CI: 45, 55 months). Among the 31 patients with stage IV disease, there were 25 deaths during the study period, and median survival was 41 months (95% CI: 27, 58 months). Survival did not differ significantly between patients with stage III disease and those with stage IV disease (P = .105) (Fig 2).
Figure 2:
Graph shows survival curves stratified by stage.
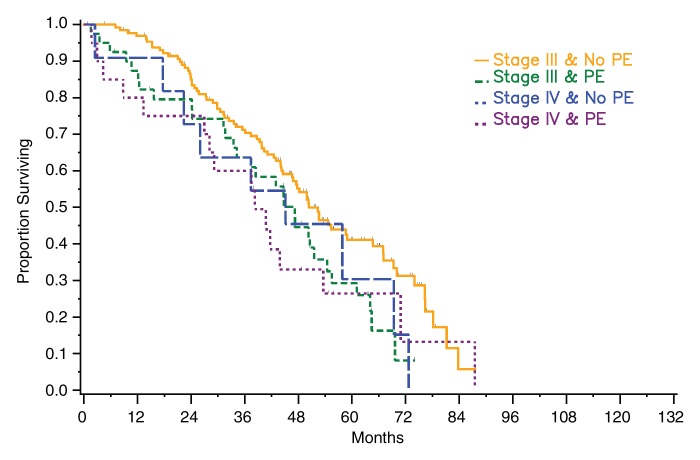
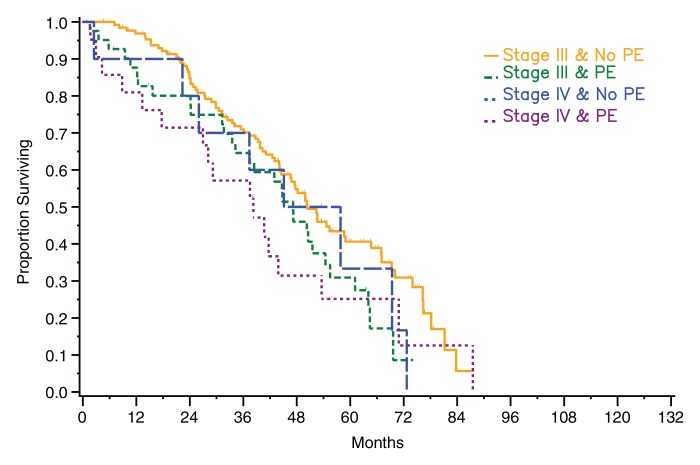
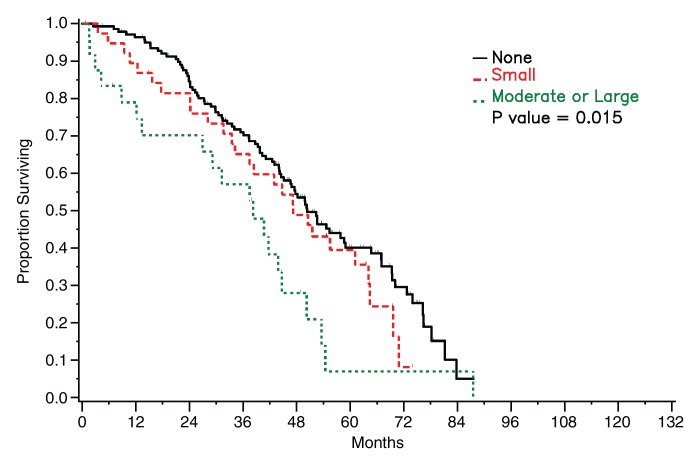
Among patients with stage III disease, median survival was 47 months (95% CI: 34, 54 months) for those with pleural effusion on CT images versus 50 months (95% CI: 45, 65 months) for those without pleural effusion for both readers. The difference was significant for reader 1 (P = .04) and not significant for reader 2 (P = .07). Among patients with stage IV disease, median survival periods for patients with and those without pleural effusion on CT images were 38 months (95% CI: 13, 54 months) and 45 months (95% CI: 18, 69 months), respectively, for reader 1 and 38 months (95% CI: 18, 54 months) and 51 months (95% CI: 2, 73 months), respectively, for reader 2. The difference was not statistically significant for either reader (reader 1: P = .82, reader 2: P = .58) (Fig 3). However, when data from all patients (regardless of stage) were combined and effusions were broken down by size, a significant difference in survival was found between patients with no or a small effusion versus those with a moderate-to-large effusion as assessed by reader 1 (P = .006) and by reader 2 (P = .015) (Fig 4).
