Abstract
Objective
To describe the temporal bone histopathology in children with congenital toxoplasmosis.
Background
Toxoplasmosis is a parasitic infection caused by Toxoplasma gondii. If fetal infection occurs early in gestation severe inflammation and necrosis can cause brain lesions, chorioretinitis and hearing loss. Hearing loss in congenital toxoplasmosis may be preventable with early diagnosis and treatment.
Materials and Methods
The temporal bones of nine subjects with congenital toxoplasmosis were removed at autopsy and studied under light microscopy. Cytocochleograms were constructed for hair cells, the stria vascularis and cochlear neuronal cells.
Results
Three of nine subjects (33%) were found to have parasites in the temporal bone. The organism was identified in the internal auditory canal, the spiral ligament, stria vascularis and saccular macula. The cystic form of the parasite was not associated with the inflammatory response seen in the active tachyzoite form.
Conclusions
We infer that the hearing loss of toxoplasmosis is likely the result of a postnatal inflammatory response to the tachyzoite form of T gondii. Our findings have implications for the early identification and management of Toxoplasmosis.
Keywords: Temporal bone, histopathology, hearing loss, Toxoplasmosis
Introduction
Toxoplasmosis is a parasitic infection caused by Toxoplasma gondii, an obligate intracellular protozoan. Cats are hosts of T gondii. During infection millions of oocysts are shed in the cat feces. The infection is acquired when oocysts are ingested by consumption of contaminated food or water. Tachyzoites, a motile form of the parasite, enter nucleated cells and are disseminated through the blood stream. The tachzyoites elicit an inflammatory response. The tachyzoites can form bradyzoites that are contained within cysts that may not elicit an immune response in the host. Bradyzoites can transform back to tachyzoites resulting in recurrent infection.
The seroprevalence of T. gondii in the United States is 22.5% and the incidence of toxoplasmosis is 1–10 per 10,000 (1,2). If infection occurs around the time of pregnancy transplacental transmission may occur. Infection in the immunocompetent adult is often mild but it is more severe in newborns and the immunodeficient patient. If fetal infection occurs early in gestation severe inflammation and necrosis can be observed particularly in the central nervous system. Most newborns with congenital toxoplasmosis have subclinical infections at birth however (3). Untreated patients may go on to develop chorioretinitis, cognitive and motor dysfunction, seizures and hearing loss.
The prevalence of sensorineural hearing loss is as high as 28% in children who do not receive treatment. Remarkably this number can be reduced to 0% in patients that are identified early and are compliant with treatment (4). The described hearing loss of toxoplasmosis is sensorineural. Given the paucity of histopathological data the mechanism of hearing loss is unknown. We describe the temporal bone histopathological findings of 3 infants with congenital toxoplasmosis to better understand the mechanisms of hearing loss.
Material and Methods
The temporal bones were removed at autopsy and fixed in 10% formalin solution, decalcified with EDTA and embedded in celloidin. Serial sectioning was performed in the axial plane at a thickness of 20 um, staining every tenth section with hematoxylin and eosin (5). All stained sections were examined under light microscopy. Graphic reconstruction of the cochlea was performed to quantify hair cells, the stria vascularis, and cochlear neuronal cells. This was compared to normative data as described by Schuknecht (5).
Immunohistochemistry
Celloidin was removed from unstained sections as previously described (6). Immunostaining was performed using polyclonal anti-Toxoplasma gondii antibody (Abcam Cambridge, MA) at a dilution of 1:50. After 14-hour primary antibody incubations, sections were rinsed in 3 washes of PBS. A goat anti rabbit secondary antibody was used at a dilution of 1:200 and incubated for 1 hour. After 3 rinses in PBS, avidin-biotin-horseradish peroxidase (Standard ABC Kit, Vector Laboratories, Burlingame, California) was applied to the sections and left to incubate for 1 hour, followed by 3 washes with PBS. The sections were colorized with 0.01% diaminobenzidine and 0.01% hydrogen peroxide for 5 minutes, rinsed, and dehydrated.
