Abstract
Biomonitoring studies show that humans carry a body burden of multiple classes of contaminants which are not often studied together. Many of these chemicals may be hepatotoxic. We used the 2003–2004 National Health and Nutrition Examination Survey to evaluate the relationship between alanine aminotransferase (ALT) and 37 environmental contaminants, comprising heavy metals, non-dioxin-like polychlorinated biphenyls (PCBs), and dioxin-like compounds, using a novel method. Linear regression models were constructed for each chemical separately, then as a class, using quartiles to represent exposure and adjusting for age, sex, race, income, and BMI. We then used an optimization approach to compile a weighted sum of the quartile scores, both within and across chemical classes. Using the optimization approach to construct weighted quartile scores, the dioxin like PCB, the non-dioxin like PCB and metal class-level scores were significantly associated with elevated ALT. A significant interaction was detected between the class-level score for metals, and the score for non-dioxin-like PCBs. When including all chemicals in one model, 3 chemicals accounted for 78 % of the weight (Mercury, PCB 180, 3,3’,4,4’,5-PNCB) with the remaining 22% associated with 4 chemicals (a dioxin and 3 PCBs). Validation with a holdout dataset indicated that the weighted quartile sum estimator efficiently identifies reproducible significant associations.
Keywords: Biomonitoring, ALT, liver, mixtures, cumulative
Introduction
Biomonitoring of human tissues and fluids has shown that virtually all individuals, worldwide, carry a “body burden” of synthetic chemicals (Thornton et al. 2002; CDC 2009). Although the measurement of an environmental chemical in a person’s tissues or fluids is an indication of exposure, it does not by itself mean that the chemical or the exposure concentration is sufficient to cause a disease or an adverse effect. However, since humans are exposed to multiple chemicals, there may be a combination effect (e.g., additive, synergistic) on health risks associated with exposure even at low levels (Kortenkamp 2008). Further, biomonitoring studies show that humans carry a body burden of multiple classes of contaminants, which are often not studied together.
Some examples of environmental chemical classes include metals, polychlorinated biphenyls (PCBs) and dioxins. Each is thought to impact human health, and these chemicals are generally ubiquitous exposures. Among the metals, several including cadmium, mercury and lead have been the focus of much research. Cadmium is mainly used in batteries and solar panels, and is also one of the components of cigarette smoke (ATSDR 2008). Exposure to lead has decreased over time due to removal from gasoline, individuals are still exposed through its presence through automotive and industrial emissions, older paint formulations, ammunition (EPA 2006), and moonshine (Chisolm 1971; Needleman 2004). Mercury is mainly used for industrial chemical production and electronics, but is also present in old thermometers and dental amalgams; for the general population, the main source of exposure is dietary (seafood; Figure 1) (ATSDR 1999). Polychlorinated biphenyls (PCBs) and dioxins are persistent organic pollutants (POPs). PCBs are a group of 209 different compounds formerly used in electronic equipment manufacture for their insulative and conductive properties. Although production in the US was banned in 1979 due to health concerns, PCBs persist in the environment, and the main source of exposure for the general population is diet (fish; Figure 1) (Johnson et al. 2008; ATSDR 2000). Similarly, dioxins enter the general population almost exclusively from ingestion of food, specifically through the consumption of fish, meat, and dairy products since dioxins are fat-soluble and readily bioaccumulate up the food chain (Schecter et al. 2001).
Figure 1.
Warning sign for contaminated fish
The liver is the principal organ that detoxifies or excretes a large number of xenobiotics and other foreign substances that enter the body. It therefore follows that long-term environmental exposures will lead to chronic intrahepatic exposure to these substances; such chemical exposures may affect not only the expression of genes involved in their metabolism but also other genes which may have either adaptive or harmful consequences. A priori, one may thus hypothesize that chronic environmental exposures may manifest as altered liver function and future liver disease.
In conducting risk assessments, there is an increasing recognition that multiple environmental exposures may impact a common adverse outcome (e.g. liver toxicity). However, classical analytical methods may not be appropriate, due to (1) high dimensional exposure data (relative to number of observations), (2) correlated exposures, (3) low level exposures (particularly multiple compounds below an individual observable effect level, but which in combination produce an observable effect), and (4) differences in potency (i.e. chemicals with the highest body burdens are not necessarily the most potent/toxic). There is a need for straightforward, easily interpretable methods to assess the relationship between relevant environmental exposure profiles and risk of common adverse outcomes.
One study which considered multiple chemical classes in relation to liver function is from Cave et al. (Cave et al. 2010). The National Health and Nutrition Examination Survey (NHANES) data were used to evaluate the risk of elevated alanine aminotransferase (ALT, a measure of liver function) and exposure to chemicals in 17 different subclasses. Participants were assigned a rank for each chemical, and these ranks were summed within each of the 17 subclasses. The summed ranks were divided into quartiles; if the test for trend across the quartiles was statistically significant, the chemicals in the subclass were further evaluated in what appear to be single-chemical models. The use of summed ranks (and quartiles of this measure) has the advantage of accommodating relatively low level exposures, as well as potential differences in potency, across chemicals. Although this approach also effectively accounts for all chemicals in a subclass, it is possible that the noise induced by considering only a subclass-level measure (i.e. the summed ranks) in the screening step would mask associations with individual members of the subclass. We propose a weighted quartile score method to represent exposure to multiple chemicals, both within and across classes, and demonstrate the method using epidemiologic data. In the present study, we tested the hypothesis that inter-class mixtures of environmental chemicals/metabolites are dose-dependently associated with increased risk for liver toxicity in the US population.