Figure 3a:
Graphs show survival curves for patients stratified by disease stage and presence of pleural effusion (PE) on CT images according to readers (a) 1 and (b) 2.
Figure 4a:
Graphs show survival curves for patients stratified by pleural effusion (PE) size on CT images as assessed by readers (a) 1 and (b) 2.
Figure 3b:
Graphs show survival curves for patients stratified by disease stage and presence of pleural effusion (PE) on CT images according to readers (a) 1 and (b) 2.
Figure 4b:
Graphs show survival curves for patients stratified by pleural effusion (PE) size on CT images as assessed by readers (a) 1 and (b) 2.
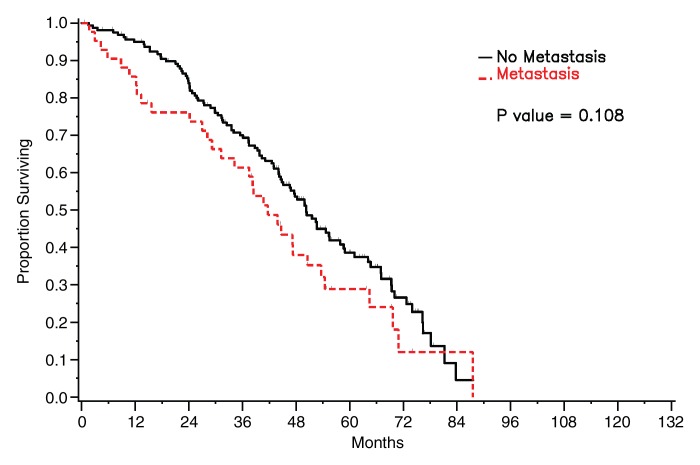
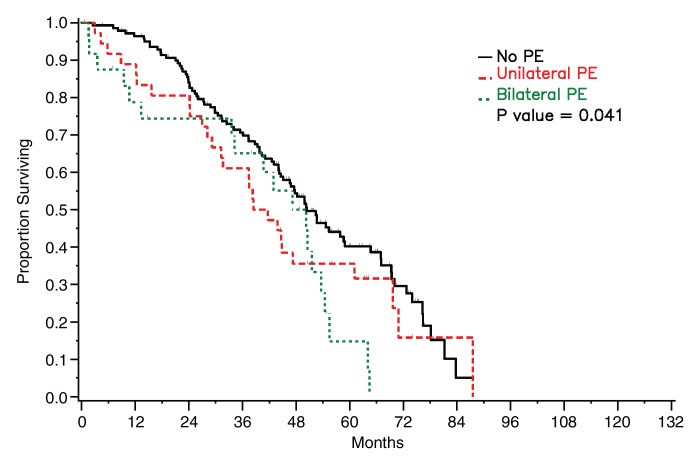
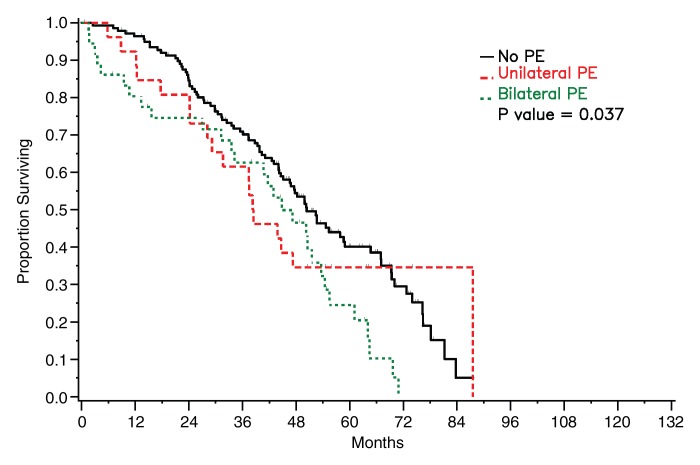
Survival did not differ significantly between patients who had detectable pleural metastasis on CT images and those who did not for either of the radiologists (reader 1: P = .108, reader 2: P = .388) (Fig 5). Survival was significantly worse in patients with bilateral pleural effusions than in patients with no or unilateral effusion on CT images (reader 1: P = .041, reader 2: P = .037) (Fig 6).
