Abstract
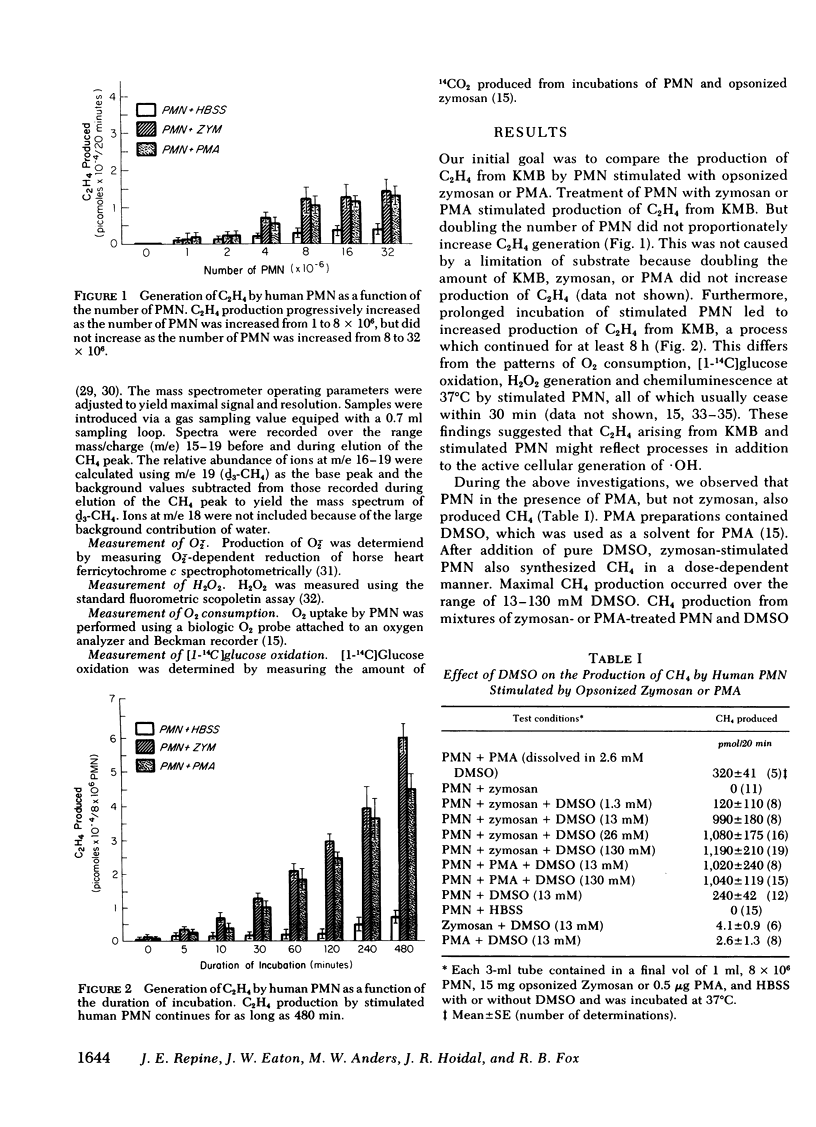
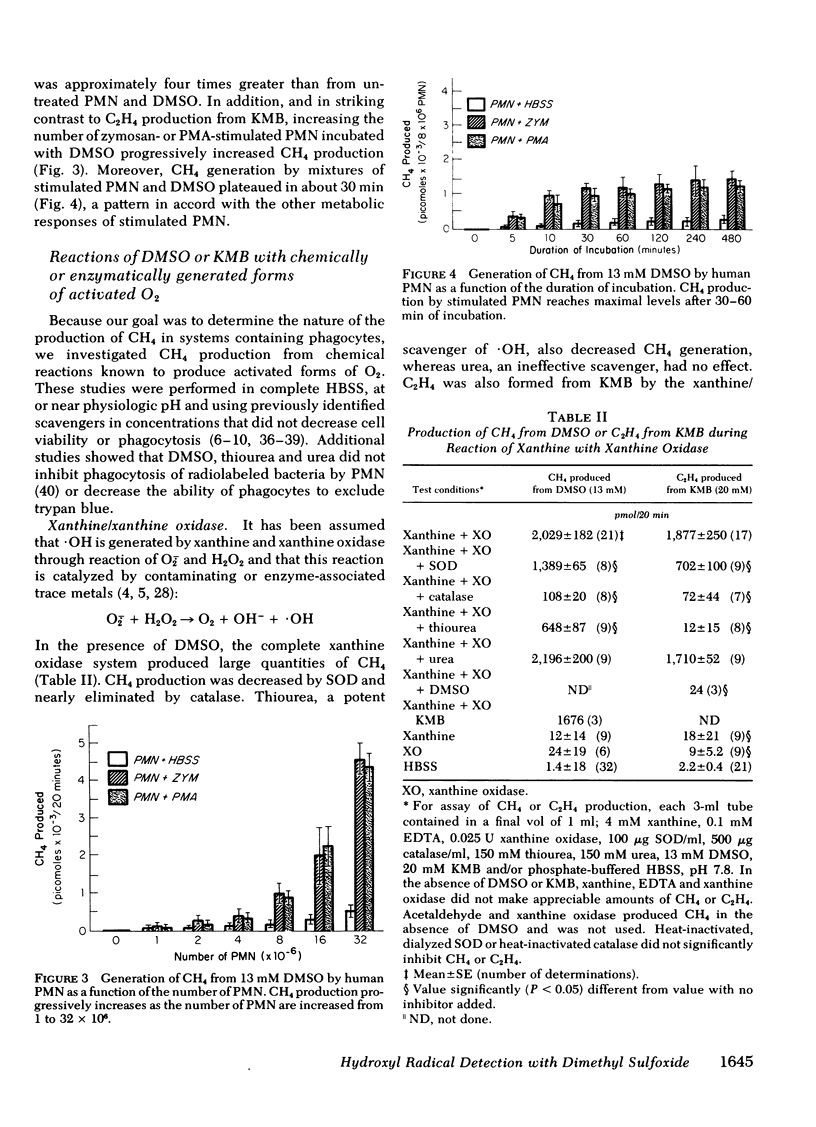
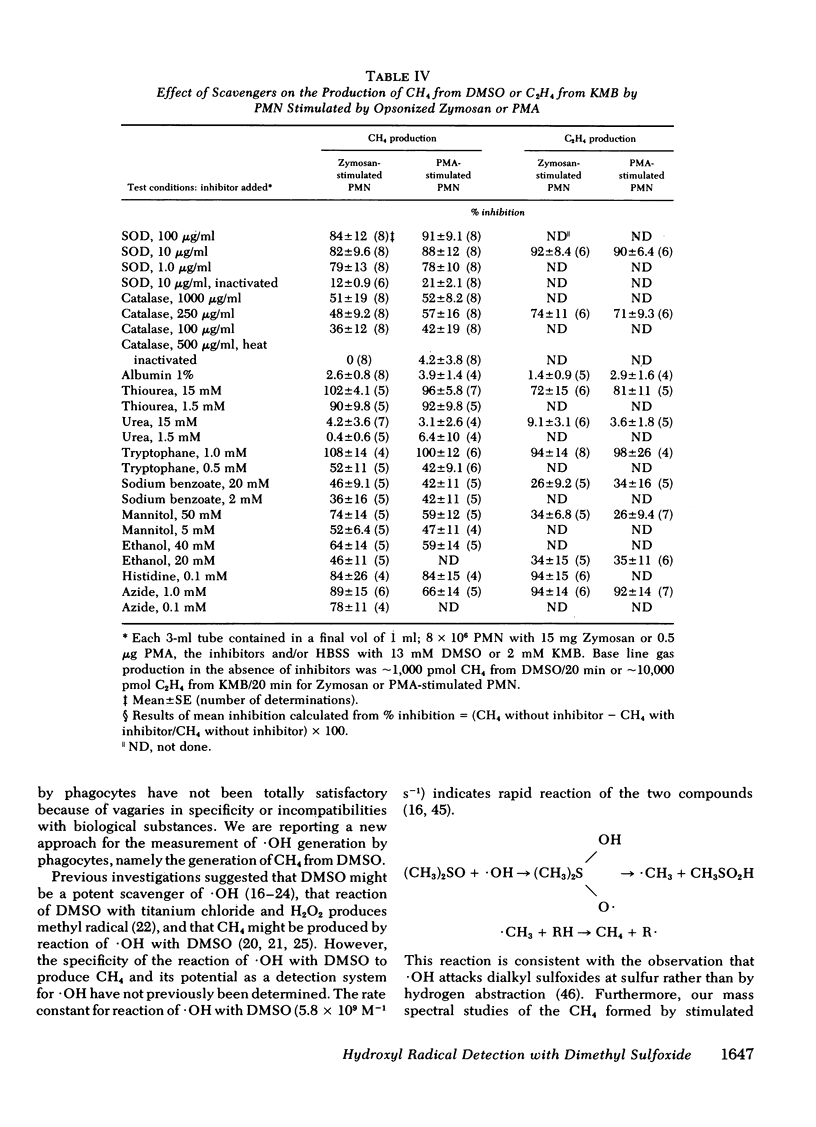
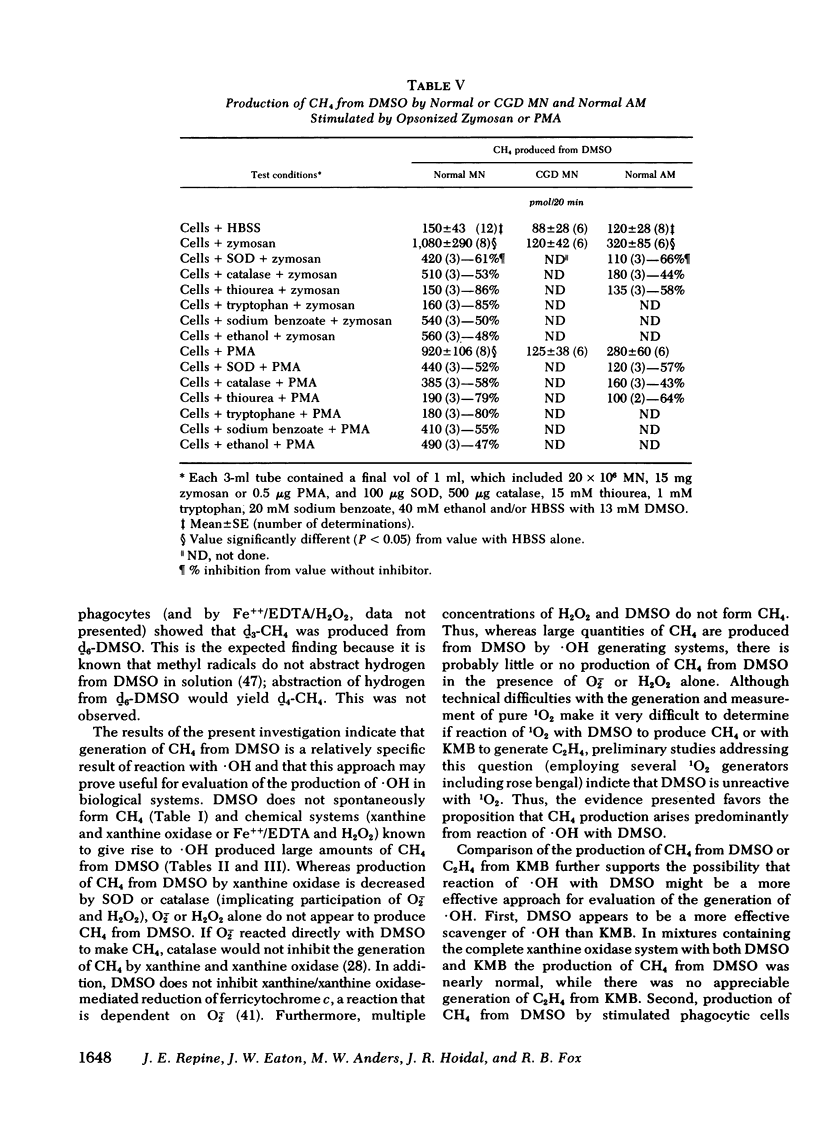
Methane (CH4) production from the anti-inflammatory agent, dimethyl sulfoxide (DMSO), was used to measure ·OH from chemical reactions or human phagocytes. Reactions producing ·OH (xanthine/xanthine oxidase or Fe++/EDTA/H2O2) generated CH4 from DMSO, whereas reactions yielding primarily O-2̇ or H2O2 failed to produce CH4. Neutrophils (PMN), monocytes, and alveolar macrophages also produced CH4 from DMSO. Mass spectroscopy using d6-DMSO showed formation of d3-CH4 indicating that CH4 was derived from DMSO. Methane generation by normal but not chronic granulomatous disease or heat-killed phagocytes increased after stimulation with opsonized zymosan particles or the chemical, phorbol myristate acetate. Methane production from DMSO increased as the number of stimulated PMN was increased and the kinetics of CH4 production approximated other metabolic activities of stimulated PMN. Methane production from stimulated phagocytes and DMSO was markedly decreased by purportedly potent ·OH scavengers (thiourea or tryptophane) and diminished to lesser degrees by weaker ·OH scavengers (mannitol, ethanol, or sodium benzoate). Superoxide dismutase or catalase also decreased CH4 production but urea, albumin, inactivated superoxide dismutase, or boiled catalase had no appreciable effect. The results suggest that the production of CH4 from DMSO may reflect release of ·OH from both chemical systems and phagocytic cells. Interaction of the nontoxic, highly permeable DMSO with ·OH may explain the anti-inflammatory actions of DMSO and provide a useful measurement of ·OH in vitro and in vivo.
