Abstract
The advent of optogenetics provides a new direction for the field of neuroscience and biotechnology, serving both as a refined investigative tool and as potential cure for many medical conditions via genetic manipulation. Although still in its infancy, recent advances in optogenetics has made it possible to remotely manipulate in vivo cellular functions using light. Coined Nature Methods’ ‘Method of the Year’ in 2010, the optogenetic toolbox has the potential to control cell, tissue and even animal behaviour. This optogenetic toolbox consists of light-sensitive proteins that are able to modulate membrane potential in response to light. Channelrhodopsins (ChR) are light-gated microbial ion channels, which were first described in green algae. ChR2 (a subset of ChR) is a seven transmembrane a helix protein, which evokes membrane depolarization and mediates an action potential upon photostimulation with blue (470 nm) light. By contrast to other seven-transmembrane proteins that require second messengers to open ion channels, ChR2 form ion channels themselves, allowing ultrafast depolarization (within 50 milliseconds of illumination). It has been shown that integration of ChR2 into various tissues of mice can activate neural circuits, control heart muscle contractions, and even restore breathing after spinal cord injury. More compellingly, a plethora of evidence has indicated that artificial expression of ChR2 in retinal ganglion cells can reinstate visual perception in mice with retinal degeneration.
Introduction
In 1979, Francis Crick expressed that a cardinal challenge facing neuroscience is to develop the ability to control a single type of cell in the brain, while leaving other cells in the vicinity unaltered [1].
Traditional approaches such as electrical stimulation methods are incapable of such precise discrimination amongst cells while pharmacological methods, albeit specific, present the problem of having poor temporal and spatial resolution.
The solution to Crick’s challenge is ‘optogenetics’. This involves using genetic-engineering techniques to enable the expression of light-sensitive proteins, such as channelrhodopsins (ChR) or halorhodopsins (NpHR), on neurons. ChR and NpHR are akin to on and off light switches, respectively. In the presence of blue light, ChRs undergo a conformational change to permit the influx of cations, activating the cell. On the contrary, when a yellow light is shone NpHRs (which are chloride pumps), hyperpolarize the cell, thereby inactivating it [2]. The discovery of these two optogenetic tools allow for modulation of neurons and control over nerve circuits with an unprecedented degree of spatial, temporal and neuro-chemical accuracy (Fig. 1) [3,4].
FIGURE 1.
Light sensitive proteins channelrhodopsin (left) and halorhodopsin (right).
Copyright © Elsevier. Reproduced with permissions from Refs. [2,3].
In November 2012, researchers successfully suppressed epileptic seizures in rats following delivery of the gene encoding NpHR into a pyramidal cell via a viral vector [5,6]. This demonstrates the huge potential applicability of optogenetics, which will almost definitely be used as a tool to map functional circuits of the nervous system in the near future [7]. It could also be a possible therapeutic avenue for neurological disorders in humans including epilepsy, Parkinson’s disease, and spinal cord injuries [8].
Apart from these, optogenetics has a huge therapeutic potential in retinal degenerative diseases. Using a photoreceptor-deficient mouse model, Bi et al. established that the expression of ChR2 in retinal ganglion cells enabled the regeneration of visually elicited responses in the visual striate cortex in addition to encoding light signals [9]. Therefore in this review, the scope of optogenetics will focus on ChRs in the field of visual regeneration.
Channelrhodopsins: an optogenetic tool
ChR2 (737 amino acids) is a member of the rhodopsin (Rh) family. These are retinal-binding membrane proteins with a seven trans-membrane α helix motif (Fig. 2) [10,11]. Retinal is a bound chromophore that absorbs photons, hence enabling the photo-cycle to occur [12]. Mutations in the ChR2 gene that cause certain amino acid changes could alter properties such as the absorption, conductance or kinetics of the channel (Fig. 3), generating novel ChR variants [13].
FIGURE 2.
FIGURE 3.
(A) Three-dimensional structure of ChR2. Retinal is shown in yellow. The residues in blue are conserved in the four known ChR, while those that differ are coloured grey. (B) Structures of 2 possible chromophore dark state isomers.
Copyright © 2011 Nature Publishing Group. Reproduced with permissions from Ref. [13].
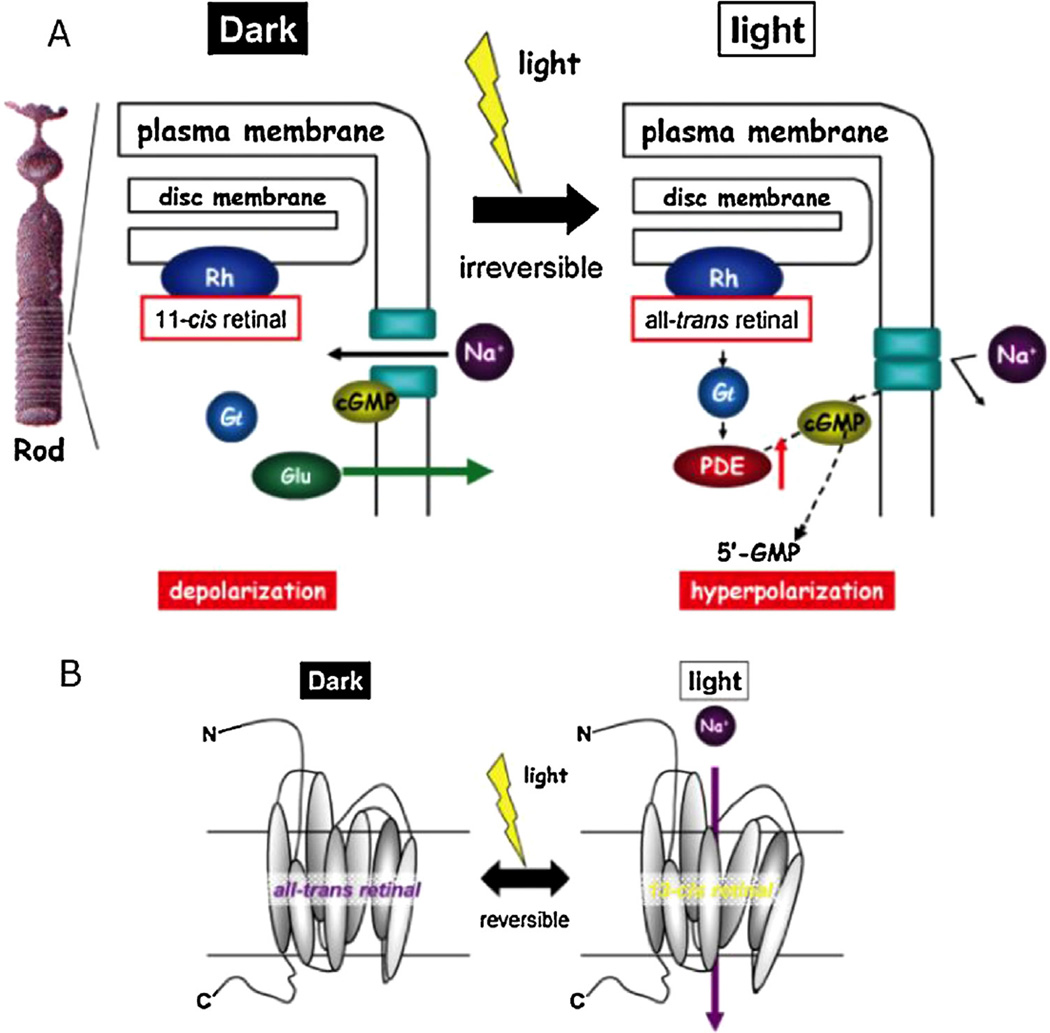
Discovered in 1984 [14], this light-gated cation channel is found intrinsically in the eyespot of the unicellular green alga Chlamydomonas reinhardtii. Its innate photoperceptive function gives rise to its phototactic behaviour. Phototaxis occurs because of the action potential generation, evoked by membrane depolarization upon photostimulation with blue light (470 nm) [10].
