Abstract
Recent in vitro studies have implicated galectin-3 as an important receptor in host recognition and response to specific Candida species; however its role in protection against disseminated candidiasis in vivo has not been evaluated. This study investigated the importance of galectin-3 in host defense against systemic infection with the highly virulent species Candida albicans (C. albicans), and the less virulent species, Candida parapsilosis (C. parapsilosis). Mice deficient in galectin-3 (gal3−/−) were more susceptible to infection than wild type (WT) mice. When infected with C. albicans, gal3−/− mice died significantly faster and exhibited a trend towards increased fungal burden and increased abscess formation in infected brains compared to WT mice. When infected with C. parapsilosis, gal3−/− mice had significantly higher renal fungal burdens and abscess formation compared to WT mice. To evaluate whether galectin-3 may contribute to susceptibility to candidiasis in human infants, galectin-3 levels in sera of newborn infants, a patient population uniquely susceptible to infections with both C. albicans and C. parapsilosis, were compared to serum galectin-3 levels of adults. Galectin-3 levels were significantly lower in newborn infant sera compared to adult sera. These data indicate that galectin-3 plays an important role in a murine model of disseminated candidiasis and suggest a potential mechanism of neonatal susceptibility to these infections.
Keywords: Galectin-3, Candida albicans, Candida parapsilosis, candidiasis, neonate
INTRODUCTION
Candida species are the fourth most common cause of nosocomial bloodstream infections and are the leading cause of invasive fungal disease [1,2]. Historically, C. albicans has been the leading cause of invasive fungal infections; however the frequency of infections caused by non-albicans species has dramatically increased [3–5]. Numerous studies have reported that infections caused by C. parapsilosis account for 15–67% of invasive candidiasis in premature newborn infants, and in some centers this species surpasses C. albicans as the predominant cause of neonatal candidiasis [6]. Interestingly, C. parapsilosis is often associated with lower mortality rates in the human host and is significantly less virulent than C. albicans in mouse models of infections [7–12]. Nevertheless, systemic infections caused by both species are associated with significant morbidity and mortality rates, even with antifungal treatment [2,4,13]. Understanding how the host defends against these infections will further our understanding of patient susceptibilities and aid in the development of novel therapeutics.
To defend against pathogenic fungi, the host’s immune system must respond to the invading pathogens through recognition of specific fungal pathogen associated molecular patterns (PAMPs). These PAMPs are recognized by pathogen recognition receptors (PRRs) which are found on a wide array of cells [14,15]. PRRs include C-type lectin receptors, toll-like receptors (TLRs), integrins, and scavenger receptors. Among C-type lectin receptors, dectin-1 recognizes β-(1,3)-glucan, dectin-2 recognizes high mannose structures, and the mannose receptor recognizes N-linked mannan. Among TLRs, TLR2 recognizes β-(1–2)-mannose structures and phospholipid mannan, TLR4 recognizes O-linked mannan, and TLR6 recognizes phospholipid mannan. Mac-1, a β2 integrin comprised of CD11b and CD18 subunits, recognizes and binds β-(1,6)-glucan and the mannoprotein, pH-regulated antigen-1 protein (Prap10). Recognition of Candida by these receptors leads to a wide array of responses that influence both innate and adaptive immunity.
Recently, galectin-3, an S-type lectin receptor which recognizes β-(1–2) oligomannan [16,17], has emerged as an important receptor responsible for distinguishing non-pathogenic fungi from pathogenic fungi [18,19]. In addition, galectin-3 kills Candida species expressing specific β-(1–2) oligomannan epitopes [17]. Galectin-3 is found in numerous cell types and influences the function of many innate immune cells including neutrophils, monocytes, macrophages, endothelial cells, and epithelial cells [20,21] and can be secreted into the microenvironment by activated or damaged cells [22]. Galectin-3 plays a critical role in influencing immunity to many different types of microbial infections [23–27]; however, its role in host protection during disseminated candidiasis has not been evaluated.
Numerous studies have utilized knockout mice to elucidate the roles of specific receptors and downstream signaling components in host defense against disseminated candidiasis, including TLR1, TLR2, TLR4, TLR6, TLR9 dectin-1, dectin-2, mac-1, myD88, and Vav proteins [28–38]. In this study, we evaluate the role of galectin-3 during disseminated candidiasis by infecting mice deficient in galectin-3 (gal3−/−) with C. albicans. Mice were also infected with C. parapsilosis because of its increased prevalence in immunocompromised patients, especially the neonatal population. We demonstrate that galectin-3 plays an important role in host defense against disseminated candidiasis when mice are infected with either C. albicans or C. parapsilosis.
MATERIALS AND METHODS
Growth, Maintenance, and Preparation of Organisms
C. albicans strain SC5314 and a C. parapsilosis clinical isolate, 15-72391-101 [39], referred to here as JMB81, were used throughout this study. Strains were maintained on YPD plates (1% yeast extract, 2% peptone, 2% dextrose, 2% agar). To prepare strains for injection, overnight cultures were grown for 16 hrs at 37°C with vigorous agitation in YPD broth. Cells were harvested and washed six times by centrifugation in sterile saline. C. albicans was adjusted to 5 × 106 cells/ml and C. parapsilosis was adjusted to 5 × 108 cells/ml in sterile saline. Prepared cell suspensions were used immediately to infect mice. Suspension concentrations were confirmed by serial dilution onto YPD plates incubated at 37°C for 48 hours.
Murine Model of Disseminated Candidiasis
Following review and approval by the Institutional Animal Welfare Committee, 4–8 week old female gal3−/− mice (B6.Cg-Lgals3 tm Poi/J) and their WT counterparts (C57BL/6J) were obtained from Jackson Laboratories. Mice were infected with 1 × 105 CFU of C. albicans strain SC5314 or 1 × 108 CFU of C. parapsilosis strain JMB81 by injecting 200 μl of the cell suspension prepared above via tail vein. Survival was monitored up to 21 days post infection. All procedures involving animals conformed to the ILAR Guide for the Care and Use of Laboratory Animals (2011 edition) of the Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council.