Full text
PDF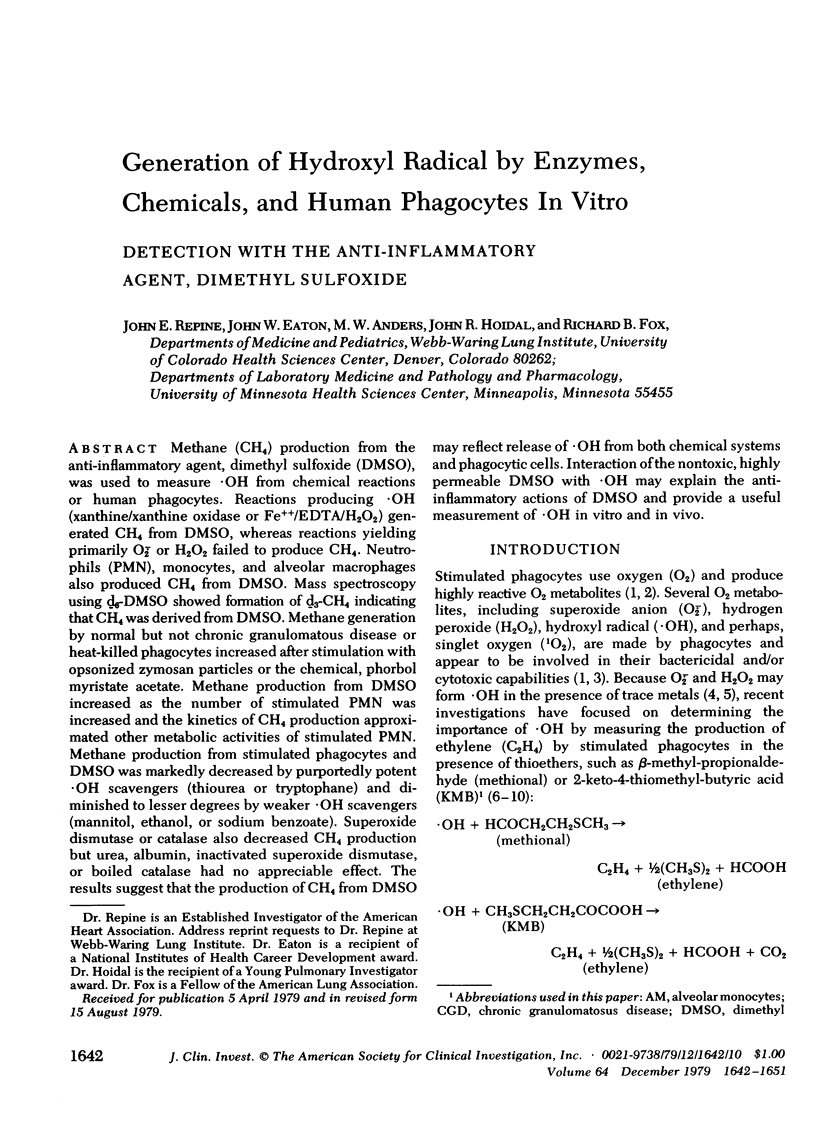





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. R., Brendzel A. M. Use of a unique chemiluminescence spectrometer in a study of factors influencing granulocyte light emission. J Immunol Methods. 1978;19(2-3):279–287. doi: 10.1016/0022-1759(78)90187-4. [DOI] [PubMed] [Google Scholar]
- Ashwood-Smith M. J. Current concepts concerning radioprotective and cryoprotective properties of dimethyl sulfoxide in cellular systems. Ann N Y Acad Sci. 1975 Jan 27;243:246–256. doi: 10.1111/j.1749-6632.1975.tb25363.x. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Bors W., Lengfelder E., Saran M., Fuchs C., Michel C. Reactions of oxygen radical species with methional: a pulse radiolysis study. Biochem Biophys Res Commun. 1976 May 3;70(1):81–87. doi: 10.1016/0006-291x(76)91111-6. [DOI] [PubMed] [Google Scholar]
- Brownlee N. R., Huttner J. J., Panganamala R. V., Cornwell D. G. Role of vitamin E in glutathione-induced oxidant stress: methemoglobin, lipid peroxidation, and hemolysis. J Lipid Res. 1977 Sep;18(5):635–644. [PubMed] [Google Scholar]
- Cederbaum A. I., Dicker E., Cohen G. Effect of hydroxyl radical scavengers on microsomal oxidation of alcohols and on associated microsomal reactions. Biochemistry. 1978 Jul 25;17(15):3058–3064. doi: 10.1021/bi00608a018. [DOI] [PubMed] [Google Scholar]
- Cederbaum A. I., Dicker E., Rubin E., Cohen G. The effect of dimethylsulfoxide and other hydroxyl radical scavengers on the oxidation of ethanol by rat liver microsomes. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1254–1262. doi: 10.1016/0006-291x(77)91428-0. [DOI] [PubMed] [Google Scholar]
- Chapman J. D., Reuvers A. P., Borsa J., Greenstock C. L. Chemical radioprotection and radiosensitization of mammalian cells growing in vitro. Radiat Res. 1973 Nov;56(2):291–306. [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G., Cederbaum A. I. Chemical evidence for production of hydroxyl radicals during microsomal electron transfer. Science. 1979 Apr 6;204(4388):66–68. doi: 10.1126/science.432627. [DOI] [PubMed] [Google Scholar]
- Cohen G. The generation of hydroxyl radicals in biologic systems: toxicological aspects. Photochem Photobiol. 1978 Oct-Nov;28(4-5):669–675. doi: 10.1111/j.1751-1097.1978.tb06993.x. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Farrant J. Human red cells under hypertonic conditions; a model system for investigating freezing damage. 3. Dimethylsulfoxide. Cryobiology. 1972 Apr;9(2):131–136. doi: 10.1016/0011-2240(72)90020-x. [DOI] [PubMed] [Google Scholar]