Materials and Methods
We used data from the 2003–2004 cycle of the NHANES for this analysis (CDC 2008). The NHANES is a cross-sectional, complex sample survey, which is designed to provide a nationally representative sample of the non-institutionalized, civilian US population. Included in these analyses are participants aged 12 years and older, who were included in one of the laboratory assessments of the NHANES. We excluded individuals who were missing information on serum alanine amino-transferase (ALT, used as a measure of liver function), on important covariates including body mass index (BMI), poverty income ratio (PIR) and alcohol intake. Further exclusion criteria were designed to exclude individuals with NAFLD due to identifiable causes (Cave et al. 2010; Clark et al. 2003). Thus, we excluded those who had a positive test result for serum hepatitis B surface antigen or for serum hepatitis C antibody, and women with elevated transferrin saturation (defined as >50%; transferrin saturation was not measured in adult men in the NHANES). We also excluded individuals whose self-reported alcohol intake was high enough, that they may have experienced alcohol-related changes in ALT. This included those with an intake of ≥20 g/day for men or ≥10 g/day for women as assessed by a two-day dietary recall (all participants included), or who reported regularly having >2 drinks/day for men or >1 drink/day for women (based on the alcohol questionnaire administered to those aged 20 years and older). Finally, those with self-reported liver disease, or who had ALT levels above the 99th percentile of the distribution (>81 U/L) were excluded, on the assumption that these individuals are likely to have frank liver disease and are not representative of the general population with regards to the associations between environmental chemical exposure and liver function. The total 2003–2004 NHANES sample was 10,122 individuals; after the inclusions and exclusions described above, the sample available for these analyses was 1,345 individuals (Table 1).
Table 1.
NHANES 2003–2004 sample selection.
| 2003–2004 |
| (N=10122)
|
| Included in Lab C (aged 12 years or older) |
| (N=2285)
|
| ALT measured |
| (N=2121)
|
| Exclusions (possibly overlapping) |
| Missing BMI: 45 |
| Missing PIR: 100 |
| Missing Alcohol Information: 285 |
| Positive Hepatitis B Surface Antigen: 9 |
| Positive Hepatitis C Antibody +: 35 |
| Elevated Transferrin Saturation: 16 |
| High Alcohol Intake: 562 |
| Self-reported Liver Disease: 56 |
| ALT >99th percentile (81 U/L): 21
|
| Final Sample: N= 1345 |
| Test sample: N=672, Validation Sample: N=673 |
The environmental chemical exposures were grouped into three classes—metals (cadmium, lead, total mercury), co-planar PCBs along with dioxins and furans (PCBs 28, 66, 74, 105, 118 and 156; 1,2,3,6,7,8-HXCDD; 1,2,3,4,6,7,8-HPCDD; 1,2,3,4,6,7,8,9-OCDD; 1,2,3,4,6,7,8-HPCDF; 3,3',4,4',5-PNCB), and non-dioxin-like PCBs (PCBs 44, 49, 52, 87, 99, 101, 110, 138, 146, 149, 151, 153, 170, 177, 178, 180, 183, 187, 194, 196, 199, 206, 209). Each of these analytes was measured in serum; for PCBs, dioxins and furans, lipid adjusted values were used. We included only analytes detectable in ≥60% of samples. For samples where values were below the limit of detection, we used the NHANES substituted value of (limit of detection / √2) (CDC 2008).
Serum ALT was measured in all NHANES participants aged 12 years and older. Important covariates identified in the literature on liver function, and included in multivariate analyses were: age in years (continuous), sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, other Hispanic, Other/mixed/missing), and body mass index (BMI, continuous). We also investigated a measure of socio-economic status, the poverty income ratio (PIR), which is the ratio of family income to poverty threshold.
The NHANES data were divided into two equally sized samples, one used in initial analyses (‘training’ set), and one for validation of the final model (‘validation’ set). The training and validation sets were created using simple random sampling with a set seed for replicability. The first set of analyses was linear regression modeling of each chemical class separately. First, all analytes in the class were included in a multivariate model, and stepwise selection used to determine which were associated with elevated ALT after adjusting for the covariates described above. Next, recognizing that the analytes varied in range and level, we categorized individuals according to quartiles of each analyte. The quartile scores (0, 1, 2, or 3) were then entered into multivariate linear regression models separately, then in combination. Finally, we used an optimization approach to compile a weighted sum of the quartile scores, both within a chemical class, and across chemical classes. The NLP procedure in SAS was used to construct a set of weights applied to the quartile scores, which maximized the likelihood of the non-linear regression model where regression coefficients and the weights are estimated (i.e., in a nonlinear model). The set of weights was constrained to sum to one—using this approach, the weight assigned to a given analyte score reflects the ‘importance’ of that analyte in terms of association with risk of elevated ALT. The weighted sum was constrained to have a positive coefficient since our primary interest is identifying stressors which may increase the risk of elevated ALT. The weights generated in this set of analyses were then used to construct weighted sums in the validation dataset, and the weighted score evaluated according to its coefficient estimate.
In each cycle of the NHANES, most environmental pollutants are measured in one of 3 laboratory samples (A, B or C), while some pollutants (including certain heavy metals) are measured in all participants. The lab samples are comprised of a 1/3 randomly assigned group of survey participants. Therefore, only 1/3 of participants will have measurements available for a given chemical. As lab subsamples are non-overlapping, no one individual will have exposure information for all chemical classes. Since our goal is to assess the joint contribution of chemicals in different classes, as an exploratory analysis we imputed serum and urine concentrations of additional pollutants of interest across lab samples—these included polyfluorinated compounds (PFCs), phthalates, phenols, along with the dioxins, furans and PCBs included in the main analysis. Imputation was performed using the geometric mean of each analyte, stratified into 72 groups based on sex, age group (12–19, 20–39, 40–64, ≥65 years), race (non-Hispanic White, non-Hispanic Black, Other) and BMI (≤24.9, 25–29.9, ≥30). The weighted score including all the analytes was constructed in the same fashion described for the main analysis, above, and compared to the results for data without imputation performed. More detailed methods and results are presented in the Supplementary Material.