Fungal Burden Analysis of Harvested Organs
To determine the fungal burdens of mice infected with C. albicans, mice were euthanized at 3 days post infection or when moribund and organs were harvested for CFU counts. To determine fungal burdens of mice infected with C. parapsilosis, mice were euthanized 3, 7 and 21 days post infection. Harvested organs were weighed, placed in 1 ml of sterile saline, and homogenized using a FastPrep®-24 Tissue and Cell Homogenizer (MP Biomedicals) with Lysing Matrix D. Homogenized organs were serially diluted and plated onto YPD plates containing streptomycin (100 μg/ml) and ampicillin (50 μg/ml). Inoculated plates were incubated at 37°C for 48 to 72 hours before enumerating CFUs. Fungal burden was expressed as the number of CFU/ml/gram of harvested organ.
Histological Examinations of Harvested Organs
Brains and kidneys of infected mice were fixed in 10% formalin at time of death. Fixed organs were sectioned and stained with Grocott’s methenamine silver (GMS) or hematoxylin and eosin (H&E). For H&E stained slides, the number of brain and renal abscesses were counted during microscopic examination at high magnification and expressed as the number of abscesses per tissue section.
Galectin-3 ELISA
Following review and approval by the Institutional Review Board, sera from peripheral blood of healthy adult volunteers and umbilical cord blood of newborns immediately following delivery of the placenta were separated in SST tubes (BD, Franklin Lakes, NJ) and stored at −80°C until use. Serum galectin-3 levels were quantified using the human galectin-3 Quantikine® enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer’s instructions.
Statistics
For parametric variables, comparisons were made by ANOVA. Between-group comparisons were made by the Holm-Sidak test. P values ≤ 0.05 were considered significant. Tests appropriate for nonparametric variables were used as indicated.
RESULTS
Deficiency of galectin-3 decreases survival of mice infected with C. albicans, but not C. parapsilosis
Wild type (WT) control mice or mice deficient in galectin-3 (gal3−/−) were infected with 1×105 CFU of C. albicans or 1×108 CFU of C. parapsilosis via tail vein injection. Survival was monitored for up to 21 days. Gal3−/− mice infected with C. albicans died significantly faster than WT mice (Figure 1A) with a mean survival rate of 6.2±5.2 days and 14.3±4.0 days, respectively. No significant difference in survival was observed when mice were infected with C. parapsilosis (Figure 1B). These data suggest that the absence of galectin-3 increases mortality in mice infected with C. albicans but not C. parapsilosis.
Figure 1. Kaplan-Meier survival curves of WT and gal3−/− mice infected with C. albicans and C. parapsilosis.
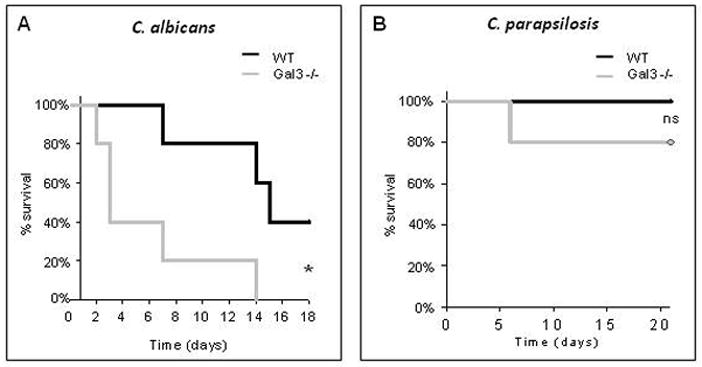
Kaplan-Meier survival curve of WT and gal3−/− mice infected with 1×105 CFU of C. albicans (A) or 1×108 CFU of C. parapsilosis (B) via tail vein injection. N = 5 mice per group. *p < 0.02 by log rank test. ns = not significant, p=0.32 by log rank test.
When infected with C. albicans, WT and gal3−/− mice exhibit different renal fungal distribution patterns and histopathology
There was no difference in mean fungal burden at time of death in kidneys of WT and gal3−/− mice infected with C. albicans, although WT animals had considerably wider variability in colony counts than gal3−/− animals (Figure 2A). Because the variability in time of death confounded assessment of tissue fungal burden, a subset of animals in each group was euthanized on post infection day 3 when all animals were still alive. WT animals had significantly lower kidney fungal burden at day 3 relative to gal3−/− animals (Figure 2A). Interestingly, the distribution of fungal elements in kidneys of WT mice was remarkably different from that of infected gal3−/− mice. Most noticeable was the formation of large fungal balls in the renal pelvis of WT mice (Figure 2B), while the majority of gal3−/− mice exhibited fungal distribution throughout the kidney (Figure 2C). Four out of the five (80%) WT mice exhibited pelvic fungal balls with no observable fungus in the fifth mouse. Two of the five (40%) infected gal3−/− mice exhibited fungal ball formation while the remaining three (60%) exhibited fungal distribution throughout the kidney. Both fungal distribution patterns were associated with yeast and hyphal morphologies (Figure 2D–E).
Figure 2. Fungal burden and distribution in kidneys infected with C. albicans.
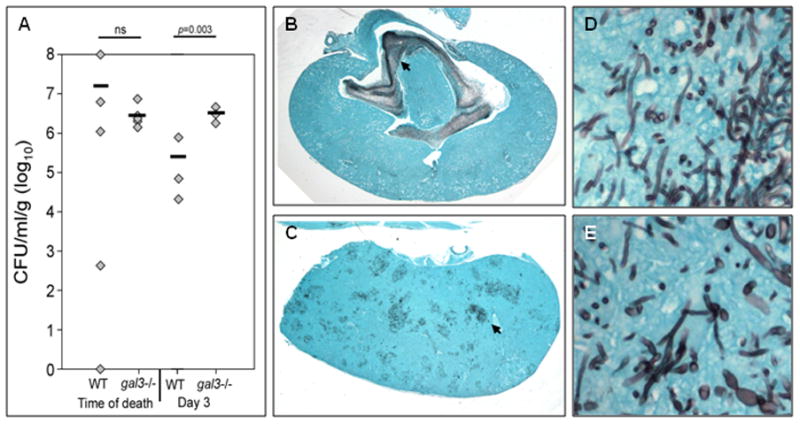
WT and gal3−/− mice were infected with 1×105 CFU of C. albicans via tail vein injection. (A) Kidney fungal burden of infected WT and gal3−/− mice at time of death and at day 3 post-infection. Comparisons of fungal burdens were made by Mann-Whitney rank-sum test when there were sufficient cases for ranking, or a negative binomial model where group n=3. Bar represents mean CFU. Low magnification view of GMS stained kidney sections of infected WT (B) and gal3−/− mice (C) at time of death. Fungal elements appear black. Black arrows point to dense areas of fungus. High magnification (×600) of GMS stain of kidney of infected WT (D) and gal3−/− (E) mouse demonstrating yeast and hyphal morphologies.