Figure 5a:
Graphs show survival curves for patients stratified by presence of pleural metastasis on CT images as assessed by readers (a) 1 and (b) 2.
Figure 6a:
Graphs show survival curves for patients stratified by pleural effusion (PE) laterality on CT images as assessed by readers (a) 1 and (b) 2.
Figure 5b:
Graphs show survival curves for patients stratified by presence of pleural metastasis on CT images as assessed by readers (a) 1 and (b) 2.
Figure 6b:
Graphs show survival curves for patients stratified by pleural effusion (PE) laterality on CT images as assessed by readers (a) 1 and (b) 2.
Results of Multivariate Analysis
The significant predictors of survival were found to be suboptimal debulking with residual disease larger than 1 cm (reader 1: hazard ratio [HR] = 2.06 [95% CI: 1.38, 3.08], P < .01; reader 2: HR = 2.10 [95% CI: 1.40, 3.16], P < .01); the presence of a moderate-to-large pleural effusion on CT images (reader 1: HR = 2.27 [95% CI: 1.31, 3.92], P < .01; reader 2: HR = 2.25 [95% CI: 1.26, 4.01], P = .02); preoperative serum CA-125 level, after Cox regression with pleural effusion (reader 1: HR = 0.86 [95% CI: 0.75, 0.98], P = .03; reader 2: HR = 0.85 [95% CI: 0.75, 0.98], P = .02); and ascites, after Cox regression with pleural effusion (reader 1: HR = 2.20 [95% CI: 1.37, 3.51], P < .01; reader 2: HR = 2.20 [95% CI: 1.38, 3.52], P < .01).
Patient age at the time of surgery and FIGO stage were not significant predictors of survival (Table 3), nor was pleural effusion laterality (reader 1: P = .36; reader 2: P = .48) at multivariate analysis (Table 4).
Table 3.
Multivariate Analysis of Survival with Pleural Effusion Classified as Absent, Small, or Moderate to Large

Data in parentheses are 95% CIs.
For an increase of 10 years.
For an increase by a multiple of e.
Table 4.
Multivariate Analysis of Survival with Pleural Effusion Classified by Laterality
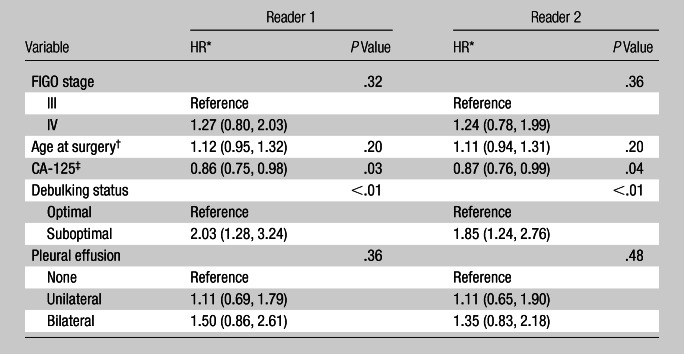
Data in parentheses are 95% CIs.
For an increase of 10 years.
For an increase by a multiple of e.
Discussion
For patients with epithelial ovarian cancer, stage is determined according to FIGO guidelines and is considered to be the main indicator of prognosis (15). Besides stage, several other prognostic factors have been identified, including age, the presence of ascites, and the amount of residual disease after surgery (16). Tumor grade has not been shown to affect prognosis and survival in ovarian cancer (P = .71) (17). Malignant pleural effusions are classified as stage IV; however, the simple presence of fluid in the pleural cavity is not. Results of a previous study (6) showed that when they are moderate or large in size, pleural effusions noted on CT images are more likely to be malignant; this finding was achieved on the basis of comparison to the results of thoracentesis. The accuracy of pleural fluid cytologic examination has previously been found to be between 40% and 87% (18–20).