Case Reports
Nine cases donated from the Hospital San Jose, Costa Rica with an autopsy diagnosis of congenital toxoplasmosis were reviewed. All cases had a history of positive Sabin Feldman testing, an indirect serological test for the detection of T gondii IgG antibodies. Three specimens were found to have T gondii organisms. Only the specimens where T gondii was positively identified were reviewed, as material from the autopsy note could not be obtained for confirmation. The histopathological and descriptive clinical findings of these cases are reviewed.
Case 1
Clinical History
The first case is that of a 2-month-old male with congenital toxoplasmosis. Prior to death he had a skin rash, ecchymosis, fever, jaundice and hepatosplenomegaly. On autopsy he was noted to have meningoencephalitis. The gross description of the brain describes obstruction of the Sylvian aqueduct and T gondii cysts in the frontal lobe. No hearing test is available.
Histopathology
Both sides are similar in presentation. The external ear is unremarkable. The middle ear is bilaterally filled with eosinophilic fluid and polymorphonuclear leukocytes (PMNs). The mastoid and petrous apex show active extramedullary hematopoiesis as expected for this age. The bony and membranous labyrinths are intact. There is endolymphatic hydrops of the membranous labyrinth in the apical turn on both sides. Postmortem preservation is good. There is minimal autolysis and cellular disintegration in the organ of Corti, stria vascularis and spiral ligament. Outer hair cells (OHCs) and inner hair cells (IHCs) are preserved well throughout the cochlear duct. A cytocochleogram of the ear is shown in Figure 1A. Encysted organisms are seen in the lateral cochlear wall on both sides (Figure 2). There is no inflammatory response or necrotic material in the labyrinth. The cochlear neuron population is decreased compared to newborns on both sides (Total counts: right- 20,790, a 41% loss vs age matched controls: left- 14,139 a 60% loss vs age matched). The cochlear neuronal cells show cytoplasmic vacuolization with inconspicuous nucleoli. The vestibular sense organs appear normal. The endolymphatic sac is enlarged with normal duct morphology, on both sides. The intracanalicular meninges are thickened and infiltrated with inflammatory cells and there are few encysted parasites floating free in the Internal Auditory Canal (IAC). The neuronal cell populations seem to be normal in the geniculate and Scarpa’s ganglion.
Figure 1.
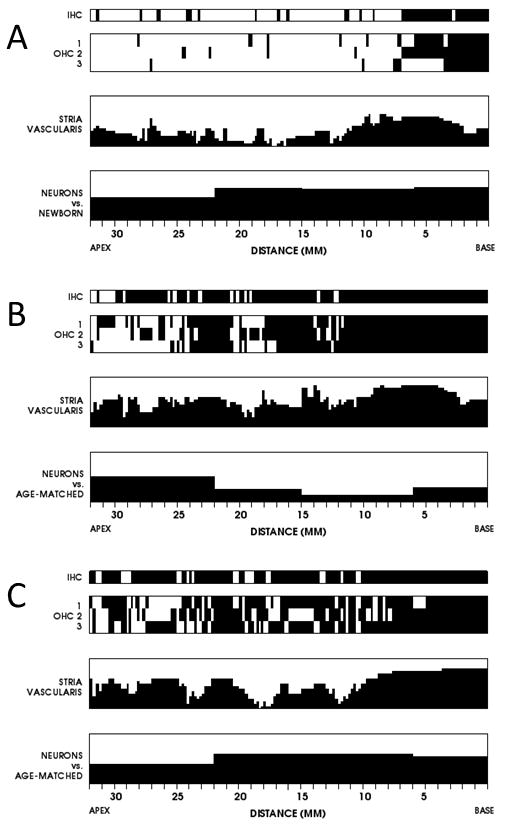
Cytocochleograms of histologic changes within the cochlea in the three individuals in this report. Black fillings indicate missing or abnormal elements. The vertical axes for the stria vascularis and neurons show percentage of loss compared to age matched controls. A. Subject 1, left ear. B. Subject 2, left ear. C. Subject 3, left ear.