Pelvic fungal balls were associated with renal papillary necrosis and diffuse dilatation of collecting and more proximal tubules, consistent with obstructive uropathy (Figure 3A and B). Fungal balls were also accompanied by a dense influx of immune cells composed mainly of neutrophils and monocytes. In comparison, the gal3−/− mice with fungal distribution throughout the kidney also exhibited widely distributed abscesses in the renal cortex (Figure 3C and D). These data suggest that the presence of galectin-3 may influence the distribution and dissemination of C. albicans in infected kidneys. However, because the majority of gal3−/− mice succumbed to infection faster than the WT mice, the possibility that differences in fungal distribution patterns may be a result of different stages of disease progression cannot be excluded.
Figure 3. Histopathology of mouse kidneys infected with C. albicans.
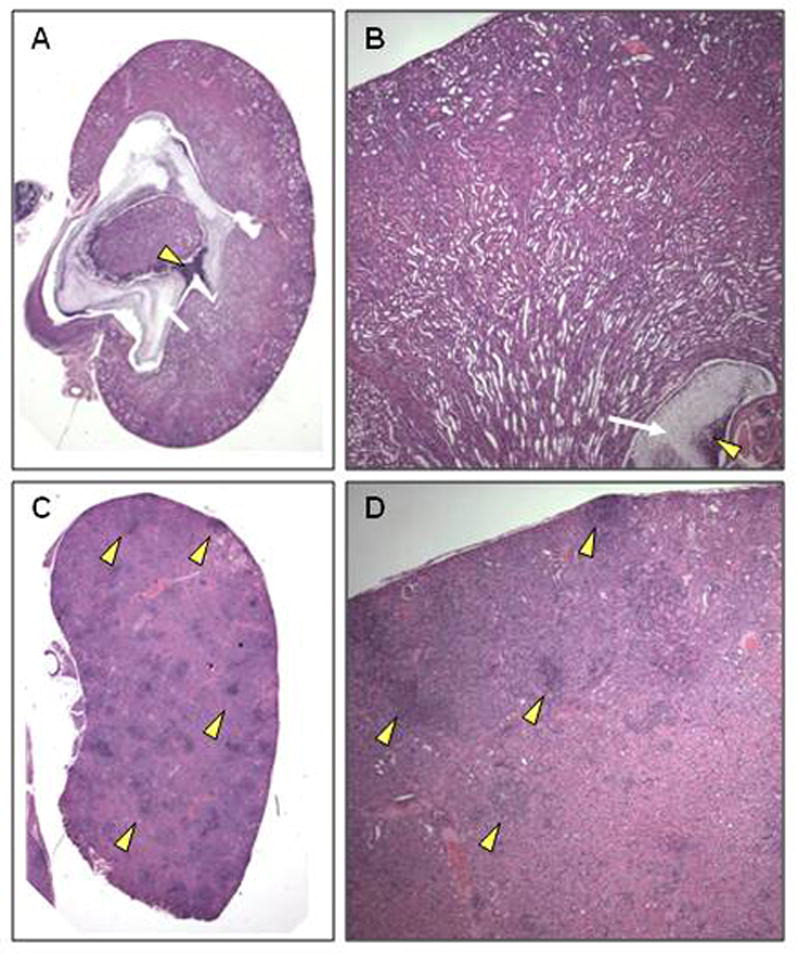
WT and gal3−/− mice were infected with 1×105 CFU of C. albicans via tail vein infection. (A) Low magnification view of hematoxylin and eosin (H&E) stained kidney sections of infected WT mouse at time of death. White arrow indicates large fungal ball and yellow arrow head indicates dense leukocytic infiltrate surrounding it. (B) Higher magnification (×40) view of H&E stain of infected WT kidney at time of death demonstrating the presence of a large fungal ball (white arrow) occluding the renal pelvis surrounded by dense leukocyte infiltrate (yellow arrow head). Renal papillary necrosis is noted. (C) Low magnification view of H&E stained kidney sections of infected gal3−/− mouse at time of death demonstrating numerous renal abscesses (yellow arrow heads). (D) Higher magnification (×40) view of H&E stained infected gal3−/− kidney at time of death demonstrating the presence of large and numerous fungal abscesses (yellow arrow heads).
Galectin-3 protects kidneys when infected with C. parapsilosis
Although there was no significant difference in mortality between the WT and gal3−/− mice infected with C. parapsilosis, kidneys from gal3−/− mice had significantly higher fungal burdens 3 and 7 days post infection, and a trend towards higher fungal burden on day 21 (Figure 4A). Increased fungal burden was easily apparent on histological examination of kidney sections. Aggregates of C. parapsilosis yeast were seen throughout the renal cortex of infected gal3−/− kidneys at low magnification (Figure 4C). These aggregates were not observed in infected WT kidneys (Figure 4B). The fungal distribution in kidneys of mice infected with C. parapsilosis was also remarkably different from that of C. albicans. On higher magnification, C. parapsilosis yeast were detected in infected WT kidneys (Figure 4D), but were most often observed as single yeast or small aggregates scattered in the interstitium. In contrast, closer examination of infected gal3−/− kidneys revealed large intravascular conglomerates of yeast (Figure 4E).
Figure 4. Fungal burden and distribution in kidneys infected with C. parapsilosis.