- French J. E., Flor W. J., Grissom M. P., Parker J. L., Sajko G., Ewald W. G. Recovery, structure, and function of dog granulocytes after freeze-preservation with dimethylsulfoxide. Cryobiology. 1977 Feb;14(1):1–14. doi: 10.1016/0011-2240(77)90117-1. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen metabolism in Lactobacillus plantarum. J Bacteriol. 1974 Jan;117(1):166–169. doi: 10.1128/jb.117.1.166-169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila R. E., Winston B., Cohen G. Alloxan-induced diabetes-evidence for hydroxyl radical as a cytotoxic intermediate. Biochem Pharmacol. 1976 May 1;25(9):1085–1092. doi: 10.1016/0006-2952(76)90502-5. [DOI] [PubMed] [Google Scholar]
- JACOB S. W., BISCHEL M., HERSCHLER R. J. DIMETHYL SULFOXIDE (DMSO): A NEW CONCEPT IN PHARMACOTHERAPY. Curr Ther Res Clin Exp. 1964 Feb;6:134–135. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Klebanoff S. J. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1974 Jun 25;249(12):3724–3728. [PubMed] [Google Scholar]
- Klebanoff S. J., Rosen H. Ethylene formation by polymorphonuclear leukocytes. Role of myeloperoxidase. J Exp Med. 1978 Aug 1;148(2):490–506. doi: 10.1084/jem.148.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake C. D. Dimethyl sulfoxide. Science. 1966 Jun 17;152(3729):1646–1649. doi: 10.1126/science.152.3729.1646. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- Miller J. S., Cornwell D. G. The role of cryoprotective agents as hydroxyl radical scavengers. Cryobiology. 1978 Oct;15(5):585–588. doi: 10.1016/0011-2240(78)90082-2. [DOI] [PubMed] [Google Scholar]
- Mironescu S. Hyperosmotic injury in mammalian cells. Volume and alkali cation alterations of CHO cells in unprotected and DMSO-treated cultures. Cryobiology. 1978 Apr;15(2):178–191. doi: 10.1016/0011-2240(78)90022-6. [DOI] [PubMed] [Google Scholar]
- Panganamala R. V., Sharma H. M., Heikkila R. E., Geer J. C., Cornwell D. G. Role of hydroxyl radical scavengers dimethyl sulfoxide, alcohols and methional in the inhibition of prostaglandin biosynthesis. Prostaglandins. 1976 Apr;11(4):599–607. doi: 10.1016/0090-6980(76)90063-0. [DOI] [PubMed] [Google Scholar]
- Pribor D. B. Biological interactions between cell membranes and glycerol or DMSO. Cryobiology. 1975 Aug;12(4):309–320. doi: 10.1016/0011-2240(75)90004-8. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Tang R. H. Ethylene formation from methional. Biochem Biophys Res Commun. 1978 Mar 30;81(2):498–503. doi: 10.1016/0006-291x(78)91562-0. [DOI] [PubMed] [Google Scholar]
- ROSENBAUM E. E., JACOB S. W. DIMETHYL SULFOXIDE (DMSO) IN MUSCULOSKELETAL INJURIES AND INFLAMMATIONS. II. DIMETHYL SULFOXIDE IN RHEUMATOID ARTHRITIS, DEGENERATIVE ARTHRITIS AND GOUTY ARTHRITIS. Northwest Med. 1964 Apr;63:227–229. [PubMed] [Google Scholar]
- Repine J. E., Clawson C. C., Friend P. S. Influence of a deficiency of the second component of complement on the bactericidal activity of neutrophils in vitro. J Clin Invest. 1977 May;59(5):802–809. doi: 10.1172/JCI108702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Clawson C. C. Influence of surface proteins and separation techniques on neutrophil unstimulated and stimulated locomotion in vitro. J Reticuloendothel Soc. 1978 Sep;24(3):217–226. [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. M. Effects of phorbol myristate acetate on the metabolism and ultrastructure of neutrophils in chronic granulomatous disease. J Clin Invest. 