Figure 2.

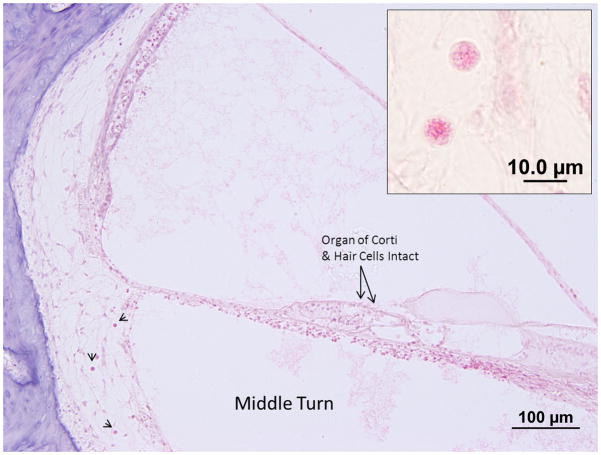
Photomicrograph of a Hematoxylin and Eosin stained section of the left cochlea of Subject 1. The middle turn of the cochlea shows the cystic form of the parasite within the spiral ligament. The organ of Corti appears normal. Inset, higher magnification view of a cyst in the spiral ligament. The diameter of the cyst is 8.5 um.
Case 2
Clinical History
This 2-month-old male was noted to have encephalitis, severe hydrocephalus, bilateral frontal lobe cysts and subependymal cysts at autopsy. No hearing testing is available.
Histopathology
Both sides are similar in presentation. The mastoid cells are infiltrated with inflammatory cells and there are hemorrhagic areas in the mastoid bone and middle ear. The middle ear mucosa is hypertrophied and infiltrated with lymphocytes and round cells. There is no necrosis or T. gondii in the middle ear or the mastoid bone. The external ear, tympanic membrane and ossicles are unremarkable. The bony and membranous labyrinths are intact. Postmortem preservation is poor with significant postmortem autolysis in the organ of corti and stria vascularis in both sides. There is significant loss of outer hair cells in middle and apical regions and severe loss in the remaining cochlear duct. Inner hair cells are absent throughout the cochlear duct. A cytocochleogram of the ear is shown in Figure 1B. No organisms are identified within the labyrinth. The cochlear neuron population is decreased compared to newborns on both sides (Total counts: right- 23,348, a 34% loss vs age matched controls: left- 26,028 a 27% loss vs age matched). Cochlear neuronal cell losses are more prominent in the apical part of Rosenthal’s canal. There is mild autolysis in the vestibular sense organs. The population of hair cells in the vestibular epithelium appear normal however. The internal auditory canal (IAC) is unremarkable on the right side. The left IAC has a necrotic focus with Tachyzoites and an inflammatory cell response situated between the posterior wall of the IAC and vestibular nerve (Figure 3). The vestibular nerve is displaced anteriorly and there is patchy destruction with PMN infiltration. The cochlear nerve appears normal.
Figure 3.