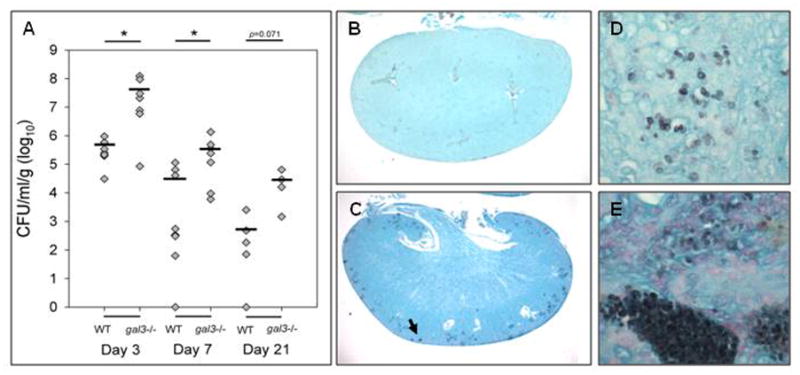
WT and gal3−/− mice were infected with 1×108 CFU of C. parapsilosis via tail vein injection. (A) Fungal burden of kidneys of WT and gal3−/− mice; 3, 7 and 21 days post infection. Bar represents mean CFU. Comparisons of fungal burdens were made by Wilcoxon rank-sum test, *p<0.05. Low magnification view of GMS stained kidney sections of infected WT (B) and gal3−/− (C) mice 3 days post infection. Fungal elements appear black. Black arrow indicates dense area of yeast. Higher magnification (×600) of yeast of infected WT (D) and gal3−/− (E) mice demonstrating yeast present in renal parenchyma.
H&E stains of infected kidneys also revealed an increased number of renal abscesses in infected gal3−/− kidneys compared to WT kidneys (Figure 5A). Inflammation was mainly localized to the renal cortex. On low magnification, renal abscesses are easily seen scattered throughout the renal cortex of infected gal3−/− kidneys (Figure 5C) but not WT kidneys (Figure 5B). On higher magnification, areas of loose abscess formation, comprised of mixed lymphohistiocytic and neutrophilic infiltrates, occasionally associated with frank granulomatous reactions, were noted in WT and gal3−/− mice, however they were much larger and more numerous in gal3−/− mice (Figure 5E) compared to WT (Figure 5D). Abscesses in WT and gal3−/− kidneys often surrounded glomeruli and renal vessels. Taken together, these data suggest that the presence of galectin-3 affords some protection in kidneys when mice are infected with C. parapsilosis.
Figure 5. Histopathology of mouse kidneys infected with C. parapsilosis.
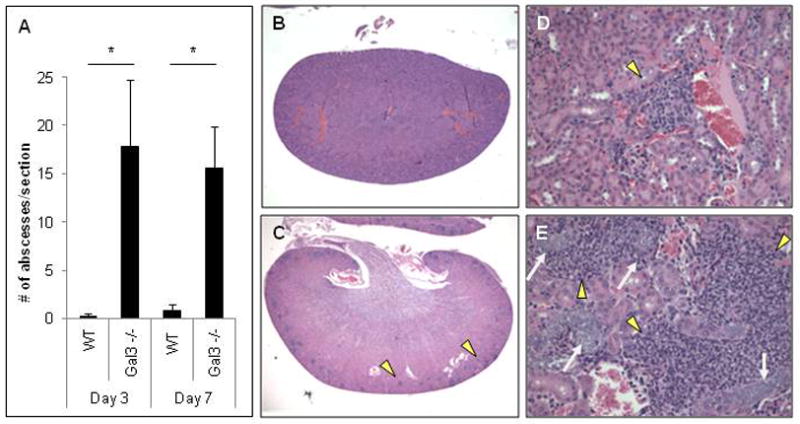
WT and gal3−/− mice were infected with 1×108 CFU of C. parapsilosis via tail vein injection. (A) Number of renal abscesses per kidney section of infected WT and gal3−/− mice 3 and 7 days post infection. Results are mean ± SEM. Comparisons of renal abscesses were made by Kruskal-Wallis ANOVA on Ranks, *p<0.05. Low magnification view of H&E stains of kidney sections from infected WT (B) and gal3−/− mice (C) 3 days post infection. Yellow arrow heads indicate abscesses. High magnification (×200) of H&E stained kidney sections of infected WT (D) and gal3−/− (E) mice 3 days post infection. Yellow arrow heads indicate abscesses. White arrows point to large conglomerates of yeast within renal vessels.
Galectin-3 protects brains when infected with C. albicans
Compared to WT mice, gal3−/− mice infected with C. albicans exhibited a trend toward higher brain fungal burden at time of death (Figure 6A). In the animals euthanized on post infection day 3, gal3−/− mice had significantly increased fungal burden relative to WT (Figure 6A). Both infected WT and gal3−/− brains exhibited yeast and hyphal morphologies (Figure 6B), albeit at a higher frequency in gal3−/− animals. Additionally, abscesses were observed in both infected WT and gal3−/− brains (Figure 6D), however gal3−/− brains exhibited a trend toward increased number of abscesses at time of death (Figure 6C). These observations suggest that galectin-3 may play a role in protecting brains during infection with C. albicans.
Figure 6. Fungal burden, fungal distribution, and histopathology of brains infected with C. albicans.