In conventional metabotropic rhodopsins, the photo-isomerization of 11-cis-retinal to all-trans-retinal evokes a G-protein mediated signalling cascade. The channel activation leads to consequential activation of GTP-binding protein transducing (Gt) which goes on to activate the next protein in the cascade, cGMP phosphodiesterase (PDE) (Fig. 4) [15]. The hydrolysis of cGMP to 5′-GMP by PDE closes the channel, inactivating it. The isomerization of 11-cis-retinal is irreversible and regenerating it requires multiple enzymatic reactions.
FIGURE 4.
Schematic diagram of photo-stimulated signal transduction pathways in (A) Rh and (B) ChR2.
Copyright © 2009 Springer. Reproduced with permissions from Ref. [15].
By contrast to metabotropic rhodopsins, channelrhodopsins are ionotropic (i.e. they form ion channels themselves). The conformational change to be directly coupled to light, allowing ultrafast depolarization (within 50 milliseconds of illumination) [16], irrespective of extracellular pH. Moreover, as ChR2 is a microbial type (or type I) rhodopsin, the chromophore contains all-trans-retinal in the ground (dark) state, and not 11-cis-retinal like rhodopsins in vertebrate retina [17]. Upon visual stimulation, the concomitant binding and isomerization of all-trans-retinal to 13-cis-retinal elicits a conformational change to open the non-selective cation channel. This photo-isomerization is a reversible process as both isomers remain attached to the protein. This is unlike animal visual pigments that lose their chromophore after photo-isomerization from 11-cis to all-trans [18]. Additionally, all-trans-retinal is an endogenous chemical found in the mammalian nervous system [19]. Thus, this eliminates the need to introduce exogenous mediators (Table 1).
TABLE 1.
Summary of ChR2s structure and properties
| Structure | Function |
|---|---|
|
|
Fundamental optogenetics
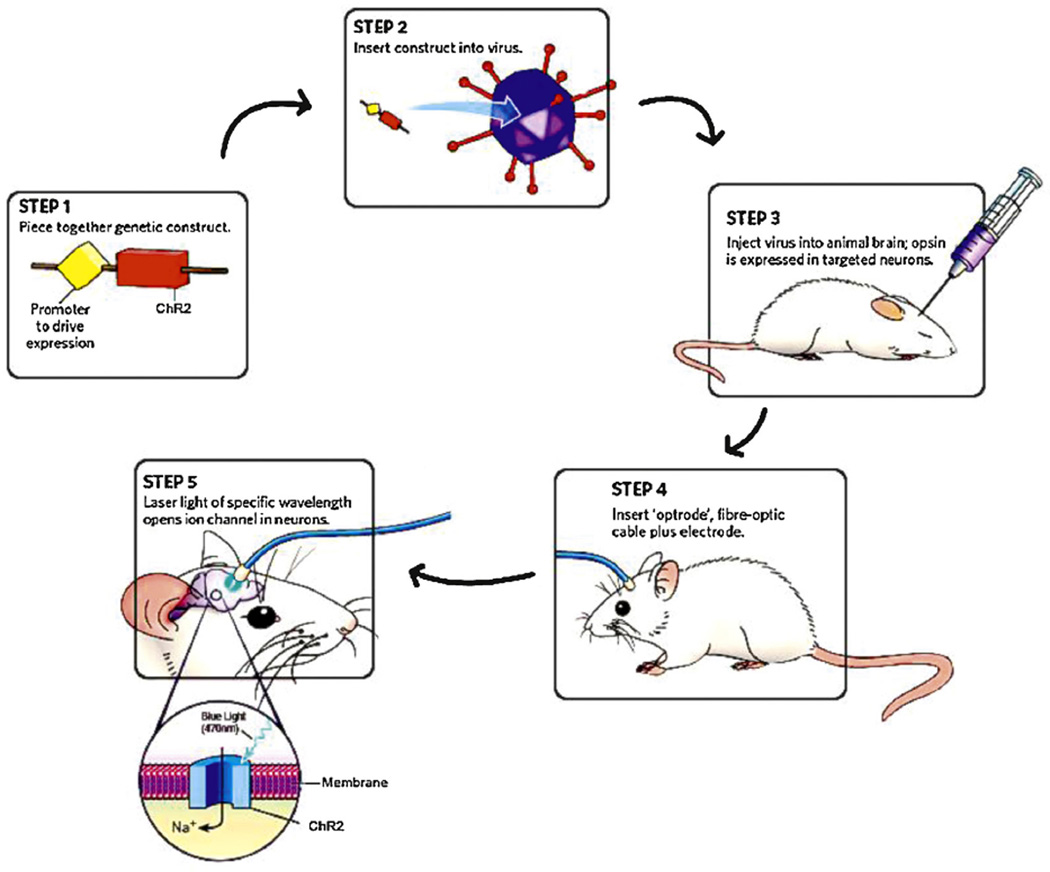
Optogenetics employs the basic principles of gene therapy – to compensate the loss of function by targeting DNA itself. Upon obtaining the exogenous gene (e.g. ChR2) along with a specific promoter sequence, it is re-packaged into an appropriate vector and introduced into the target cell (Fig. 5) [20]. Subsequently, cellular mechanisms transcribe and translate the inserted gene, resulting in the synthesis of ChR2 protein, which is then incorporated into the host cell membrane. These proteins are activated by illumination and thus, cellular activity can be altered by controlling the light switch.