In our study, the presence of a moderate-to-large pleural effusion on CT images was an adverse prognostic factor in patients with stage III or IV epithelial ovarian cancer when controlling for other variables. Since many patients who had pleural effusions in our study did not have the pleural fluid removed and evaluated for malignant cells, it is unclear if the poor prognosis associated with moderate-to-large effusions was owing to the size of the effusions, the likelihood that there were malignant cells in the effusions, the possibility of the effusions being associated with bulky intrathoracic disease, or a combination of the above. Clinical practice at our institution is now changing. Researchers in several recent clinical studies (10,12,13) (including video-assisted thoracic surgery studies) have demonstrated that the thorax is frequently involved in advanced ovarian cancer, harboring undiagnosed pleural disease at the time of diagnosis, and that this is likely to affect survival even in cases of optimal debulking.
At our institution, we now perform video-assisted thoracic surgery in patients with moderate-to-large pleural effusions. We have previously reported that as many as 40% of patients with advanced ovarian carcinoma and moderate-to-large pleural effusions may harbor gross intrathoracic disease, which is extremely useful information since patients with gross intrathoracic disease are triaged to intrathoracic cytoreduction or neoadjuvant chemotherapy depending on the resectability of the intrathoracic disease (12,13,21).
It is becoming increasingly clear that ovarian cancer is a disease that is amenable to local control and treatment, which distinguishes it from other cancers. To optimize survival, we must strive to identify the tumor deposits with imaging and to surgically eradicate the bulk of the tumor in all possible locations, aiming for optimal cytoreduction. Aletti et al (9) showed that patients with pleural effusion at initial diagnosis were more likely to have either pleural or chest disease at the time of first recurrence (56.3% vs 15.6%, P < .001) and at the time of last follow-up (74.2% vs 27.3%, P < .001). In the future, given the success of intraperitoneal chemotherapy for epithelial ovarian cancer, it may be reasonable to further investigate intrapleural chemotherapy in optimally debulked advanced ovarian carcinoma with documented malignant effusion (22,23).
Our study had a number of limitations. Selection bias may have occurred because we included only patients who had CT studies performed before primary cytoreductive surgery. We did not include patients who were treated with neoadjuvant chemotherapy or any other type of therapy, but since primary cytoreduction is currently our standard of care, we felt this was a reasonable choice. Additionally, we chose a somewhat arbitrary period between imaging and surgery of 45 days or less. However, we reviewed only the most recent imaging study for each patient because we wanted the interval between CT examination and surgery to be as short as possible so that the analysis of the relationship between imaging and surgical findings would be valid. Furthermore, our reference standard was limited, as most of the effusions identified on CT images did not have a pathologic diagnosis. Therefore, our results only indicated that, in patients with stage III or IV ovarian cancer, the presence of a moderate to large (but not a small) effusion on CT images was an adverse prognostic factor.
Another possible limitation was that we combined patients with stage III disease with those with stage IV disease in some of our analyses. We considered this reasonable since many patients with stage III disease and pleural effusions did not undergo throacentesis and consequently could have been understaged, whereas most patients with stage IV disease and pleural effusions underwent thoracentesis with the finding of malignant cells, which is what caused them to be assigned stage IV disease. We also combined stage III and IV disease because, for the past several years, stage IV disease has been managed similarly to stage III disease. Cytoreduction has been shown to improve overall outcome in patients with stage IV disease, particularly if based on positive pleural cytology, even though the optimal abdominal debulking rate is lower than that in patients with stage III disease, and optimal cytoreduction may not be possible for all patients (9). We also did not account for the effect of histologic tumor type on survival. However, the number of nonserous tumors was so low that meaningful subdivision was impossible (173 [85%] of the 203 patients had papillary-serous carcinoma).
To keep the number of eligible patients high, we used all available CT images, even though some were obtained at outside institutions with varying CT techniques. CT technology evolved substantially between 1997 and 2004; however, determination of the size and laterality of pleural effusion is unlikely to be affected by scanning technique or equipment. Detection of pleural metastasis could have been difficult on digitized images from outside institutions, and this could account for the low interreader agreement between the two radiologists. Most likely, positron emission tomography (PET)/CT is a more sensitive tool for the detection of pleural metastasis (24). Since a considerable number of the CT scans were obtained at outside institutions, it was not possible to apply a computer-assisted method of effusion volume estimation, which may be more accurate than the visual method that was used by the radiologists in our study.