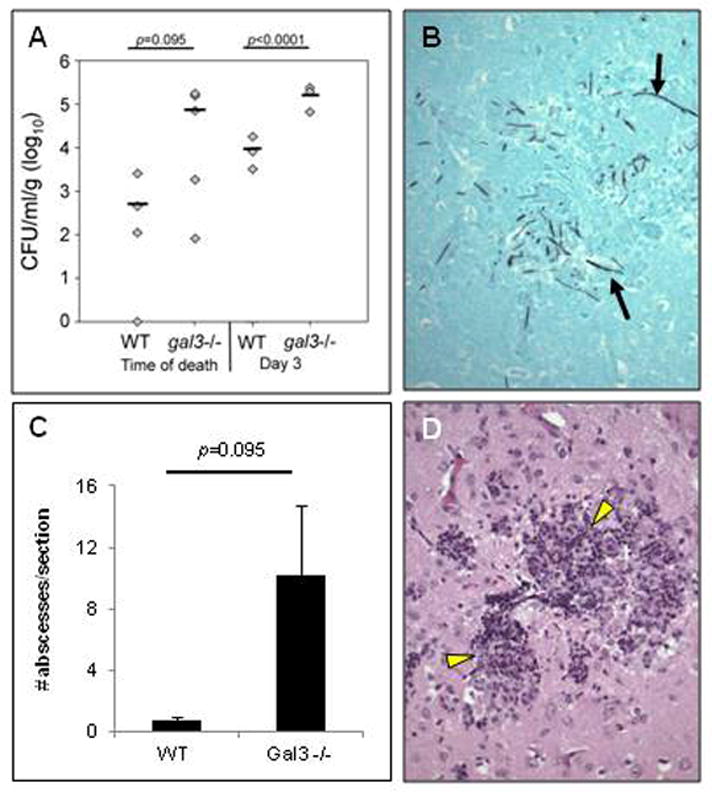
WT and gal3−/− mice were infected with 1×105 CFU of C. albicans via tail vein injection. (A) Brain fungal burden from WT and gal3−/− mice at time of death and at day 3 post-infection. Bar represents mean CFU. (B) High magnification (×200) of GMS stain of brain section from an infected gal3−/− mouse at time of death. Black arrows indicate hyphae. (C) Number of brain abscesses per section from WT and gal3−/− mice at time of death. Results are means ± SEM. (D) High magnification (×200) of H&E stained brain section of gal3−/− mice at time of death. Yellow arrow heads indicate abscesses. Comparisons of fungal burdens and abscesses were made by Mann-Whitney rank-sum test when there were sufficient cases for ranking, or a negative binomial model where group n=3.
C. parapsilosis infects the brain during disseminated candidiasis
The brains of WT and gal3−/− mice infected with C. parapsilosis had high fungal burdens on days 3 and 7 post infection (Figure 7A). In addition, yeast were still detected 21 days post infection in some mice. C. parapsilosis yeast had successfully traversed from blood vessels into brain parenchyma (Figure 7B). However, no significant difference in brain fungal burden was observed between infected gal3−/− and WT mice on days 3, 7 or 21 post infection (Figure 7A). Similar patterns of yeast dissemination in WT and gal3−/− mice were seen (Figure 7B). Small abscesses were present in WT and gal3−/− mice (Figure 7C), but the number and size of abscesses were similar (data not shown). Taken together, these data indicate that C. parapsilosis is capable of infecting brain tissue during disseminated candidiasis; however, galectin-3 does not play a role in protecting mice from this process.
Figure 7. Fungal burden, fungal distribution, and histopathology of brains infected with C. parapsilosis.
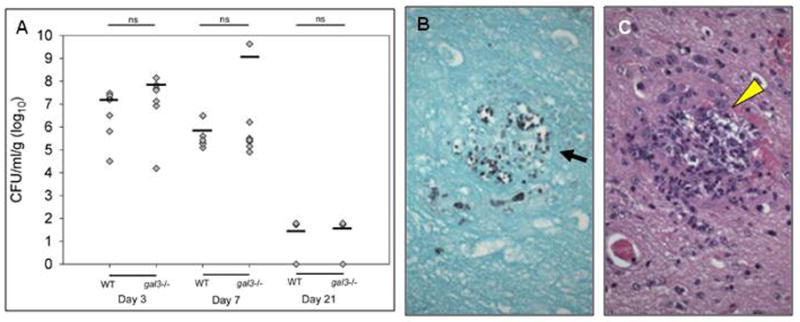
WT and gal3−/− mice were infected with 1×108 CFU of C. parapsilosis via tail vein injection. (A) Brain fungal burden from WT and gal3−/− mice at 3, 7, and 21 days post infection. Bars represent mean CFU. Comparisons of fungal burden were made by Wilcoxon rank-sum test. (B) High magnification (×400) of GMS stained brain section of infected gal3−/− mouse 3 days post infection. Black arrow indicates yeast. (C) High magnification (×600) of H&E stain of brain section of gal3−/− mice 3 days post infection. Yellow arrow heads indicate abscesses. Yeast were observed intravascularly, often outlining the contours of capillaries or larger vessels. Yeast were also observed in the parenchyma.
Term and preterm infants have reduced serum galectin-3 levels at birth compared to adults
Because of the role galectin-3 played in protecting mice from disseminated candidiasis, the levels of galectin-3 was evaluated in newborn infants; a human patient population especially vulnerable to candidiasis. Premature infants are at increased risk for invasive candidiasis and mucocutaneous candidiasis is prevalent in healthy, term newborns. To compare levels of galectin-3 in newborns to adults, serum samples from umbilical cord blood were collected immediately following delivery of term and preterm newborns, and galectin-3 concentrations was compared to serum samples collected from healthy adult volunteers (n=13). Newborns were separated into groups based on gestational age, term (≥ 37 weeks, n=12), 28 –36 weeks (n=8), or <28 weeks (n=6). When compared to adults, serum galectin-3 levels were significantly lower in all neonatal groups (Figure 8). These data suggest that the increased susceptibility of infants to Candida infections may be due, in part, to reduced galectin-3 levels.
Figure 8. Serum levels of galectin-3 are reduced in umbilical cord blood of newborns.