Results
Characteristics of the study participants are shown in Table 2. Comparison of demographic characteristics shows the training and validation groups are generally comparable. The proportion of females was slightly higher in the test dataset, and the proportion of non-Hispanic whites and other Hispanic individuals somewhat lower, in comparison to the validation dataset. However, the distribution of ALT values was very similar, with mean (standard deviation) values of 20.35 (9.19) and 21.04 (10.23) U/L in the test and validation sets, respectively; medians were identical at 18 U/L.
Table 2.
Characteristics of NHANES participants, 2003–2004 (all values are unweighted).
| Test Dataset | Validation Dataset | |
|---|---|---|
| Characteristic | N (%) | |
| Total | 672 (100) | 673 (100) |
| Sex | ||
| Male | 310 (46.1) | 337 (50.1) |
| Female | 362 (53.9) | 336 (49.9) |
| Race/ethnicity | ||
| Non-Hispanic White | 267 (39.7) | 291 (43.2) |
| Non-Hispanic Black | 181 (26.9) | 176 (26.2) |
| Mexican-American | 172 (25.6) | 158 (23.5) |
| Other Hispanic | 18 (2.68) | 22 (3.27) |
| Other/Mixed/Missing | 34 (5.06) | 26 (3.86) |
| Age (years) | ||
| 12–19 | 316 (47.0) | 296 (44.0) |
| 20–39 | 93 (13.8) | 101 (15.0) |
| 40–64 | 123 (18.3) | 146 (21.7) |
| ≥65 | 140 (20.8) | 130 (19.3) |
| BMI (kg/m2) | ||
| <18.5 | 47 (6.99) | 49 (7.28) |
| 18.5–24.9 | 271 (40.3) | 265 (39.4) |
| 25.0–29.9 | 190 (28.3) | 173 (25.7) |
| ≥30.0 | 164 (24.4) | 189 (27.6) |
| Family PIR | ||
| < 2 | 339 (50.5) | 365 (54.2) |
| ≥ 2 | 333 (49.6) | 308 (45.8) |
| Mean (SD)Median (25th, 75th percentiles) | ||
| ALT (IU/L) | 20.35 (9.19) 18 (15, 23) |
21.04 (10.23) 18 (15, 24) |
In the preliminary analysis examining single analytes in separate multivariate logistic regression models (adjusting for potential confounders), several analytes were associated with changes in ALT (i.e. p<0.10; Table 3). Among the metals, higher exposure to lead was associated with lower ALT, while mercury was associated with higher ALT. Two dioxin-like compounds (1,2,3,4,6,7,8-HPCDD and 3,3’,4,4’,5-PNCB) and three non-dioxin-like PCBs (PCBs 177, 178 and 180) were associated with higher ALT. PCB 149 (non-dioxin-like) was associated with decreased ALT, although the association was inconsistent across quartiles. The disadvantage of this approach is that chemicals are included in the model as single covariates without accounting for the more relevant exposure to mixtures of chemicals. Since it is unlikely that exposure to any of these substances is actually protective, and the interest is in identifying exposures associated with increased risk, the proposed index approach (as noted in the methods) constrains the effect measure to be positive (i.e. associated with elevated ALT). This allows the identification of the combined effect of multiple ‘bad actors,’ effectively decreasing the noise from un-associated compounds.
Table 3.
Results from single-chemical models, for association with ALT, NHANES 2003–2004.
| Analyte | Q2 vs. Q1 | Q3 vs. Q1 | Q4 vs. Q1 | |||
|---|---|---|---|---|---|---|
| B (SE) | p-value | B (SE) | p-value | B (SE) | p-value | |
| Metals (ug/mL serum) | ||||||
| Cadmium | 0.0604 (0.0395) | 0.1269 | −0.0421 (0.0350) | 0.2293 | −0.0623 (0.0396) | 0.1158 |
| Lead | −0.0680 (0.0369) | 0.0656 | −0.0390 (0.0376) | 0.2991 | −0.1030 (0.0416) | 0.0135 |
| Mercury | 0.0327 (0.0356) | 0.3597 | 0.0133 (0.0375) | 0.7228 | 0.0914 (0.0387) | 0.0183 |
| Dioxin-like compounds (ng/g lipid) | ||||||
| PCB 28 | 0.0591 (0.0384) | 0.1250 | −0.0288 (0.0383) | 0.4527 | 0.0328 (0.0384) | 0.3957 |
| PCB 66 | −0.0144 (0.0386) | 0.7011 | 0.0182 (0.0386) | 0.6367 | −0.0045 (0.0405) | 0.9109 |
| PCB 74 | 0.0415 (0.0396) | 0.2952 | 0.0453 (0.0451) | 0.3159 | 0.0776 (0.0637) | 0.2236 |
| PCB 105 | 0.0372 (0.0394) | 0.3444 | 0.0352 (0.0419) | 0.4018 | 0.0377 (0.0504) | 0.4554 |
| PCB 118 | 0.0367 (0.0392) | 0.3495 | 0.0217 (0.0424) | 0.6092 | 0.0517 (0.0549) | 0.3470 |
| PCB 156 | 0.0638 (0.0394) | 0.1058 | 0.0148 (0.0489) | 0.7616 | 0.0244 (0.0725) | 0.7363 |