FIGURE 5.
Schematic diagram of optogenetic techniques.
Copyright © 2010 Nature Publishing Group. Reproduced with permissions from Ref. [20].
Obtaining gene of interest
Channelopsin-1 and -2 (Chop1 and Chop2) were successfully cloned from C. reinhardtii [21–23]. In the presence of all-trans-retinal, these cloned opsins demostrated the ability to form directly light-sensitive ion channels when expressed in Xenopus laevis oocytes or HEK293 cells. Chop2 is favoured as its functional ion-channel, ChR2 (ChR2 refers to Chop2 with a chromophore attached), is permeable to physiological cations. Although many novel ChR variants possessing improved properties have been engineered [19], the current challenge is to develop the optimal ChR variant – one that balances between being kinetically rapid and having high light sensitivity, that is not at risk of desensitization [24].
Delivery of ChR2 gene to target cell
There are three main methods for deliver of genes coding for light-sensive proteins into target cells, each with their own advantages and limitations. They are transfection, viral transduction, and creating transgenic animal lines. Targeted cellular expression can be achieved by using specific promoters, recombinase-based conditional systems, delivering a location specific injection, or by restricting activation through targeted light delivery [25].
Transfection
Transfection refers to non-viral methods of introducing the gene into host cells. This includes electroporation, DNA microinjection, liposomal transfection, and calcium phosphate precipitation. Such methods are relatively safe as it does not involve using infectious biological agents [19], However, two significant limitations are its effectiveness in achieving a high level of expression on the host cell as well as practicality issues for use in vivo.
Viral transduction
Viral transduction is currently the most popular method used. This technique involves using a recombinant virus containing the ChR2 gene, which is injected into a specific region on the gene and hence expressed either selectively or broadly, depending on the aim. A multitude of viral vectors, including adenoviruses [26], adenoassociated viruses (AAV) [27], retroviruses [28] and lentiviruses [29,30], have been used experimentally.
By using viral vectors, precise cells can be targeted by other means apart from using specific promoters. For instance, gene delivery to specific cells can be achieved through targeting populations of neurons depending on their topological connections.
However, one significant limitation of using viral delivery is that that there is an upper limit to the length of the gene used. The promoter chosen needs to be small (less than 4 kb), precise, and strong [25], and therefore this downside can be circumvented by using Cre-driver animals and Cre-dependent viruses. This technique is designed such that the expression of channelrhodopsin is dependent on the coexpression of a tyrosine recombinase enzyme, Cre recombinase. The presence of Cre enables strong expression of channelrhodopsin with for instance, the elongation factor 1-alpha (EF1a) promoter.
Another limitation is that viral transduction could lead to undesirable side effects such as triggering an adverse immune response, causing unspecific systemic disemination of viral vectors, or potentially even lead to an overexpression of the ChR2 gene [15].
Nevertheless, these considerations are not a concern as the eye is considered to be an immunologically protected space. The blood-retinal barrier, coupled with the eye’s unique immune surveillance system, enables such protection to be attained. Systemic dissemination of the viral vector is twarted by the presence of the blood-retinal barrier while the eye-specific immune system may play a role in activating an antibody-mediated immune reaction in response to the foreign viral antigens. Hence, these features make viral vectors a good option for gene delivery.
Transgenic animal lines
Another popular method involves the use of transgenic or knockin animals. The first example of using this technique in optoge-netics involves the generation of a transgenic ChR2-YFP (fusion protein) expressing mouse line under the control of the Thy1 promoter [7]. This involves cloning cDNA encoding ChR2-YFP fusion protein to the Xhol site of the mouse Thy1 vector [31]. Standard pronuclear injection techniques were then applied to create transgenice mouse lines [32]. One feature of this method is that the transgene expression increases with animal maturation, contributing to the high level of ChR2 expression in an adult mouse.
Other transgenic animals used in optogenetic experimentation involving ChR2 include Caenorhabditis elegans [33], zebrafish [34], Drosophila melanogaster [35] and primates [36]. Furthermore, Weick et al. showed that ChR2 was functional in human embryonic stem cell-derived neurons [37].
Techniques involving the use of transgenic animals have an advantage over that involving viral transduction in that there are no viral payload limitations and larger promoters can be used which confer tigher control over transgene expression. Additionally, it allows for a more widespread expression of ChR2 in various locations within the brain. One limitation is the inability for blue light to penetrate into deeper regions, such as deep brain tissues [38,39].
Target cell
Most strategies use either ON bipolar cells [40,41] or retinal ganglion cells [9,42,43] as target cells for ChR2 gene transfer.
When the ChR2 gene is transduced into ON bipolar cells, there is selective activation of the retinal ON pathway in the presence of light. However, some problems, such as those relating to the mechanism of gene transfer into ON bipolar cells, were faced using this method [15]. Nevertheless, Lagali et al. managed to achieve successful expression of ChR2 gene onto ON bipolar cells (Fig. 6) [41]. The technique involved using a 200 base pair promoter sequence from the Grm6 gene found in mice. This gene encodes for a receptor specific to ON-bipolar cells, the metabotropic glutamate receptor 6 (mGluR6) (Fig. 7) [41]. In the normal wild-type retina, the Rh molecules are involved in photoreception and are the only light-sensitive cells in the retina. Bipolar cells receives direct synaptic input from the photoreceptors which is then passed on to retinal ganglion cells (RGCs) via synapses found in the inner plexiform layer (IPL). RGCs are the sole output from the retina and transmit this information to the optic nerve. Such a mechanism is not possible in retinal degenerative conditions because of the loss of photoreceptors. However, this method bypasses the photoreceptors by expressing ChR2 on ON bipolar cells. Thus, the inner retinal circuitry can be activated through the photo-mediated activation of ChR2-expressing ON bipolar cells.
FIGURE 6.
Illustration demostrating retinal activity in wild-type retinas in comparison with ChR2-expressing ON bipolar cells in degenerated retinas.
Copyright © 2008 Nature Publishing Group. Reproduced with permissions from Ref. [41].
FIGURE 7.
ChR2-expressing ON bipolar cells in wild-type and rd1 mouse retinas.