1974 Jul;54(1):83–90. doi: 10.1172/JCI107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuvers A. P., Greenstock C. L., Borsa J., Chapman J. D. Letter: Studies on the mechanism of chemical radioprotection by dimethyl sulphoxide. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Nov;24(5):533–536. doi: 10.1080/09553007314551431. [DOI] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J Exp Med. 1979 Jan 1;149(1):27–39. doi: 10.1084/jem.149.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. F. Toxicity of dimethyl sulfoxide, alone and in combination. Ann N Y Acad Sci. 1975 Jan 27;243:98–103. doi: 10.1111/j.1749-6632.1975.tb25348.x. [DOI] [PubMed] [Google Scholar]
- Sagone A. L., Jr, Mendelson D. S., Metz E. N. The effect of sodium azide on the chemiluminescence of granulocytes--evidence for the generation of multiple oxygen radicals. J Lab Clin Med. 1977 Jun;89(6):1333–1340. [PubMed] [Google Scholar]
- Shirley S. W., Stewart B. H., Mirelman S. Dimethyl sulfoxide in treatment of inflammatory genitourinary disorders. Urology. 1978 Mar;11(3):215–220. doi: 10.1016/0090-4295(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Spector J. I., Yarmala J. A., Marchionni L. D., Emerson C. P., Valeri C. R. Viability and function of platelets frozen at 2 to 3 C per minute with 4 or 5 per cent DMSO and stored at -80 C for 8 months. Transfusion. 1977 Jan-Feb;17(1):8–15. doi: 10.1046/j.1537-2995.1977.17177128894.x. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. Evaluation of opsonic and leukocyte function with a spectrophotometric test in patients with infection and with phagocytic disorders. Blood. 1973 Jul;42(1):121–130. [PubMed] [Google Scholar]
- Tauber A. I., Babior B. M. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977 Aug;60(2):374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., King G. W., LoBuglio A. F. Evidence for hydroxyl radical generation by human Monocytes. J Clin Invest. 1977 Aug;60(2):370–373. doi: 10.1172/JCI108785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. C., Wood J. Pharmacologic and biochemical considerations of dimethyl sulfoxide. Ann N Y Acad Sci. 1975 Jan 27;243:7–19. doi: 10.1111/j.1749-6632.1975.tb25339.x. [DOI] [PubMed] [Google Scholar]
- Yang S. F. Biosynthesis of ethylene. Ethylene formation from methional by horseradish peroxidase. Arch Biochem Biophys. 1967 Nov;122(2):481–487. doi: 10.1016/0003-9861(67)90222-6. [DOI] [PubMed] [Google Scholar]
- Yang S. F. Further studies on ethylene formation from alpha-keto-gamma-methylthiobutyric acid or beta-methylthiopropionaldehyde by peroxidase in the presence of sulfite and oxygen. J Biol Chem. 1969 Aug 25;244(16):4360–4365. [PubMed] [Google Scholar]