| 1,2,3, 6,7,8-HXCDD | −0.0138 (0.0398) | 0.7287 | 0.0049 (0.0446) | 0.9128 | −0.0479 (0.0619) | 0.4400 |
| 1,2,3,4,6,7,8-HPCDD | −0.0070 (0.0392) | 0.8590 | 0.1026 (0.0406) | 0.0117 | 0.0809 (0.0466) | 0.0829 |
| 1,2,3,4,6,7,8,9-OCDD | 0.0334 (0.0396) | 0.3996 | 0.0642 (0.0433) | 0.1385 | 0.0029 (0.0514) | 0.9555 |
| 1,2,3,4,6,7,8-HPCDF | −0.0099 (0.0396) | 0.8018 | −0.0306 (0.0401) | 0.4466 | −0.0699 (0.0410) | 0.0888 |
| 3,3',4,4',5-PNCB | 0.0574 (0.0402) | 0.1540 | 0.0860 (0.0432) | 0.0471 | 0.1046 (0.0518) | 0.0439 |
| Non-dioxin-like PCBs (ng/g lipid) | ||||||
| PCB 44 | −0.0290 (0.0389) | 0.4573 | 0.0078 (0.0396) | 0.8442 | −0.0400 (0.0399) | 0.3171 |
| PCB 49 | −0.0162 (0.0388) | 0.6761 | −0.0120 (0.0395) | 0.7609 | −0.0300 (0.0400) | 0.4542 |
| PCB 52 | −0.0197 (0.0385) | 0.6075 | −0.0138 (0.0394) | 0.7268 | −0.0132 (0.0397) | 0.7386 |
| PCB 87 | −0.0225 (0.0387) | 0.5614 | 0.0004 (0.0400) | 0.9921 | −0.0332 (0.0387) | 0.3908 |
| PCB 99 | 0.0615 (0.0395) | 0.1196 | 0.0148 (0.0419) | 0.7247 | 0.0194 (0.0497) | 0.6956 |
| PCB 101 | 0.0018 (0.0387) | 0.9631 | 0.0007 (0.0394) | 0.9867 | 0.0030 (0.0393) | 0.9396 |
| PCB 110 | −0.0438 (0.0388) | 0.2608 | −0.0196 (0.0397) | 0.6207 | −0.0281 (0.0395) | 0.4767 |
| PCB 138 | 0.0139 (0.0394) | 0.7251 | 0.0401 (0.0477) | 0.4013 | 0.0628 (0.0645) | 0.3308 |
| PCB 146 | (0.0395) | 0.4602 | 0.0500 (0.0489) | 0.3073 | 0.0867 (0.0685) | 0.2059 |
| PCB 149 | −0.0421 (0.0382) | 0.2710 | 0.0127 (0.0385) | 0.7424 | −0.0654 (0.0395) | 0.0985 |
| PCB 151 | −0.0366 (0.0387) | 0.3440 | 0.0352 (0.0394) | 0.3721 | −0.0251 (0.0401) | 0.5323 |
| PCB 153 | 0.0304 (0.0394) | 0.4409 | 0.0516 (0.0481) | 0.2840 | 0.1030 (0.0688) | 0.1345 |
| PCB 170 | 0.0568 (0.0392) | 0.1484 | 0.0391 (0.0489) | 0.4242 | 0.0533 (0.0727) | 0.4641 |
| PCB 177 | 0.0781 (0.0391) | 0.0462 | 0.0811 (0.0468) | 0.0836 | 0.0332 (0.0630) | 0.5991 |
| PCB 178 | 0.0648 (0.0388) | 0.0953 | 0.0783 (0.0481) | 0.1037 | 0.1153 (0.0705) | 0.1024 |
| PCB 180 | 0.0560 (0.0395) | 0.1561 | 0.0917 (0.0494) | 0.0642 | 0.1250 (0.0736) | 0.0902 |
| PCB 183 | 0.0462 (0.0393) | 0.2400 | 0.0741 (0.0476) | 0.1205 | 0.0966 (0.0636) | 0.1292 |
| PCB 187 | 0.0448 (0.0391) | 0.2525 | 0.0717 (0.0475) | 0.1321 | 0.1621 (0.0665) | 0.0151 |
| PCB 194 | 0.0352 (0.0391) | 0.3684 | 0.0487 (0.0477) | 0.3070 | 0.0282 (0.0712) | 0.6915 |
| PCB 196 | 0.0322 (0.0389) | 0.4075 | 0.0504 (0.0470) | 0.2841 | 0.0639 (0.0700) | 0.3406 |
| PCB 199 | 0.0037 (0.0398) | 0.9254 | 0.0510 (0.0490) | 0.2989 | 0.0428 (0.0788) | 0.5871 |
| PCB 206 | 0.0349 (0.0400) | 0.3833 | 0.0898 (0.0473) | 0.0580 | 0.0606 (0.0668) | 0.3642 |
| PCB 209 | 0.0310 (0.0390) | 0.4281 | 0.0513 (0.0461) | 0.2662 | −0.0121 (0.0644) | 0.8510 |
Adjusted for sex, race/ethnicity, age (continuous), poverty-income ratio (continuous), and BMI (continuous)
Next, quartile scores were combined within each class of chemicals (Table 4). When simply adding quartile scores within a class (unweighted quartile score), there was not a significant association between metals, dioxin-like, or non-dioxin-like PCBs, and ALT. However, when the optimization approach was used to construct weighted quartile scores, each of the class-level scores was significantly associated with elevated ALT in separate models using the test data. To assess the joint effects of chemicals across classes, we used these weighted class-level scores, adjusting for covariates (gender, race, BMI, PIR, age), in a multiple regression model of ln(ALT) with the validation data. The model was initially parameterized to include linear terms and pairwise interactions across the class-level scores. Both before and after eliminating non-significant interaction product terms, there was a significant positive interaction between the metals quartile score and the non-dioxin-like PCBs quartile score (p=0.022). Figure 2 depicts this interaction, illustrating that higher values of both the metals and non-dioxin-like PCBs quartile scores are associated with a greater than additive increase in ALT.