Copyright © 2008 Nature Publishing Group. Reproduced with permissions from Ref. [41].
Another aspect that can be targeted is the RGC layer. RGCs are in fact better candidates, as target genes are easily transducible into these cells. Tomita et al. demonstrated that after a single intravitreal injection of AAV2/2 vector containing the ChR2 gene, about 30% of RGCs became photosensitive owing to the expression of ChR2 [15]. This is consistent with experiments that used ChR2 rd1/rd1 transgenic mice expressing ChR2-YFP (yellow fluorescent protein), which also showed similar results (Fig. 8) [44].
FIGURE 8.
(A) ChR2-expressing RGCs are able to absorb the photon directly, omitting bipolar cell-mediated pathways. (B) Retinal ganglion cells (RGC) expressing ChR2. (C) Visually evoked potential in non-dystrophic rat, dystrophic rat and dystrophic rat + AAV ChR2. Copyright © 2009 Springer. (D) Transgenic mice expressing ChR2-YFP; Cholinergic amarcrine cells stained with ChAT, which do not express ChR2-YFP. Copyright © 2010 Society for Neuroscience. Reproduced with permissions from Refs [15,44].
In the normal visual pathway, photoreceptors absorb a photon and transmit signals to the RGC via second order neurons such as bipolar cells. RGCs expressing ChR2 possess the advantage of displaying a quicker response to light as transmit signals directly to the lateral geniculate nucleus (LGN), omitting bipolar cell-mediated pathways. This is demonstrated by the experiment by Lagali et al., which compares the visually evoked potentials in the non-dystrophic rat, dystrophic rat and dystrophic rat + AAV ChR2. The robust amplitude was recorded upon visual stimulation in dystrophic rat + AAV-ChR2 with a shorter P1 latency than the non-dystrophic rat. This can be attributed to the direct response of RGC to photo-stimulation.
Nevertheless, to make this a viable therapeutic treatment in humans, there are certain clinical agendas that need to be met. Genetic expression of ChR2 needs to confer long-term specificity and must demostrate continuous therapeutic efficacy, while remaining clinically practical [40].
Visual regeneration in animal models
Expression of ChR2 can be achieved in retinal cells, including ON bipolar cells [40] and retinal ganglion cells [9,43]. This can be delivered via plasmid or adeno-associated virus (AAV) with high transduction efficiency [45,46], or transgenic mouse lines under Thy-1.2 promoter bred to express ChR2 [44,47]. Experimental evidence demonstrated that long-term stability of ChR2 expression was achieved in vivo [48,49], with restoration of photosensitivity in almost the whole lifespan (64 weeks) of murine models. Gene delivery using viruses as vectors was generally considered safe as this did not cause neurotoxicity [50] or significant immunologically harmful reactions [51]. Expression of ChR2 and stimulation using blue LED [12] mediates depolarization and subsequent action potential in retinal ON bipolar cells, generating visual signals sufficient for animals to perform optomotor tasks [41] (Table 2).
TABLE 2.
Summary of optogentic experiments involving ChR2 and variants
| Gene | Delivery method |
Illumination method |
Recipient cell |
In vivo/ in vitro |
Overview | Reference |
|---|---|---|---|---|---|---|
| Chop2-GFP | AAV | Xenon lamp with 400–580 nm filter | RGC | In vivo |
|
[9] |
| hChR2 | rAAV | Blue LED (470 nm) | ON bipolar cells | In vivo |
|
[40] |
| Chop2 | AAV |
|
RGC | In vivo |
|
[43] |
| hChR2 | Plasmid | Metal halide lamp-based epifluorescent illuminator (460 ± 40 nm) | Soma and dendrites of RGC | In vivo |
|
[45] |
| ChR2 | AAV | Blue LED (470 nm) | RGC | In vivo |
|
[46] |
| Chop2-GFP | rAAV2 | Blue LED (455 nm) | GCL | In vivo |
|
[48] |
| Chop2-GFP | rAAV2 | Blue LED (455 nm) | Retinal cells and optic nerve | In vivo |
|
[49] |
| Chop2-GFP | rAAV2 | Blue LED (455 nm) | Retinal neurons | In vivo |
|
[50] |
| ChR2 | Plasmid | Epifluorescent mercury lamp-based illuminator | Retinal ON bipolar cells | In vivo |
|
[41] |
| ChR2 | rAAV | Blue LED (470 nm) | Retinal cells | In vivo |
|
[51] |
| ChR2 | Transgenic mouse lines under Thy-1.2 promoter | Video projector (blue gun component) | Retinal cells | In vivo |
|
[44] |
| ChR2 | AAV | Blue LED (470 nm) | RGC | In vivo |
|
[12] |
Neural activation through illumination
The control over the neuron lies in with the light switch (Fig. 9). This precise control is achieved by altering the illumination light temporally (e.g. by using an ultrafast shutter with constant light source or high-speed LED flashes) or spatially (e.g. light-patterning methods that enable the illumination of a subset of cells) [1]. Other factors include the physical size of the light source, intensity and illumination volume [19].
FIGURE 9.
Neuronal activation following photo-stimulation (top). ChR2s expressed on neuronal membrane (bottom).
Copyright © 2012 Elsevier. Reproduced with permissions from Ref. [14].