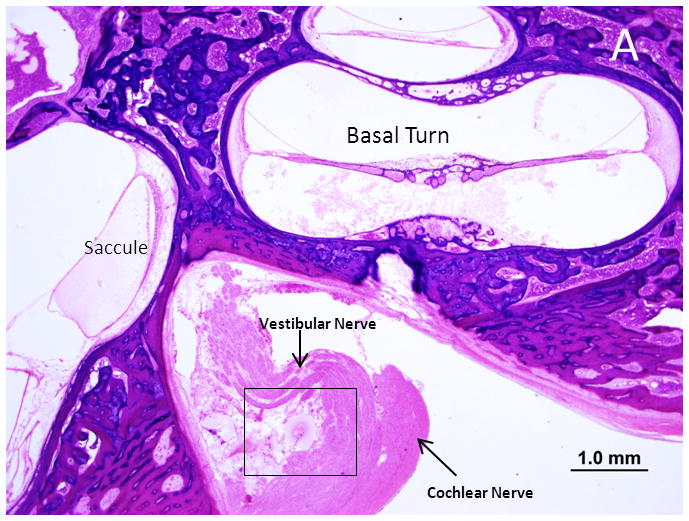
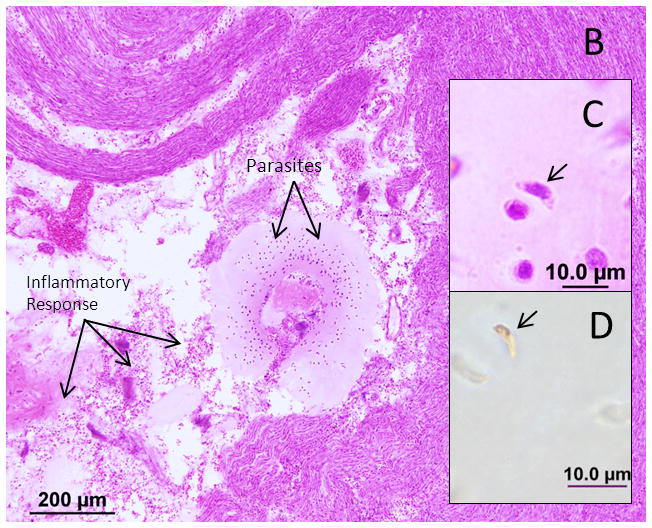
Photomicrograph of the right internal auditory canal from Subject 2. A. A necrotic lesion with T. gondii tachzyoites displacing the vestibular nerve. B. The boxed area in A is shown at higher magnification with labeled arrows pointing to the cellular response and parasites. C. A tachyzoite by H&E stain (arrow) D. A tachyzoite is shown after immunohistochemistry with polyclonal IgG anti-Toxoplasma gondii antibody (arrow).
Case 3
Clinical History
This case is of a 12-day-old male with chlamydial conjunctivitis, jaundice and generalized seizures who died of respiratory failure. On autopsy he was found to have meningoencephalitis with stenosis of the aqueduct of Sylvius and T gondii brain cysts. Additional findings noted on the autopsy report were chorioretinitis, cardiomegaly and intracranial calcifications. No hearing testing is available.
Histopathology
Both sides are very similar in presentation. The external ear, the mastoid and petrous bones are unremarkable. The middle ear space is partly filled with eosinophilic substance with round cells and keratin debris (amniotic fluid). There is minimal mesenchymal tissue in the middle ear.
The bony and membranous labyrinths are intact and there is no sign of endolymphatic hydrops. Postmortem preservation is fair. There is significant cellular loss and autolysis of the cochlear neurosensory elements and of the stria vascularis as shown in the cytocochleogram (Figure 1C.) On the left side there are T gondii cysts seen in the lateral cochlear wall (Figure 4). No significant tissue necrosis or inflammatory reaction can be seen. The cochlear neuron population is decreased compared to newborns on both sides (Total counts: right- 20,889, 41% loss vs age matched controls: left- 16,497, a 54% loss vs age matched). Cochlear neuronal cells show significant pre-mortem changes such as cytoplasmic vacuolization and loss of distinction between nuclei and nucleoli. The vestibular sense organs and population of hair cells epithelium is well preserved. Few encysted parasites are seen in the saccular macula on the left ear (Figure 5). The crista of all three semicircular canals, the macula of the utricle and the saccule are unremarkable on the right side. The neuronal cell population appears normal in Scarpa’s ganglion on both sides.
Figure 4.
A photomicrograph of the left cochlea from subject 3. Arrowheads point to cysts within the spiral ligament. The organ of Corti appears normal with intact hair cells. Inset: two cysts within the spiral ligament.
Figure 5.