Table 4.
Beta coefficients (SE; p-value) and weights assigned to quartile scores, for association with ALT, separate models for each analyte class, NHANES 2003–2004.
| Analyte | Unweighted quartile score | Weighted quartile score |
|---|---|---|
| Metals (ug/mL serum) | −0.007 (0.007; 0.30) | 0.02 (0.01; 0.04) |
| Cadmium | -- | 0 |
| Lead | -- | 0 |
| Mercury | -- | 1 |
| Dioxin-like compounds (ng/g lipid) | −0.003 (0.002; 0.21) | 0.05 (0.02; 0.009) |
| PCB 28 | -- | 0 |
| PCB 66 | -- | 0 |
| PCB 74 | -- | 0 |
| PCB 105 | -- | 0 |
| PCB 118 | -- | 0 |
| PCB 156 | -- | 0 |
| 1,2,3, 6,7,8-HXCDD | -- | 0 |
| 1,2,3,4,6,7,8-HPCDD | -- | 0.30 |
| 1,2,3,4,6,7,8,9-OCDD | -- | 0 |
| 1,2,3,4,6,7,8-HPCDF | -- | 0 |
| 3,3',4,4',5-PNCB | -- | 0.70 |
| Non-dioxin-like PCBs (ng/g lipid) | 0.0006 (0.001; 0.64) | 0.05 (0.02; 0.03) |
| PCB 44 | -- | 0 |
| PCB 49 | -- | 0 |
| PCB 52 | -- | 0 |
| PCB 87 | -- | 0 |
| PCB 99 | -- | 0 |
| PCB 101 | -- | 0.10 |
| PCB 110 | -- | 0 |
| PCB 138 | -- | 0 |
| PCB 146 | -- | 0 |
| PCB 149 | -- | 0 |
| PCB 151 | -- | 0 |
| PCB 153 | -- | 0 |
| PCB 170 | -- | 0 |
| PCB 177 | -- | 0 |
| PCB 178 | -- | 0 |
| PCB 180 | -- | 0.04 |
| PCB 183 | -- | 0 |
| PCB 187 | -- | 0.86 |
| PCB 194 | -- | 0 |
| PCB 196 | -- | 0 |
| PCB 199 | -- | 0 |
| PCB 206 | -- | 0 |
| PCB 209 | -- | 0 |
Adjusted for sex, race/ethnicity, poverty-income ratio (continuous), age (continuous), and BMI (continuous)
Figure 2.
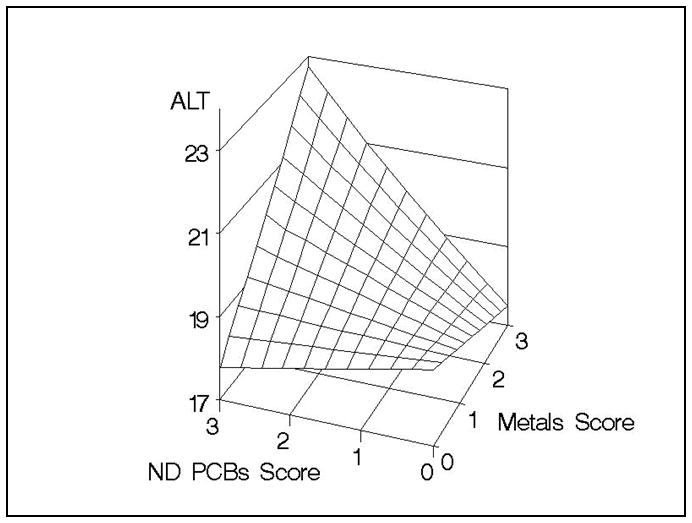
Predicted response surface, adjusted for covariates and the dioxin-like PCBs score with centered prediction at covariate averages, depicting the interaction (p=0.022) between the weighted metals quartile score and the weighted non-dioxin-like (ND) PCBs quartile score using the validation data.
Finally, the optimization procedure was repeated, but including all analytes as potential contributors. The chemicals with non-zero weights were: mercury (29%),1,2,3,4,6,7,8-HPCDD (9%), 3,3’,4,4’,5-PNCB (24%), PCB 52 (4%), PCB 101 (3%), PCB 180 (25%), PCB 187 (6%). This score was also highly associated with increased odds of elevated ALT (p= 0.003). The weighted scores were also evaluated in the validation dataset (Table 5 and Figure 3). Both the estimated beta coefficients and standard errors were very similar to those generated from the test dataset. In general, the “significant” weighted scores in the test dataset were significant, conditioning on the weights, in the validation dataset – indicating reproducible results.
Table 5.