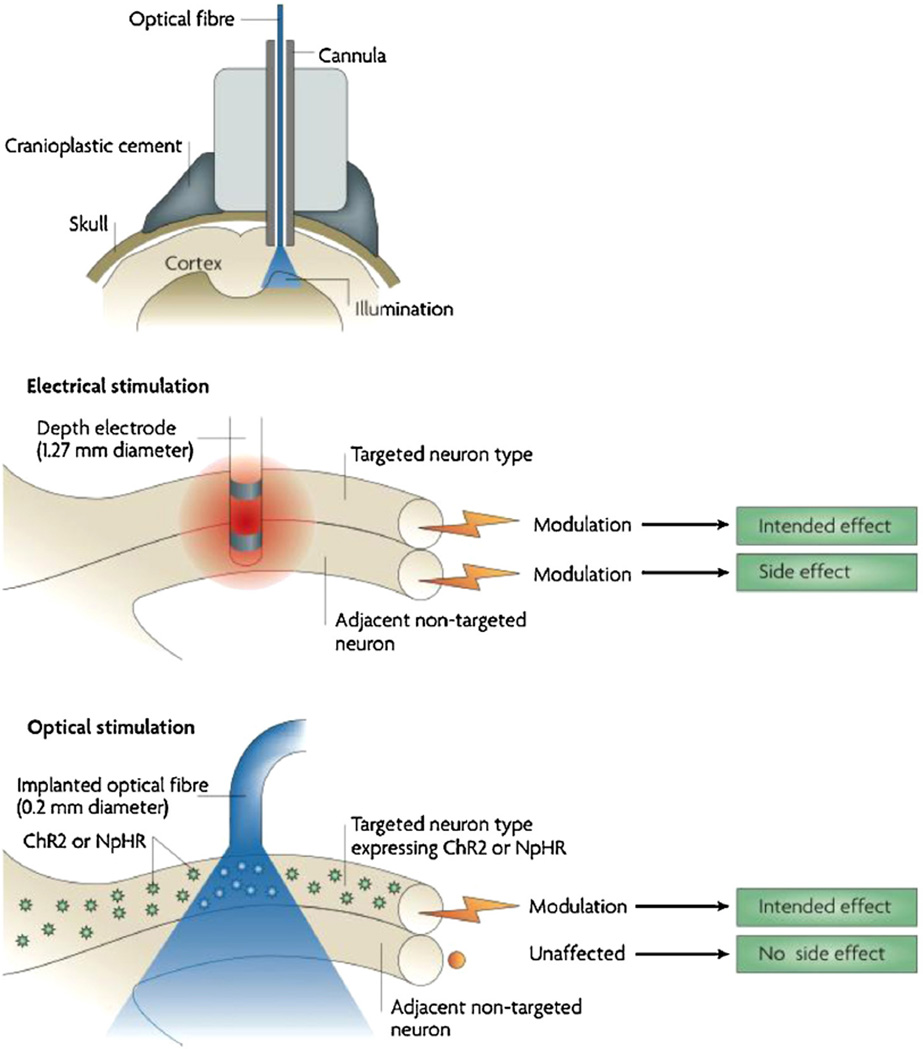
Successful photostimulation requires at least 5 mW/mm2 of blue light at sample [52]. Typically, the light source for in vitro experimentation used includes arc lamps (xenon, mercury, mercury–xenon or metal halide) [53–55], laser-based systems and more recently, light-emitting diodes (LED) [56]. In vivo, stimulation is commonly done by with laser light coupled to optical fibres which are guided through cannulas [57], to deliver light to the transduced tissue. Alternatively, fibre-coupled LEDs can also be used (Fig. 10).
FIGURE 10.
Light delivered through optic fibre to rodent’s brain.
Copyright © 2012 Elsevier. Reproduced with permissions from Ref. [85].
The main advantages of arc lamps are that it is easily accessible as it can be made via modifications to the fluorescence microscope (available in most laboratories) and that it emits continuous light at high intensity. However, its large size and poor efficiency in coupling light into the optic fibre makes it a less practical option for in vivo usage.
Laser-based systems possess the twin-advantages of being efficient at coupling light into the optic fibre, and the ability to deliver high intensity brightness per unit area of illumination, although this is at the expense of a relatively higher cost and power requirement.
LEDs, on the other hand, are compact and can be easily mounted on the rodent, enabling free and unhindered movement (Fig. 11). In 2011, Wentz et al. successfully developed a wireless headborne device [58]. Notwithstanding, LEDs are at present, weaker in terms of light intensity as compared to its arc lamp and laser-based counterparts. It is predicted that this challenge could be overcome in future with technological advancements in this aspect (Table 3).
FIGURE 11.
Illustration of optogenetic control in animal models.
Copyright © 2007 Nature Publishing Group. Reproduced with permissions from Ref. [57].
TABLE 3.
Benefits and limitations of light sources used in ChR2-related optogenetic excitation experimentsAdapted from Ref. [19].
| Light source | Benefits | Limitations |
|---|---|---|
| Arc lamp |
|
|
| Laser-based system |
|
|
| Light-emitting diode (LED) |
|
|
A panacea for retinal disorders
Retinitis pigmentosa (RP) is one of the most common hereditary degenerative eye disease. It is attributed to the progressive loss of photoreceptors, which are the rods and cones. Initial symptoms include night blindness, which sequentially escalates to the loss of peripheral vision, causing ‘tunnel vision’ [59]. Being the only retinal neurons possessing the ability to absorb photons, the complete degeneration of photoreceptor cells would ultimately lead to the loss of central vision [15]. Another retinal dystrophy accounting for over 45% of visual impairment in patients over 65 years of age is macular degeneration (MD), which spares peripheral but not central vision [60]. Unfortunately, there is currently no effective treatment for either of these conditions. Methods currently employed include the use of retinal prostheses, gene replacement therapy and either retinal cell [61–67] or stem cell [68–72] transplantations.
Retinal prostheses are implantable biomedical devices. They function by utilizing a photo-sensitive video camera [73] to generate visual percepts by neuro-electrical stimulation of spared neurons in the inner retina [40]. A myriad of designs are named in accordance to their location – including cortical, subretinal and epiretinal [74]. Although electrical stimulation of the retina or optic nerve could help restore light perception, it non-specifically activates multiple cell types, affecting spatial and temporal processing [75,76].
Gene-replacement therapy has shown to success in treating a rare form of RP, Leber’s congentinal amaurosis (LCA), involving mutations in the RPE65 gene [77,78]. Although it is a promising strategy, one significant disadvantage is that retinal degeneration is not caused by a straightforward single gene mutation which can be easily replaced. The genetic heterogeneity of retinal degeneration renders such a strategy unfeasible [79]. Furthermore, studies have shown that the use of gene-replacement therapy in visual regeneration is often complicated by retinal reorganization [80] and photoreceptor death [40].
Transplantation of the retinal pigment epithelium (RPE) provides a transducing interface, enabling visual perception. However, technical difficulties are often encountered in establishing synaptic connection from the transplanted cells to host retina [73]. Moreover, it is only applicable during early stages of disease as its effectiveness is limited once there is severe or complete loss of photoreceptors [81]. The transplantation of embryonic stem cells (ESCs) is another strategy being researched in the field of visual regeneration. The goal is to enable these unspecialized ESCs differentiate into photoreceptors, replacing the ones lost in retina dystrophies [82]. One significant disadvantage is that the use of ESCs is controversial [83]. Admittedly, the discovery of induced pluripotent stem cells (iPSCs) could overcome such ethical issues [72]. Nevertheless, there are still challenges faced with methods employing the use of stem cells such as the inability to direct and control the correct differentiation fate of ESCs into specialized adult cell types [83].