In summary, we found that, in patients with stage III or IV epithelial ovarian cancer, the presence of a moderate-to-large pleural effusion on CT images was independently associated with poorer overall survival after controlling for age, stage, preoperative serum CA-125 level, and debulking status. In our study, readers of different experience levels displayed substantial agreement in determining the size of pleural effusion, suggesting that the assessment of this important imaging finding in patients with advanced epithelial ovarian cancer is reproducible. Therefore, when reading preoperative CT scans in patients with stage III or IV epithelial ovarian cancer, radiologists should be sure to report the presence and size of pleural effusions. Patients with moderate-to-large effusions may benefit from further evaluation with PET/CT imaging or even video-assisted thoracic surgery to detect pleural metastasis and facilitate appropriate management.
Advance in Knowledge.
In patients with stage III or IV epithelial ovarian cancer, a moderate-to-large pleural effusion on preoperative CT was independently associated with poorer overall survival after controlling for age, preoperative CA-125 level, surgical stage, presence of ascites, and cytoreductive status.
Implication for Patient Care.
When reading preoperative CT scans in patients with stage III or IV epithelial ovarian cancer, radiologists should be sure to report the presence and size of pleural effusions, pointedly noting any that are moderate to large.
Disclosures of Potential Conflicts of Interest: O.M. No potential conflicts of interest to disclose. N.M.I. No potential conflicts of interest to disclose. S.M. No potential conflicts of interest to disclose. H.A.V. No potential conflicts of interest to disclose. J.Z. No potential conflicts of interest to disclose. C.S.M. No potential conflicts of interest to disclose. Y.S. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a consultant for Genzyme. Other relationships: none to disclose. R.S.P. No potential conflicts of interest to disclose. D.S.C. No potential conflicts of interest to disclose. H.H. No potential conflicts of interest to disclose.
Acknowledgments
The authors thank Ada Muellner, MS, for editing the manuscript and Carolina Montalvo, BA, and Michael J. Sohn, BS, for preparing the figures.
Received January 22, 2010; revision requested February 12; revision received July 20; accepted July 30; final version accepted September 28.
Funding
This work was supported by the National Institutes of Health [grant number 5T32CA009529.
Abbreviations:
- CI
- confidence interval
- FIGO
- International Federation of Obstetrics and Gynecology
- HR
- hazard ratio
References
- 1.American Cancer Society Cancer facts & figures 2009. Atlanta, Ga: American Cancer Society, 2009 [Google Scholar]
- 2.Benedet JL, Bender H, Jones H, 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers: FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet 2000;70(2):209–262 [PubMed] [Google Scholar]
- 3.Ozols RF. Update on Gynecologic Oncology Group (GOG) trials in ovarian cancer. Cancer Invest 2004;22(suppl 2):11–20 [DOI] [PubMed] [Google Scholar]
- 4.Traill ZC, Davies RJ, Gleeson FV. Thoracic computed tomography in patients with suspected malignant pleural effusions. Clin Radiol 2001;56(3):193–196 [DOI] [PubMed] [Google Scholar]
- 5.Leung AN, Müller NL, Miller RR. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol 1990;154(3):487–492 [DOI] [PubMed] [Google Scholar]
- 6.Kim KW, Choi HJ, Kang S, et al. The utility of multi-detector computed tomography in the diagnosis of malignant pleural effusion in the patients with ovarian cancer. Eur J Radiol 2010;75(2):230–235 [DOI] [PubMed] [Google Scholar]