Beta coefficients (p-value) and weights assigned to quartile scores, for association with ALT when all analyte classes are included in the same model, NHANES 2003–2004.
| Analyte | Unweighted quartile score | Weighted quartile score | VALIDATION DATASET: Weighted quartile score |
|---|---|---|---|
| All analytes included in weighted sum (Model 2) | |||
| All analytes together | 0.0004 (0.68) | 0.08 (0.003) | 0.06 (0.04) |
| Weights | Weights from test data set (see column at left) | ||
| Cadmium | -- | 0 | |
| Lead | -- | 0 | |
| Mercury | -- | 0.29 | |
| PCB 28 | -- | 0 | |
| PCB 66 | -- | 0 | |
| PCB 74 | -- | 0 | |
| PCB 105 | -- | 0 | |
| PCB 118 | -- | 0 | |
| PCB 156 | -- | 0 | |
| 1,2,3, 6,7,8-HXCDD | -- | 0 | |
| 1,2,3,4,6,7,8-HPCDD | -- | 0.09 | |
| 1,2,3,4,6,7,8,9-OCDD | -- | 0 | |
| 1,2,3,4,6,7,8-HPCDF | -- | ||
| 3,3',4,4',5-PNCB | -- | 0.24 | |
| PCB 44 | -- | 0 | |
| PCB 49 | -- | 0 | |
| PCB 52 | -- | 0.04 | |
| PCB 87 | -- | 0 | |
| PCB 99 | -- | 0 | |
| PCB 101 | -- | 0.03 | |
| PCB 110 | -- | 0 | |
| PCB 138 | -- | 0 | |
| PCB 146 | -- | 0 | |
| PCB 149 | -- | 0 | |
| PCB 151 | -- | 0 | |
| PCB 153 | -- | 0 | |
| PCB 170 | -- | 0 | |
| PCB 177 | -- | 0 | |
| PCB 178 | -- | 0 | |
| PCB 180 | -- | 0.25 | |
| PCB 183 | -- | 0 | |
| PCB 187 | -- | 0.06 | |
| PCB 194 | -- | 0 | |
| PCB 196 | -- | 0 | |
| PCB 199 | -- | 0 | |
| PCB 206 | -- | 0 | |
| PCB 209 | -- | 0 | |
Adjusted for sex, race/ethnicity, poverty-income ratio (continuous), age (continuous), and BMI (continuous)
Figure 3.
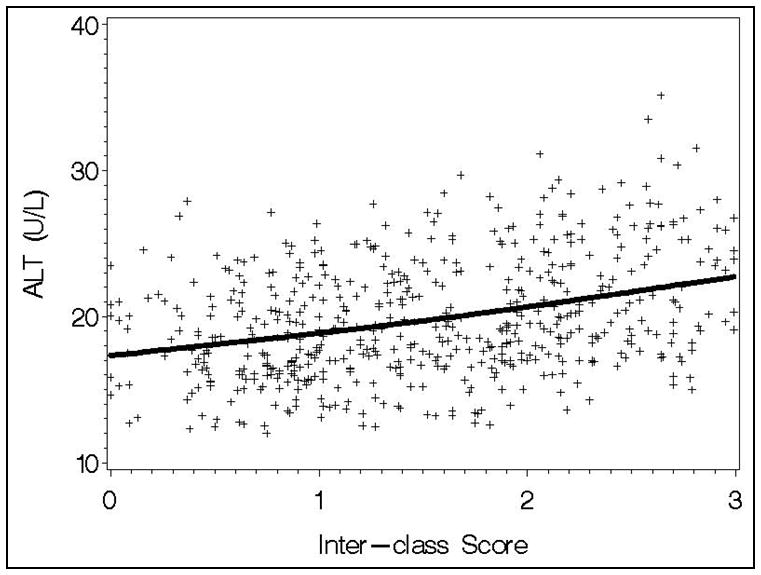
Observed and model predicted association between the inter-class score (with weights determined in the test dataset) and ALT (p=0.031) in the validation data, after adjusting for covariates (PIR, race, age, gender, and BMI).
In the exploratory analysis using both observed and imputed exposure data, the coefficient for the weighted score variable was significantly associated with elevated ALT, both in the test and validation datasets. Further, the largest weight was, as in the main analysis, placed on mercury concentration in serum. However, there are additional chemicals which received substantial weight that we did not consider in the main analysis—namely PFOA (weight=0.28) and the three environmental phenols (weights ranging from 0.09 to 0.12).
Discussion
The NHANES data demonstrate the ubiquitous exposure of multiple environmental chemicals and chemical classes in the US population. Although exposure is clearly indicated from biomonitoring data, the impact of exposure to complex chemical mixtures on human health is not as clear. Due to the high-dimensionality of the problem with correlated data, standard statistical methods are limited. Some authors have proposed to sum components in a mixture to accommodate the mixture effect. However, the least potent chemicals in the mixture may dominate the sum and mask the potential effect of more potent chemicals. Thus, we have used a weighted sum approach (analogous to (Gennings et al. 2010)) to estimate empirical weights in a weighted quartile sum index. In this population, 78% of the effect on ALT was due to three environmental chemicals (total mercury, PCB 180, and 3,3’,4,4’5-PNCB). We also found evidence of possible interaction among heavy metals and non-dioxin-like PCBs, underscoring the importance of considering multiple chemicals when evaluating common health outcomes such as liver function. In this case, the interaction was greater than additive—the effect on ALT levels was stronger for individuals with higher body burdens of heavy metals and non-dioxin-like PCBs than would be predicted in a no-interaction model. Further research is needed to identify and confirm this and other potential interactions among chemicals, and the underlying mechanisms.
There is evidence from previous studies on the liver toxicity of the chemicals evaluated in this study. Among the heavy metals, studies in rats demonstrated an increase in both AST and ALT of more than 200% after short duration (24 and 48 hour) exposure to a non-lethal dose of mercury (Patnaik et al. 2010). Male mice exposed to a mixture of lead, mercury, cadmium and copper in their drinking water for seven weeks had significant increases in blood markers of liver function including ALT, AST, alkaline phosphatase (ALP), and γ-glutamyltranspeptidase (GGT) (Al-Attar 2011). The hepatotoxic potential of PCB mixtures is well documented in animals by oral and other routes of exposure. The spectrum of observed hepatic effects in animals is broad and includes microsomal enzyme induction, liver enlargement, increased serum levels of liver enzymes and lipids, and histopathologic alterations that progress to fatty and necrotic lesions and tumors (ATSDR 2000). The findings of human studies, however, are not as obvious. Many of the human studies involving worker and other populations with high body burdens of PCBs report associations between PCBs and hepatic indices such as liver enzymes, lipids, and cholesterol (ATSDR 2000). Studies of people exposed to PCBs by ingestion of contaminated fish or PCBs contaminated rice oil in the Yusho or Yu-Cheng incidents have reported increases in serum levels of some liver enzymes (e.g., GGT, AST, ALT) that are suggestive of microsomal enzyme induction or possible liver damage (ATSDR 2000).