Another method is neuroprotection, which involves the over-expression of neurotrophic factors (e.g. GDNF) to slow down genetically determined photoreceptor cell death, regardless of the mechanism of cell death [84]. Although successfully tested in animal models, there was no significant benefit over the placebo in human trials [83]. Furthermore, it is not applicable for advanced disease stages where all the photoreceptors are lost [73].
However, the burgeoning field of optogenetics and discovery of ChR2 has conjured hope as a novel therapeutic option for these medical conditions, as it overcomes the key problems of methods that are currently employed (Table 4).
TABLE 4.
Evaluation of current methods of treatment for retinal dystrophies in humans.
| Method | Features | Limitation |
|---|---|---|
| Retinal prostheses |
|
|
| Gene therapy |
|
|
| Retinal pigment layer (RPE) transplantation |
|
|
| Stem cell (SC) Transplantation |
|
|
Apart from being a specific and precise potential therapeutic option, optogenetics could solve the problem of genetic heterogeneity in these retinal degenerative diseases, which has made treatment complicated. Indeed, RP is associated with nearly 200 different genetic mutations [79]. Observations from experiments suggest that the optogenetic method has the potential to be used as therapeutic intervention independently of the aetiology of the retinal degeneration. Experiments performed have used many animal models [85], with different mutations that mimic various retinal degenerative disorders in humans. For instance, some different etiologies include mice homozygous for rd mutation (rd/rd) with a null mutation in a cyclic GMP phosphodiesterase, PDE6 [86,87], older dystrophic Royal College of Surgeons (RCS) rats (rdy/rdy) [88], as well as recessive models for inherited retinitis pigmentosa [89,90]. Hence, this demonstrates its broad applicability as a therapeutic intervention for multifactorial, polygenic retinal disorders.
Conclusions and future directions
Optogenetics is still in its nascent stages, with a potential to be developed into therapeutic strategies, and perhaps might even hold the key to numerous unanswered questions in neuroscience.
The optogenetic toolbox is ever expanding, with newer improved proteins being developed that have enhanced sensitivity, ionic selectivity and temporal resolution. Currently, the challenge is to create tools that are of greater precision, to target subcellular compartments such as dendrites or axons [89], tools for two-photon activation, as well as tools that expand the optical control further [91].
There is most certainly light at the end of the tunnel in the field of optogenetics, with many opportunities, both as a research tool and therapeutic strategy. The implications of this field might even be translated to actual visual regeneration in humans in the near future, and reach new frontiers in the realm of regenerative medicine.
References
- 1.Deisseroth K. Optogenetics. Nature Methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dugue GP, Akemann W, Knopfel T. A comprehensive concept of optogenetics. Progress in Brain Research. 2012;196:1–28. doi: 10.1016/B978-0-444-59426-6.00001-X. [DOI] [PubMed] [Google Scholar]
- 3.LaLumiere RT. A new technique for controlling the brain: optogenetics and its potential for use in research and the clinic. Brain Stimulation. 2011;4:1–6. doi: 10.1016/j.brs.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Britt JP, McDevitt RA, Bonci A. Use of channelrhodopsin for activation of CNS neurons. Current Protocols in Neuroscience. 2012 doi: 10.1002/0471142301.ns0216s58. Chapter 2, Unit2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feilden T. Switching on a light in the brain. 2012 http://www.bbc.co.uk/news/science-environment-20513292.
- 6.Wykes RC, et al. Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Science Translational Medicine. 2012;4 doi: 10.1126/scitranslmed.3004190. 161ra152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arenkiel BR, et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji ZG, Ishizuka T, Yawo H. Channelrhodopsins – their potential in gene therapy for neurological disorders. Neuroscience Research. 2012 doi: 10.1016/j.neures.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Bi A, et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller M, Bamann C, Bamberg E, Kuhlbrandt W. Projection structure of channelrhodopsin-2 at 6 Å resolution by electron crystallography. Journal of Molecular Biology. 2011;414:86–95. doi: 10.1016/j.jmb.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 11.Abilez OJ, et al. Multiscale computational models for optogenetic control of cardiac function. Biophysical Journal. 2011;101:1326–1334. doi: 10.1016/j.bpj.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomita H, et al. Channelrhodopsin-2 gene transduced into retinal ganglion cells restores functional vision in genetically blind rats. Experimental Eye Research. 2010;90:429–436. doi: 10.1016/j.exer.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Hegemann P, Moglich A. Channelrhodopsin engineering and exploration of new optogenetic tools. Nature Methods. 2011;8:39–42. doi: 10.1038/nmeth.f.327. [DOI] [PubMed] [Google Scholar]
- 14.Holmes D. Is neurology ready to see the light? Lancet Neurology. 2012;11:663–664. doi: 10.1016/S1474-4422(12)70170-9. [DOI] [PubMed] [Google Scholar]
- 15.Tomita H, Sugano E, Isago H, Tamai M. Channelrhodopsins provide a breakthrough insight into strategies for curing blindness. Journal of Genetics. 2009;88:409–415. doi: 10.1007/s12041-009-0062-6. [DOI] [PubMed] [Google Scholar]
- 16.Nagel G, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ullrich S, Nagel G, Nagel G. Degradation of channelopsin-2 in the absence of retinal and degradation resistance in certain mutants. Biological Chemistry. 2012 doi: 10.1515/hsz-2012-0256. [DOI] [PubMed] [Google Scholar]
- 18.Oesterhelt D. The structure and mechanism of the family of retinal proteins from halophilic archaea. Current Opinion in Structural Biology. 1998;8:489–500. doi: 10.1016/s0959-440x(98)80128-0. [DOI] [PubMed] [Google Scholar]
- 19.Lin JY. Optogenetic excitation of neurons with channelrhodopsins: light instrumentation, expression systems, and channelrhodopsin variants. Progress in Brain Research. 2012;196:29–47. doi: 10.1016/B978-0-444-59426-6.00002-1. [DOI] [PubMed] [Google Scholar]
- 20.Buchen L. Neuroscience: illuminating the brain. Nature. 2010;465:26–28. doi: 10.1038/465026a. [DOI] [PubMed] [Google Scholar]