- 7.Bonnefoi H, A’Hern RP, Fisher C, et al. Natural history of stage IV epithelial ovarian cancer. J Clin Oncol 1999;17(3):767–775 [DOI] [PubMed] [Google Scholar]
- 8.Penson RT, Skates SJ, Fuller AJ, Jr, Seiden MV. Clinical course of stage IV epithelial ovarian cancer [letter]. J Clin Oncol 1999;17(10):3361–3362 [DOI] [PubMed] [Google Scholar]
- 9.Aletti GD, Podratz KC, Cliby WA, Gostout BS. Stage IV ovarian cancer: disease site-specific rationale for postoperative treatment. Gynecol Oncol 2009;112(1):22–27 [DOI] [PubMed] [Google Scholar]
- 10.Eitan R, Levine DA, Abu-Rustum N, et al. The clinical significance of malignant pleural effusions in patients with optimally debulked ovarian carcinoma. Cancer 2005;103(7):1397–1401 [DOI] [PubMed] [Google Scholar]
- 11.Copeland LJ. Epithelial ovarian carcinoma. In: DiSaia PJ, Creasman WT, eds. Clinical gynecologic oncology. 7th ed. Philadelphia, Pa: Mosby-Elsevier, 2007 [Google Scholar]
- 12.Chi DS, Abu-Rustum NR, Sonoda Y, et al. The benefit of video-assisted thoracoscopic surgery before planned abdominal exploration in patients with suspected advanced ovarian cancer and moderate to large pleural effusions. Gynecol Oncol 2004;94(2):307–311 [DOI] [PubMed] [Google Scholar]
- 13.Juretzka MM, Abu-Rustum NR, Sonoda Y, et al. The impact of video-assisted thoracic surgery (VATS) in patients with suspected advanced ovarian malignancies and pleural effusions. Gynecol Oncol 2007;104(3):670–674 [DOI] [PubMed] [Google Scholar]
- 14.Mergo PJ, Helmberger T, Didovic J, Cernigliaro J, Ros PR, Staab EV. New formula for quantification of pleural effusions from computed tomography. J Thorac Imaging 1999;14(2):122–125 [DOI] [PubMed] [Google Scholar]
- 15.Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary: FIGO 6th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet 2006;95(suppl 1):S161–S192 [DOI] [PubMed] [Google Scholar]
- 16.Curtin JP, Malik R, Venkatraman ES, Barakat RR, Hoskins WJ. Stage IV ovarian cancer: impact of surgical debulking. Gynecol Oncol 1997;64(1):9–12 [DOI] [PubMed] [Google Scholar]
- 17.Winter WE, 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 2007;25(24):3621–3627 [DOI] [PubMed] [Google Scholar]
- 18.Grunze H. The comparative diagnostic accuracy, efficiency and specificity of cytologic techniques used in the diagnosis of malignant neoplasm in serous effusions of the pleural and pericardial cavities. Acta Cytol 1964;8:150–163 [PubMed] [Google Scholar]
- 19.Sallach SM, Sallach JA, Vasquez E, Schultz L, Kvale P. Volume of pleural fluid required for diagnosis of pleural malignancy. Chest 2002;122(6):1913–1917 [DOI] [PubMed] [Google Scholar]
- 20.Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997;10(8):1907–1913 [DOI] [PubMed] [Google Scholar]
- 21.Diaz JP, Abu-Rustum NR, Sonoda Y, et al. Video-assisted thoracic surgery (VATS) evaluation of pleural effusions in patients with newly diagnosed advanced ovarian carcinoma can influence the primary management choice for these patients. Gynecol Oncol 2010;116(3):483–488 [DOI] [PubMed] [Google Scholar]
- 22.Tokumitsu S, Oka H, Nozaki A, et al. A case of ovarian cancer in which a remarkable effect was seen with intrathoracic administration of CDDP and CPT-11. [in Japanese]. Gan To Kagaku Ryoho 2008;35(12):2237–2238 [PubMed] [Google Scholar]
- 23.Mitamura T, Hosaka M, Takeda M, Watari H, Sakuragi N. Intrathoracic injection of paclitaxel for a patient with stage IV serous ovarian cancer: a case report. Cancer Chemother Pharmacol 2009;64(1):169–170 [DOI] [PubMed] [Google Scholar]
- 24.Orki A, Akin O, Tasci AE, et al. The role of positron emission tomography/computed tomography in the diagnosis of pleural diseases. Thorac Cardiovasc Surg 2009;57(4):217–221 [DOI] [PubMed] [Google Scholar]