Although the epidemiologic evidence for interactions between environmental contaminants and effect on liver function is limited, there are several potential mechanisms that may explain the synergy between mercury and PCBs observed in this study. Mercury inhibits thioredoxin reductase, which is a key anti-oxidant in cells, while PCBs activate Cyp1a and damage mitochondria, thereby directly contributing to reactive oxygen species formation (Branco et al. 2012a; Branco et al. 2012b; Shen et al. 2011; Yamazaki et al. 2011). By affecting both reactive oxygen species formation and inhibiting anti-oxidant defenses, mercury and PCBs may have synergistic effects in terms of observed hepatotoxicity. Also, mercury has been found to be a contaminant in high fructose corn syrup, a common food additive which is implicated in the current epidemic of obesity (Dufault et al. 2009; Collison et al. 2009). The liver is the principal organ that metabolizes fructose, which promotes steatosis and oxidative stress (Cortez-Pinto et al. 1999; Kawasaki et al. 2009). We speculate that exposure to mercury and PCBs in the background of high levels of fructose consumption will further worsen oxidative stress and deplete glutathione thus further promoting hepatotoxicity. These possibilities now await experimental verification.
Definitive conclusions regarding human hepatotoxiciy are hampered by limitations in study design of available studies, such as exposure misclassification, lack of controls, lack of correction for common confounding variables (e.g., age and alcohol consumption), and natural partitioning of PCBs to serum lipids (ATSDR 2000). The lack of unequivocal evidence in humans that is seen in laboratory animals may result from many factors, including species differences in susceptibility or sensitivity to PCBs, and dissimilarities in exposure levels, durations, and mixture compositions. For example, PCB congeners have been classified in various groupings based on structure and functionality (e.g.,(Wolff et al. 1997; Goncharov et al. 2011). The four congeners identified with non-zero weights in the weighted sum analysis (PCB 52, 101, 180, and 187; Table 5) were classified as potentially estrogenic and weak phenobarbital inducers (PCB 52, 101, and 187) or as phenobarbital, CYP1A and CYP2B inducers (PCB 180) using the Wolff et al. classification scheme. Goncharov et al described all four as di- (PCB 52, 101, and 180) or tri-/tetra- ortho (PCB 187) substituted PCBs, which are non-coplanar and do not show dioxin-like properties.
Identifying environmental exposures which adversely impact liver function is an important public health issue. Nonalcoholic fatty liver disease (NAFLD), the most common liver condition, is estimated to affect fully one third of the US population (Browning et al. 2004). It is closely linked to the presence and severity of obesity and is generally considered to be the hepatic manifestation of the metabolic syndrome (Marchesini et al. 2001; Sanyal et al. 2001). The implications are severe, as nonalcoholic steatohepatitis (NASH) can progress to cirrhosis in 15–20% of subjects (Ekstedt et al. 2006). The pathophysiologic mechanisms underlying the development of NASH, and which drive disease progression in the 15–20% of subjects who develop cirrhosis, are not fully known, but it is possible that exposure to environmental chemicals plays a role.
There are some limitations to this study. Due to the cross-sectional nature of the NHANES, there is no way to assess temporality of exposure and response. We adjusted for several important covariates in our analyses, but there are methodologic factors that may affect measurement of ALT, including the specific assay used, laboratory variability, time from sample collection to analysis, and sample handling (such as freeze-thaw cycles). In the NHANES however, a consistent assay technique was used. In this analysis, we used ALT as a measure of liver function; the ‘gold standard’ for assessment of NAFLD would be a liver biopsy, but this is clearly not feasible for population-based studies (such as the NHANES). In this context, ALT is frequently considered to be a surrogate marker for NAFLD once other common causes of liver injury are excluded. In our study, we excluded those who had serologic markers for viral hepatitis or a history of clinically significant alcohol consumption; thus, individuals with elevated ALTs in this population are likely to represent cases of NAFLD. Strengths of this study include the large sample size, and the ability to simultaneously consider potential confounders and many chemical exposures. Results from an exploratory analysis using imputed data demonstrate that imputation of exposures may be an option when data are scarce, and consideration of many different chemical compounds is desired.
Conclusions
An important feature of our research is a method for evaluating inter-class chemical/metabolite mixtures with a common adverse effect on liver toxicity. The optimization indicated that 78% of the effect was due to total mercury, PCB 180, and 3,3’,4,4’5-PNCB. Research is ongoing to fully characterize this optimization approach; preliminary results indicate that the weighted sum estimator efficiently identifies true associations rather than spurious effects of confounders, with good coverage and power.
Supplementary Material
Acknowledgments
Source of funding: The authors gratefully acknowledge the support from #R01ES015276, #T32ES007334, and #UL1TR000058.