- 21.Nagel G, et al. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 22.Sineshchekov OA, Jung KH, Spudich JL. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii . Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8689–8694. doi: 10.1073/pnas.122243399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki T, et al. Archaeal-type rhodopsins in Chlamydomonas: model structure and intracellular localization. Biochemical and Biophysical Research Communications. 2003;301:711–717. doi: 10.1016/s0006-291x(02)03079-6. [DOI] [PubMed] [Google Scholar]
- 24.Lin JY. A user’s guide to channelrhodopsin variants: features, limitations and future developments. Experimental Physiology. 2011;96:19–25. doi: 10.1113/expphysiol.2009.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annual Review of Neuroscience. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reichel MB, et al. An immune response after intraocular administration of an adenoviral vector containing a beta galactosidase reporter gene slows retinal degeneration in the rd mouse. British Journal of Ophthalmology. 2001;85:341–344. doi: 10.1136/bjo.85.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali RR, et al. Gene transfer into the mouse retina mediated by an adeno-associated viral vector. Human Molecular Genetics. 1996;5:591–594. doi: 10.1093/hmg/5.5.591. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto T, et al. Inhibition of experimental proliferative vitreoretinopathy by retroviral vector-mediated transfer of suicide gene. Can proliferative vitreoretinopathy be a target of gene therapy? Ophthalmology. 1995;102:1417–1424. doi: 10.1016/s0161-6420(95)30850-0. [DOI] [PubMed] [Google Scholar]
- 29.Miyoshi H, Takahashi M, Gage FH, Verma IM. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lotery AJ, et al. Gene transfer to the nonhuman primate retina with recombinant feline immunodeficiency virus vectors. Human Gene Therapy. 2002;13:689–696. doi: 10.1089/104303402317322258. [DOI] [PubMed] [Google Scholar]
- 31.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 32.Feng G, Lu J, Gross J. Generation of transgenic mice. Methods in Molecular Medicine. 2004;99:255–267. doi: 10.1385/1-59259-770-X:255. [DOI] [PubMed] [Google Scholar]
- 33.Nagel G, et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Current Biology. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 34.Schoonheim PJ, Arrenberg AB, Del Bene F, Baier H. Optogenetic localization and genetic perturbation of saccade-generating neurons in zebrafish. Journal of Neuroscience. 2010;30:7111–7120. doi: 10.1523/JNEUROSCI.5193-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroll C, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Current Biology. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 36.Han X, et al. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62:191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weick JP, et al. Functional control of transplantable human ESC-derived neurons via optogenetic targeting. Stem Cells. 2010;28:2008–2016. doi: 10.1002/stem.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flusberg BA, et al. Fiber-optic fluorescence imaging. Nature Methods. 2005;2:941–950. doi: 10.1038/nmeth820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flusberg BA, Jung JC, Cocker ED, Anderson EP, Schnitzer MJ. In vivo brain imaging using a portable 3.9 gram two-photon fluorescence microendoscope. Optics Letters. 2005;30:2272–2274. doi: 10.1364/ol.30.002272. [DOI] [PubMed] [Google Scholar]
- 40.Doroudchi MM, et al. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Molecular Therapy. 2011;19:1220–1229. doi: 10.1038/mt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lagali PS, et al. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nature Neuroscience. 2008;11:667–675. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- 42.Tomita H, et al. Restoration of visual response in aged dystrophic RCS rats using AAV-mediated channelopsin-2 gene transfer. Investigative Ophthalmology and Visual Science. 2007;48:3821–3826. doi: 10.1167/iovs.06-1501. [DOI] [PubMed] [Google Scholar]
- 43.Farah N, Reutsky I, Shoham S. Patterned optical activation of retinal ganglion cells. Conference Proceedings of IEEE Engineering in Medicine and Biology Society. 2007:6368–6370. doi: 10.1109/IEMBS.2007.4353812. [DOI] [PubMed] [Google Scholar]
- 44.Thyagarajan S, et al. Visual function in mice with photoreceptor degeneration and transgenic expression of channelrhodopsin 2 in ganglion cells. Journal of Neuroscience. 2010;30:8745–8758. doi: 10.1523/JNEUROSCI.4417-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenberg KP, Pham A, Werblin FS. Differential targeting of optical neuromodulators to ganglion cell soma and dendrites allows dynamic control of center-surround antagonism. Neuron. 2011;69:713–720. doi: 10.1016/j.neuron.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 46.Isago H, et al. Age-dependent differences in recovered visual responses in Royal College of Surgeons rats transduced with the channelrhodopsin-2 gene. Journal of Molecular Neuroscience. 2012;46:393–400. doi: 10.1007/s12031-011-9599-y. [DOI] [PubMed] [Google Scholar]
- 47.Tomita H, et al. Visual properties of transgenic rats harboring the channelrhodopsin-2 gene regulated by the thy-1.2 promoter. PLoS One. 2009;4:e7679. doi: 10.1371/journal.pone.0007679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivanova E, Pan ZH. Evaluation of the adeno-associated virus mediated long-term expression of channelrhodopsin-2 in the mouse retina. Molecular Vision. 2009;15:1680–1689. [PMC free article] [PubMed] [Google Scholar]
- 49.Ivanova E, Roberts R, Bissig D, Pan ZH, Berkowitz BA. Retinal channelrhodopsin-2-mediated activity in vivo evaluated with manganese-enhanced magnetic resonance imaging. Molecular Vision. 2010;16:1059–1067. [PMC free article] [PubMed] [Google Scholar]
- 50.Ivanova E, Hwang GS, Pan ZH, Troilo D. Evaluation of AAV-mediated expression of Chop2-GFP in the marmoset retina. Investigative Ophthalmology and Visual Science. 2010;51:5288–5296. doi: 10.1167/iovs.10-5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sugano E, et al. Immune responses to adeno-associated virus type 2 encoding channelrhodopsin-2 in a genetically blind rat model for gene therapy. Gene Therapy. 2010;18:266–274. doi: 10.1038/gt.2010.140. [DOI] [PubMed] [Google Scholar]