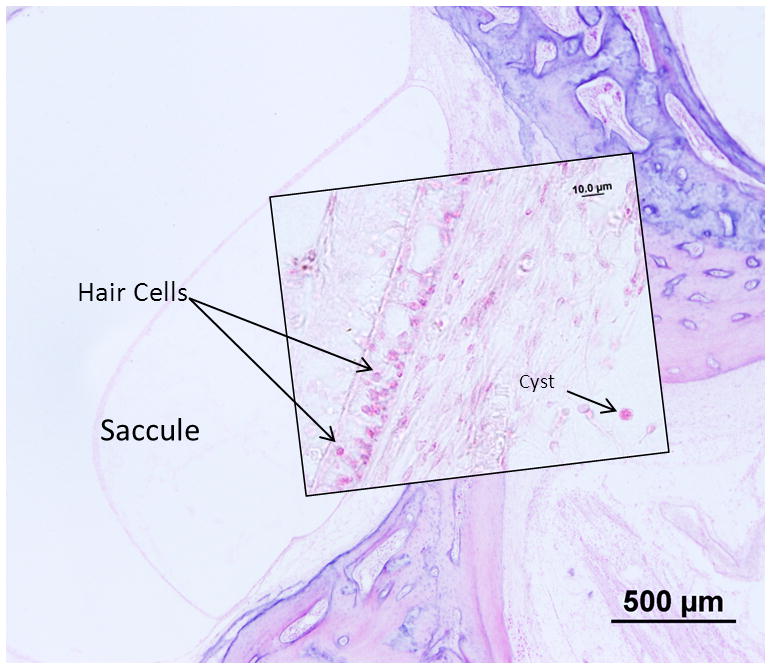
Photomicrograph of the left saccule from Subject 3. The sensory epithelium of the saccule appears intact despite the presence of the cysts beneath the epithelium. Inset: Higher power view of the sensory epithelium and cyst.
Discussion
Hearing loss related to congenital toxoplasmosis has been reported in multiple studies (7,8,9). However, less is known about how congenital T. gondii infection causes hearing loss, why not all infected newborn have hearing loss and why treatment can prevent this untoward outcome. In the present study we examined temporal bones of newborns that died secondary to congenital toxoplasmosis. In 3 of 8 (37.5%) subjects there were free or encysted organisms in the temporal bone. Two of those subjects (Case 1 and 3) had numerous encysted organisms inside the inner ear without evidence of tissue necrosis or an inflammatory cellular response. In one subject (Case 2), there was a necrotic focus with free organisms and an inflammatory response within the internal auditory canal.
Previously, Kelemen reported on the temporal bone histopathology of two cases with congenital toxoplasmosis (10). Encysted organisms were found inside the middle ear cavity residing in mesenchyme with inflammatory cellular infiltration. He observed a normal organ of Corti with intact hair cells and calcium deposits in the spiral ligament of the basal and middle turn of cochlea. He did not identify organism within the inner ear.
In this study T gondii organisms in the cystic form were identified in the stria vascularis, spiral ligament and saccular macula. The cysts were not associated with tissue necrosis or an inflammatory cell response. This is consistent with the literature on T gondii; encysted parasites do not cause tissue damage and necrosis (11). The parasites we found inside the inner ear might have entered the inner ear during parasitemia and transformed into the more quiescent cystic form. We also identified the immune response eliciting tachyzoite form in the IAC where an inflammatory response was observed.