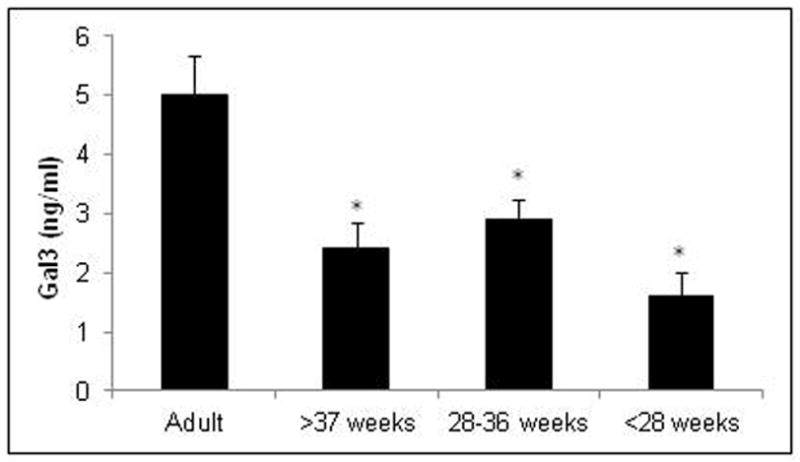
Detection of galectin-3 by ELISA in sera collected from peripheral blood of healthy adults and cord blood of neonates with gestational ages of 37 weeks or more (≥ 37 weeks), between 28 and 36 weeks (28–36 weeks), or less than 28 weeks (< 28 weeks). Results are means ± SEM. *p < 0.05.
DISCUSSION
By infecting mice deficient in galectin-3 with C. albicans and C. parapsilosis, we have demonstrated that galectin-3 plays an important role in host defense against disseminated disease with both species. When infected with C. parapsilosis, gal3−/− mice had significantly higher renal fungal burdens and increased inflammation when compared to wild type (WT) mice. When infected with C. albicans, gal3−/− mice died significantly faster than WT mice. It is unlikely that the increased susceptibility of gal3−/− mice was due to an overall decrease in gal3−/− mouse fitness, as gal3−/− mice infected with Trypansosoma brucei [40], Salmonella [41] and Rhodococcus equi [24] are more resistant to infection than their WT counterparts. One limitation of this study is the relatively small number of animals used, and these findings should be confirmed in larger studies.
The increased susceptibility of gal3−/− mice to disseminated candidiasis is likely a result of the absence of galectin-3 in multiple different cell types including macrophages, neutrophils, endothelial and epithelial cells. Both endogenous and exogenous sources of galectin-3 influence cell behavior and contribute to adaptive and innate immune modulation. By associating with TLR2 and dectin-1 in macrophages, galectin-3 has been shown to be necessary for appropriate TNF-α production when confronted with C. albicans [18,19]; therefore, reduction in galectin-3 in these cell types may significantly impact immune recruitment and regulation during Candida infections. Galectin-3 has also been shown to play an important role in neutrophil effector functions including production of reactive oxygen species, degranulation and increased phagocytosis [25,42–45]; therefore the absence of galectin-3 may severely impact neutrophil function during infection as well. Although others have found that the increased susceptibility of gal3−/− mice to streptococcal pneumonia is due to decreased neutrophil extravasation and recruitment [26,46], gal3−/− mice infected with Candida had an exaggerated amount of kidney and brain inflammatory infiltrates relative to WT mice, indicating that this mechanism may not be involved in gal3−/− mice susceptibility to disseminated candidiasis. Stimulated macrophages and infected endothelial and epithelial cells also secrete galectin-3 [46–49]. As galectin-3 has been reported to be fungicidal [17], galectin-3 secreted into the microenvironment of infected tissues may play an important role in fungal clearance. This effect may partially explain the lower kidney and brain fungal burden on day 3 in wild-type animals infected with C. albicans relative to galectin-3 deficient mice. In addition, because galectin-3 has been reported to bind to Candida species bearing specific β(1–2) linked mannose residues [16,17], galectin-3 expression by any cell type may significantly contribute to that cell’s recognition and response to Candida. Another possible mechanism leading to increased susceptibility in galectin-3 deficient animals is an exacerbated and poorly controlled inflammatory response to the infectious stimulus. Galectin-3 has been shown to be a negative regulator in the setting of inflammation induced by lipopolysaccharide (LPS), and galectin-3 deficient mice have increased susceptibility to LPS-induced shock [41]. This mechanism would also be consistent with our observation of increased abscess formation in kidneys of gal3−/− animals. Overall, the findings of this study can provide little insight to support specific mechanism(s) of susceptibility, and additional studies into the role of galectin-3 in fungal immunity are ongoing and needed.
Interestingly, disseminated disease caused by C. parapsilosis was distinct from disseminated disease caused by C. albicans. During infection with C. parapsilosis, dissemination was mainly limited to the renal cortex and often surrounded glomeruli and renal vessels. Formation of renal abscesses around C. parapsilosis yeast was also observed in both WT and gal3−/− mice, but they were larger and more numerous in gal3−/− mice. In comparison, dissemination of C. albicans in infected mice was characterized by formation of large fungal balls in the renal pelvis. Interestingly, while the majority of WT mice exhibited pelvic fungal balls, the majority of the gal3−/− mice had yeast distributed throughout the kidney. Taken together, these findings suggest that prolonged survival may be secondary to early control of fungal growth by galectin-3, based on the differences in tissue burden seen on day 3, and may also relate to the differing distribution of fungal elements within the organ. Deficiency of galectin-3 leads to disease throughout the renal parenchyma, which likely impairs renal function more acutely that the obstructive uropathy associated with fungal balls in the renal pelvis. Differences in the dissemination patterns of these separate species underline the importance of studying the host response to Candida in a species specific manner.
Galectin-3 expression has recently been implicated to play a possible role in neonatal defense against invasive infectious disease [50]. This study demonstrated a positive correlation between gestational age and galectin-3 levels in cultured whole cord blood from newborn infants. As gestational age decreased, so did galectin-3 expression levels. They also demonstrated that exposure to an invasive Streptococcus agalactiae isolate, but not a commensal isolate, increased galectin-3 expression in whole blood. Our data support these results by demonstrating that serum galectin-3 levels are significantly lower in neonates than healthy adults. The finding of reduced serum galectin-3 in a population at risk for mucocutaneous and/or disseminated candidiasis makes it tempting to speculate that galectin-3 deficiency contributes to their susceptibility, but considerably more study is needed to establish this relationship. In addition, although galectin-3 levels were reduced in neonatal serum, the contribution of specific cell types is unknown. It is unclear if the reduced galectin-3 expression is a result of suppressed cell function or if soluble factors somehow influenced expression. Insight into this process will lead to better understanding of neonatal immunity in general. Data from our group suggest that neonatal mice are more susceptible to Candida infection than adult mice [51]. In addition, increased susceptibility of neonates to Candida has been confirmed in a rat model of neonatal candidiasis [52]. Although the contribution of galectin-3 to the increased susceptibility of neonatal mice and rats to Candida infection remains to be evaluated, both models hold promise for being essential tools in understanding the role of galectin-3 in neonatal immunity. In addition, both models could be used to develop new strategies targeting galectin-3 expression in treatment and prevention of invasive candidasis. Additional research could lead to development of novel therapeutic strategies for candidiasis in neonates and other patients at risk.