Footnotes
Disclaimer: This manuscript has been reviewed by the U.S. Environmental Protection Agency and approved for publication. The views expressed in this manuscript are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Thornton JW, McCally M, Houlihan J. Biomonitoring of industrial pollutants: Health and policy implications of the chemical body burden. Public Health Rep. 2002;117(4):315–323. doi: 10.1016/S0033-3549(04)50167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Department of Health and Human Services, Centers for Disease Control and Prevention; 2009. [Google Scholar]
- Kortenkamp A. Low dose mixture effects of endocrine disrupters: implications for risk assessment and epidemiology. Int J Androl. 2008;31(2):233–237. doi: 10.1111/j.1365-2605.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Cadmium. U.S. Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry; 2008. [PubMed] [Google Scholar]
- EPA US. Final Report. Vol. 2006. Washington, DC: U.S. Environmental Protection Agency; 2006. Air Quality Criteria for Lead. [Google Scholar]
- Chisolm JJ. Lead Poisoning. Sci Am. 1971;224(2):15–23. doi: 10.1038/scientificamerican0271-15. [DOI] [PubMed] [Google Scholar]
- Needleman H. Lead poisoning. Annu Rev Med. 2004;55:209–222. doi: 10.1146/annurev.med.55.091902.103653. [DOI] [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Mercury. U.S. Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry; 1999. [Google Scholar]
- Johnson BL, Hicks HE, Cibulas W, Faroon O, Ashizawa AE, De Rosa CT, et al. Public Health Implications of Exposure to Polychlorinated Biphenyls (PCBs) Agency for Toxic Substances and Disease Registry, Division of Toxicology and Environmental Medicine; 2008. [Google Scholar]
- ATSDR. Toxicological profile for Polychlorinated Biphenyls (PCBs) Atlanta, GA: U.S. Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry; 2000. [PubMed] [Google Scholar]
- Schecter A, Cramer P, Boggess K, Stanley J, Papke O, Olson J, et al. Intake of dioxins and related compounds from food in the U.S. population. Journal of toxicology and environmental health Part A. 2001;63(1):1–18. doi: 10.1080/152873901750128326. [DOI] [PubMed] [Google Scholar]
- Cave M, Appana S, Patel M, Falkner KC, McClain CJ, Brock G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ Health Perspect. 2010;118(12):1735–1742. doi: 10.1289/ehp.1002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. National Health and Nutrition Examination Survey Data. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS); Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. 2003–2004 and 2005–2006. [Google Scholar]
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98(5):960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- Gennings C, Sabo R, Carney E. Identifying Subsets of Complex Mixtures Most Associated With Complex Diseases Polychlorinated Biphenyls and Endometriosis as a Case Study. Epidemiology. 2010;21:S77–S84. doi: 10.1097/EDE.0b013e3181ce946c. [DOI] [PubMed] [Google Scholar]
- Patnaik BB, Roy A, Agarwal S, Bhattacharya S. Induction of oxidative stress by non-lethal dose of mercury in rat liver: Possible relationships between apoptosis and necrosis. J Environ Biol. 2010;31(4):413–416. [PubMed] [Google Scholar]
- Al-Attar AM. Vitamin E attenuates liver injury induced by exposure to lead, mercury, cadmium and copper in albino mice. Saudi J Biol Sci. 2011;18(4):395–401. doi: 10.1016/j.sjbs.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco V, Canario J, Lu J, Holmgren A, Carvalho C. Mercury and selenium interaction in vivo: effects on thioredoxin reductase and glutathione peroxidase. Free radical biology & medicine. 2012a;52(4):781–793. doi: 10.1016/j.freeradbiomed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Branco V, Ramos P, Canario J, Lu J, Holmgren A, Carvalho C. Biomarkers of adverse response to mercury: histopathology versus thioredoxin reductase activity. Journal of biomedicine & biotechnology. 2012b;2012:359879. doi: 10.1155/2012/359879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Shen C, Yu J, Yu C, Chen L, Shi D, et al. PCB congeners induced mitochondrial dysfunction in Vero cells. Journal of hazardous materials. 2011;185(1):24–28. doi: 10.1016/j.jhazmat.2010.08.061. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Suzuki M, Itoh T, Yamamoto K, Kanemitsu M, Matsumura C, et al. Structural basis of species differences between human and experimental animal CYP1A1s in metabolism of 3,3',4,4',5-pentachlorobiphenyl. J Biochem. 2011;149(4):487–494. doi: 10.1093/jb/mvr009. [DOI] [PubMed] [Google Scholar]
- Dufault R, LeBlanc B, Schnoll R, Cornett C, Schweitzer L, Wallinga D, et al. Mercury from chlor-alkali plants: measured concentrations in food product sugar. Environmental health : a global access science source. 2009;8:2. doi: 10.1186/1476-069X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison KS, Saleh SM, Bakheet RH, Al-Rabiah RK, Inglis AL, Makhoul NJ, et al. Diabetes of the liver: the link between nonalcoholic fatty liver disease and HFCS-55. Obesity. 2009;17(11):2003–2013. doi: 10.1038/oby.2009.58. [DOI] [PubMed] [Google Scholar]
- Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA. 1999;282(17):1659–1664. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Igarashi K, Koeda T, Sugimoto K, Nakagawa K, Hayashi S, et al. Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. The Journal of nutrition. 2009;139(11):2067–2071. doi: 10.3945/jn.109.105858. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Camann D, Gammon M, Stellman SD. Proposed PCB congener groupings for epidemiological studies. Environ Health Perspect. 1997;105(1):13–14. doi: 10.1289/ehp.9710513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharov A, Pavuk M, Foushee HR, Carpenter DO. Blood pressure in relation to concentrations of PCB congeners and chlorinated pesticides. Environ Health Perspect. 2011;119(3):319–325. doi: 10.1289/ehp.1002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease - A feature of the metabolic syndrome. Diabetes. 2001;50(8):1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120(5):1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