- 52.Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nature Methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- 53.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neuroscience. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 54.Gunaydin LA, et al. Ultrafast optogenetic control. Nature Neuroscience. 2010;13:387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- 55.Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nature Neuroscience. 2009;12:229–234. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- 56.Adesnik H, Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature. 2010;464:1155–1160. doi: 10.1038/nature08935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nature Reviews Neuroscience. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 58.Wentz CT, et al. A wirelessly powered and controlled device for optical neural control of freely-behaving animals. Journal of Neural Engineering. 2011;8:046021. doi: 10.1088/1741-2560/8/4/046021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chader GJ. Animal models in research on retinal degenerations: past progress and future hope. Vision Research. 2002;42:393–399. doi: 10.1016/s0042-6989(01)00212-7. [DOI] [PubMed] [Google Scholar]
- 60.Michael-Titus A, Revest P, Shortland P. The nervous system: basic science and clinical conditions. Churchill Livingstone. 2010 [Google Scholar]
- 61.Lopez R, et al. Transplanted retinal pigment epithelium modifies the retinal ‘ degeneration in the RCS rat. Investigative Ophthalmology and Visual Science. 1989;30:586–588. [PubMed] [Google Scholar]
- 62.Sheedlo HJ, Gaur V, Li LX, Seaton AD, Turner JE. Transplantation to the diseased and damaged retina. Trends in Neurosciences. 1991;14:347–350. doi: 10.1016/0166-2236(91)90160-v. [DOI] [PubMed] [Google Scholar]
- 63.Lavail MM, Li L, Turner JE, Yasumura D. Retinal pigment epithelial cell transplantation in RCS rats: normal metabolism in rescued photoreceptors. Experimental Eye Research. 1992;55:555–562. doi: 10.1016/s0014-4835(05)80168-x. [DOI] [PubMed] [Google Scholar]
- 64.Lund RD, et al. Retinal degeneration and transplantation in the Royal College of Surgeons rat. Eye (London) 1998;12(Pt. 3b):597–604. doi: 10.1038/eye.1998.150. [DOI] [PubMed] [Google Scholar]
- 65.Aramant RB, Seiler MJ. Retinal transplantation – advantages of intact fetal sheets. Progress in Retinal and Eye Research. 2002;21:57–73. doi: 10.1016/s1350-9462(01)00020-9. [DOI] [PubMed] [Google Scholar]
- 66.Abe T, et al. Characterization of iris pigment epithelial cell for auto cell transplantation. Cell Transplantation. 1999;8:501–510. doi: 10.1177/096368979900800505. [DOI] [PubMed] [Google Scholar]
- 67.Kaplan HJ, Tezel TH, Berger AS, Del Priore LV. Retinal transplantation. Chemical Immunology. 1999;73:207–219. doi: 10.1159/000058747. [DOI] [PubMed] [Google Scholar]
- 68.Schraermeyer U, et al. Subretinally transplanted embryonic stem cells rescue photoreceptor cells from degeneration in the RCS rats. Cell Transplantation. 2001;10:673–680. [PubMed] [Google Scholar]
- 69.Lund RD, Ono SJ, Keegan DJ, Lawrence JM. Retinal transplantation: progress and problems in clinical application. Journal of Leukocyte Biology. 2003;74:151–160. doi: 10.1189/jlb.0103041. [DOI] [PubMed] [Google Scholar]
- 70.Yang P, Seiler MJ, Aramant RB, Whittemore SR. Differential lineage restriction of rat retinal progenitor cells in vitro and in vivo . Journal of Neuroscience Research. 2002;69:466–476. doi: 10.1002/jnr.10320. [DOI] [PubMed] [Google Scholar]
- 71.Haruta M, et al. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Investigative Ophthalmology and Visual Science. 2004;45:1020–1025. doi: 10.1167/iovs.03-1034. [DOI] [PubMed] [Google Scholar]
- 72.Dahlmann-Noor A, Vijay S, Jayaram H, Limb A, Khaw PT. Current approaches and future prospects for stem cell rescue and regeneration of the retina and optic nerve. Canadian Journal of Ophthalmology. 2010;45:333–341. doi: 10.3129/i10-077. [DOI] [PubMed] [Google Scholar]
- 73.Flannery JG, Greenberg KP. Looking within for vision. Neuron. 2006;50:1–3. doi: 10.1016/j.neuron.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 74.Margalit E, et al. Retinal prosthesis for the blind. Survey of Ophthalmology. 2002;47:335–356. doi: 10.1016/s0039-6257(02)00311-9. [DOI] [PubMed] [Google Scholar]
- 75.Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature. 2001;410:583–587. doi: 10.1038/35069068. [DOI] [PubMed] [Google Scholar]
- 76.Roska B, Molnar A, Werblin FS. Parallel processing in retinal ganglion cells: how integration of space-time patterns of excitation and inhibition form the spiking output. Journal of Neurophysiology. 2006;95:3810–3822. doi: 10.1152/jn.00113.2006. [DOI] [PubMed] [Google Scholar]
- 77.Bainbridge JW, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. New England Journal of Medicine. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 78.Hauswirth WW, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Human Gene Therapy. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Archives of Ophthalmology. 2007;125:151–158. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones BW, et al. Retinal remodeling triggered by photoreceptor degenerations. Journal of Comparative Neurology. 2003;464:1–16. doi: 10.1002/cne.10703. [DOI] [PubMed] [Google Scholar]
- 81.MacLaren RE, Bird AC, Sathia PJ, Aylward GW. Long-term results of submacular surgery combined with macular translocation of the retinal pigment epithelium in neovascular age-related macular degeneration. Ophthalmology. 2005;112:2081–2087. doi: 10.1016/j.ophtha.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 82.Tropepe V, et al. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 83.Sahni JN, et al. Therapeutic challenges to retinitis pigmentosa: from neuroprotection to gene therapy. Current Genomics. 2011;12:276–284. doi: 10.2174/138920211795860062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McGee Sanftner LH, Abel H, Hauswirth WW, Flannery JG. Glial cell line derived neurotrophic factor delays photoreceptor degeneration in a transgenic rat model of retinitis pigmentosa. Molecular Therapy. 2001;4:622–629. doi: 10.1006/mthe.2001.0498. [DOI] [PubMed] [Google Scholar]
- 85.Deisseroth K. Biological Psychiatry. 2012;71 doi: 10.1016/j.biopsych.2011.12.021. http://www.biologicalpsychiatry-journal.com/webfiles/images/journals/bps/S0006322311X00367.pdf. [DOI] [PubMed] [Google Scholar]
- 86.Bowes C, et al. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 87.Pittler SJ, Baehr W. The molecular genetics of retinal photoreceptor proteins involved in cGMP metabolism. Progress in Clinical and Biological Research. 1991;362:33–66. [PubMed] [Google Scholar]
- 88.Mullen RJ, LaVail MM. Inherited retinal dystrophy: primary defect in pigment epithelium determined with experimental rat chimeras. Science. 1976;192:799–801. doi: 10.1126/science.1265483. [DOI] [PubMed] [Google Scholar]
- 89.Lewis TL, Jr, Mao T, Svoboda K, Arnold DB. Myosin-dependent targeting of transmembrane proteins to neuronal dendrites. Nature Neuroscience. 2009;12:568–576. doi: 10.1038/nn.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.D’Cruz PM, et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Human Molecular Genetics. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 91.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]