Acknowledgments
Many thanks to Richard Tucker for assistance with statistical analysis and the Eunice Kennedy Shriver NICHD Neonatal Research Network for supplying clinical isolate 14-72391-101. This project was supported by grants from the National Center for Research Resources (5P20RR018728-10) and the National Institute of General Medical Sciences (8P20GM103537-10) from the National Institutes of Health.
Footnotes
Conflict of Interest: None.
References
- 1.Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239–244. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blyth CC, Chen SC, Slavin MA, et al. Not just little adults: candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Pediatrics. 2009;123:1360–1368. doi: 10.1542/peds.2008-2055. [DOI] [PubMed] [Google Scholar]
- 4.Bassetti M, Mikulska M, Viscoli C. Bench-to-bedside review: therapeutic management of invasive candidiasis in the intensive care unit. Crit Care. 2010;14:244. doi: 10.1186/cc9239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falagas ME, Roussos N, Vardakas KZ. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: a systematic review. Int J Infect Dis. 2010;14:e954–66. doi: 10.1016/j.ijid.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Spiliopoulou A, Dimitriou G, Jelastopulu E, Giannakopoulos I, Anastassiou ED, Christofidou M. Neonatal Intensive Care Unit Candidemia: Epidemiology, Risk Factors, Outcome, and Critical Review of Published Case Series. Mycopathologia. 2011 doi: 10.1007/s11046-011-9498-3. [DOI] [PubMed] [Google Scholar]
- 7.Bassetti M, Taramasso L, Nicco E, Molinari MP, Mussap M, Viscoli C. Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS One. 2011;6:e24198. doi: 10.1371/journal.pone.0024198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arendrup M, Horn T, Frimodt-Moller N. In vivo pathogenicity of eight medically relevant Candida species in an animal model. Infection. 2002;30:286–291. doi: 10.1007/s15010-002-2131-0. [DOI] [PubMed] [Google Scholar]
- 9.Ortega M, Marco F, Soriano A, et al. Candida species bloodstream infection: epidemiology and outcome in a single institution from 1991 to 2008. J Hosp Infect. 2011;77:157–161. doi: 10.1016/j.jhin.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Arendrup MC, Sulim S, Holm A, et al. Diagnostic issues, clinical characteristics, and outcomes for patients with fungemia. J Clin Microbiol. 2011;49:3300–3308. doi: 10.1128/JCM.00179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas PG, Rex JH, Lee J, et al. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis. 2003;37:634–643. doi: 10.1086/376906. [DOI] [PubMed] [Google Scholar]
- 12.Mellado E, Cuenca-Estrella M, Regadera J, Gonzalez M, Diaz-Guerra TM, Rodriguez-Tudela JL. Sustained gastrointestinal colonization and systemic dissemination by Candida albicans, Candida tropicalis and Candida parapsilosis in adult mice. Diagn Microbiol Infect Dis. 2000;38:21–28. doi: 10.1016/s0732-8893(00)00165-6. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 14.Cheng SC, Joosten LA, Kullberg BJ, Netea MG. Interplay between Candida albicans and the mammalian innate host defense. Infect Immun. 2012;80:1304–1313. doi: 10.1128/IAI.06146-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 16.Fradin C, Poulain D, Jouault T. beta-1,2-linked oligomannosides from Candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. Infect Immun. 2000;68:4391–4398. doi: 10.1128/iai.68.8.4391-4398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohatsu L, Hsu DK, Jegalian AG, Liu FT, Baum LG. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J Immunol. 2006;177:4718–4726. doi: 10.4049/jimmunol.177.7.4718. [DOI] [PubMed] [Google Scholar]
- 18.Jouault T, El Abed-El Behi M, Martinez-Esparza M, et al. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol. 2006;177:4679–4687. doi: 10.4049/jimmunol.177.7.4679. [DOI] [PubMed] [Google Scholar]
- 19.Esteban A, Popp MW, Vyas VK, Strijbis K, Ploegh HL, Fink GR. Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc Natl Acad Sci U S A. 2011;108:14270–14275. doi: 10.1073/pnas.1111415108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160–171. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 21.Sato S, St-Pierre C, Bhaumik P, Nieminen J. Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs) Immunol Rev. 2009;230:172–187. doi: 10.1111/j.1600-065X.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 22.Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473:172–185. doi: 10.1016/s0304-4165(99)00177-4. [DOI] [PubMed] [Google Scholar]
- 23.Bernardes ES, Silva NM, Ruas LP, et al. Toxoplasma gondii infection reveals a novel regulatory role for galectin-3 in the interface of innate and adaptive immunity. Am J Pathol. 2006;168:1910–1920. doi: 10.2353/ajpath.2006.050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferraz LC, Bernardes ES, Oliveira AF, et al. Lack of galectin-3 alters the balance of innate immune cytokines and confers resistance to Rhodococcus equi infection. Eur J Immunol. 2008;38:2762–2775. doi: 10.1002/eji.200737986. [DOI] [PubMed] [Google Scholar]
- 25.Farnworth SL, Henderson NC, Mackinnon AC, et al. Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function. Am J Pathol. 2008;172:395–405. doi: 10.2353/ajpath.2008.070870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieminen J, St-Pierre C, Bhaumik P, Poirier F, Sato S. Role of galectin-3 in leukocyte recruitment in a murine model of lung infection by Streptococcus pneumoniae. J Immunol. 2008;180:2466–2473. doi: 10.4049/jimmunol.180.4.2466. [DOI] [PubMed] [Google Scholar]
- 27.Ruas LP, Bernardes ES, Fermino ML, et al. Lack of galectin-3 drives response to Paracoccidioides brasiliensis toward a Th2-biased immunity. PLoS One. 2009;4:e4519. doi: 10.1371/journal.pone.0004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Netea MG, van de Veerdonk F, Verschueren I, van der Meer JW, Kullberg BJ. Role of TLR1 and TLR6 in the host defense against disseminated candidiasis. FEMS Immunol Med Microbiol. 2008;52:118–123. doi: 10.1111/j.1574-695X.2007.00353.x. [DOI] [PubMed] [Google Scholar]
- 29.Murciano C, Villamon E, Gozalbo D, Roig P, O’Connor JE, Gil ML. Toll-like receptor 4 defective mice carrying point or null mutations do not show increased susceptibility to Candida albicans in a model of hematogenously disseminated infection. Med Mycol. 2006;44:149–157. doi: 10.1080/13693780500294733. [DOI] [PubMed] [Google Scholar]
- 30.Netea MG, Van Der Graaf CA, Vonk AG, Verschueren I, Van Der Meer JW, Kullberg BJ. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis. 2002;185:1483–1489. doi: 10.1086/340511. [DOI] [PubMed] [Google Scholar]
- 31.Netea MG, Sutmuller R, Hermann C, et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004;172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 32.Bellocchio S, Montagnoli C, Bozza S, et al. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Utomo A, Cullere X, et al. The beta-Glucan Receptor Dectin-1 Activates the Integrin Mac-1 in Neutrophils via Vav Protein Signaling to Promote Candida albicans Clearance. Cell Host Microbe. 2011;10:603–615. doi: 10.1016/j.chom.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van de Veerdonk FL, Netea MG, Jansen TJ, et al. Redundant role of TLR9 for anti-Candida host defense. Immunobiology. 2008;213:613–620. doi: 10.1016/j.imbio.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Villamon E, Gozalbo D, Roig P, O’Connor JE, Fradelizi D, Gil ML. Toll-like receptor-2 is essential in murine defenses against Candida albicans infections. Microbes Infect. 2004;6:1–7. doi: 10.1016/j.micinf.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Saijo S, Fujikado N, Furuta T, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 37.Taylor PR, Tsoni SV, Willment JA, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saijo S, Ikeda S, Yamabe K, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Benjamin DK, Jr, Stoll BJ, Gantz MG, et al. Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics. 2010;126:e865–73. doi: 10.1542/peds.2009-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vankrunkelsven A, De Ceulaer K, Hsu D, Liu FT, De Baetselier P, Stijlemans B. Lack of galectin-3 alleviates trypanosomiasis-associated anemia of inflammation. Immunobiology. 2010;215:833–841. doi: 10.1016/j.imbio.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Komai-Koma M, Gilchrist DS, et al. Galectin-3 is a negative regulator of lipopolysaccharide-mediated inflammation. J Immunol. 2008;181:2781–2789. doi: 10.4049/jimmunol.181.4.2781. [DOI] [PubMed] [Google Scholar]
- 42.Alves CM, Silva DA, Azzolini AE, et al. Galectin-3 plays a modulatory role in the life span and activation of murine neutrophils during early Toxoplasma gondii infection. Immunobiology. 2010;215:475–485. doi: 10.1016/j.imbio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Karlsson A, Christenson K, Matlak M, et al. Galectin-3 functions as an opsonin and enhances the macrophage clearance of apoptotic neutrophils. Glycobiology. 2009;19:16–20. doi: 10.1093/glycob/cwn104. [DOI] [PubMed] [Google Scholar]
- 44.Fermino ML, Polli CD, Toledo KA, et al. LPS-induced galectin-3 oligomerization results in enhancement of neutrophil activation. PLoS One. 2011;6:e26004. doi: 10.1371/journal.pone.0026004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandez GC, Ilarregui JM, Rubel CJ, et al. Galectin-3 and soluble fibrinogen act in concert to modulate neutrophil activation and survival: involvement of alternative MAPK pathways. Glycobiology. 2005;15:519–527. doi: 10.1093/glycob/cwi026. [DOI] [PubMed] [Google Scholar]
- 46.Sato S, Ouellet N, Pelletier I, Simard M, Rancourt A, Bergeron MG. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J Immunol. 2002;168:1813–1822. doi: 10.4049/jimmunol.168.4.1813. [DOI] [PubMed] [Google Scholar]
- 47.Acosta-Rodriguez EV, Montes CL, Motran CC, et al. Galectin-3 mediates IL-4-induced survival and differentiation of B cells: functional cross-talk and implications during Trypanosoma cruzi infection. J Immunol. 2004;172:493–502. doi: 10.4049/jimmunol.172.1.493. [DOI] [PubMed] [Google Scholar]
- 48.Fowler M, Thomas RJ, Atherton J, Roberts IS, High NJ. Galectin-3 binds to Helicobacter pylori O-antigen: it is upregulated and rapidly secreted by gastric epithelial cells in response to H. pylori adhesion. Cell Microbiol. 2006;8:44–54. doi: 10.1111/j.1462-5822.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 49.Sato S, Hughes RC. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem. 1994;269:4424–4430. [PubMed] [Google Scholar]
- 50.Demmert M, Faust K, Bohlmann MK, et al. Galectin-3 in cord blood of term and preterm infants. Clin Exp Immunol. 2012;167:246–251. doi: 10.1111/j.1365-2249.2011.04509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai NY, Laforce-Nesbitt SS, Tucker R, Bliss JM. A murine model for disseminated candidiasis in neonates. Pediatr Res. 2011;69:189–193. doi: 10.1203/PDR.0b013e318206fd3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trofa D, Soghier L, Long C, Nosanchuk JD, Gacser A, Goldman DL. A rat model of neonatal candidiasis demonstrates the importance of lipases as virulence factors for Candida albicans and Candida parapsilosis. Mycopathologia. 2011;172:169–178. doi: 10.1007/s11046-011-9429-3. [DOI] [PubMed] [Google Scholar]