Wilson et al. (8) published a study of 24 cases of asymptomatic children with a mean age of 8.5 years followed without any treatment. 90% of those children developed chorioretinitis and 21% of the children were found to have some degree of sensorineural hearing loss. In 2006, The National Collaborative Chicago-Based Toxoplasma Study Group reported that children who had congenital toxoplasma infection that were given anti-parasitic treatment had favorable outcomes (12). Of sixty-eight children receiving one-year of anti-parasitic treatment, none (0%) developed hearing loss. In a meta-analysis, children with toxoplasmosis were stratified based on the duration of treatment and its effect on hearing loss (4). The prevalence of sensorineural hearing loss was 28 % with limited or no treatment. 12% of children who were given 12 months of treatment starting after 2.5 months or compliance not confirmed developed hearing loss. The rate of hearing loss was 0% among the children that had 12 months of anti-parasitic treatment prior to 2.5 months of age with serologically confirmed compliance. Early diagnosis and a full year of compliant anti-parasitic treatment is clearly associated with significantly better hearing outcomes (12,13). One can infer from this data that hearing loss may be secondary to a preventable delayed reactivation of the cystic form to the active tachyzoite form. In our 3 cases the cystic form was not associated with the inflammatory response seen with the tachyzoites. The locations of the organisms identified in this study suggest that the hearing loss may be sensory, neural or sensorineural.
The absence of audiometric data in these patients precludes drawing direct conclusions that correlate phenotype to hearing measures however. A second limitation of the study is the degree of autolytic changes observed in case 2 and 3. The loss of neurosensory elements in the labyrinth cannot be correlated to the presence of the organism in cystic form. In the first case the preservation of neurosensory elements was good but there was loss of spiral ganglion cells. This loss may have been secondary to meningoencephalitis. As the hearing loss observed clinically is predominantly in subacute infections it is unlikely that this represents a typical pattern of T gondii induced neural hearing loss.
Summary and Conclusions
We described the otopathology in 3 individuals affected with congenital Toxoplasmosis gondii, a parasitic infection that may result in hearing loss in up to one third of untreated patients. The otopathology revealed that the parasite may reside in a dormant cystic form within the stria vascularis, spiral ligament, saccular macula or internal auditory canal. The immunogenic tachzyoite form of the parasite was identified in the internal auditory canal with an associated immune response. We infer that the hearing loss in Toxoplasmosis is likely the result of a preventable postnatal inflammatory response, and the hearing loss can be sensory, neural or sensorineural. Our results have implications for the early identification and management of Toxoplasmosis.
Acknowledgments
We are grateful to Clarinda C. Northup for providing us with the temporal bones for study and Jennifer T. O’Malley for technical assistance.
Supported in part by NIH grant 1U24DC011943-01
Contributor Information
Mehti Salviz, Department of Otolaryngology, Massachusetts Eye and Ear Infirmary, Boston, MA, USA, Department of Otology and Laryngology, Harvard Medical School, Boston, MA, USA.
Jose G. Montoya, Toxoplasma Serology Laboratory, Palo Alto Medical Foundation, Palo Alto, Department of Infectious Diseases, Stanford School of Medicine, Palo Alto, CA, USA.
Joseph B. Nadol, Department of Otolaryngology, Massachusetts Eye and Ear Infirmary, Boston, MA, USA, Department of Otology and Laryngology, Harvard Medical School, Boston, MA, USA.
Felipe Santos, Department of Otolaryngology, Massachusetts Eye and Ear Infirmary, Boston, MA, USA, Department of Otology and Laryngology, Harvard Medical School, Boston, MA, USA.
References
- 1.Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, et al. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol. 2001;154:357–65. doi: 10.1093/aje/154.4.357. [DOI] [PubMed] [Google Scholar]
- 2.Lopez A, Dietz VJ, Wilson M, et al. Preventing congenital toxoplasmosis. MMWR Recomm Rep. 2000;49:59–68. [PubMed] [Google Scholar]
- 3.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–76. doi: 10.1016/S0140-6736(04)16412-X. Review. [DOI] [PubMed] [Google Scholar]
- 4.Brown ED, Chau JK, Atashband S, et al. A systematic review of neonatal toxoplasmosis exposure and sensorineural hearing loss. Int J Pediatr Otorhinolaryngol. 2009;73:707–11. doi: 10.1016/j.ijporl.2009.01.012. Review. [DOI] [PubMed] [Google Scholar]
- 5.Schuknecht HF. Pathology of the Ear. 2. 7–30. Philadelphia: Lea and Febiger; 1993. pp. 417–419. [Google Scholar]
- 6.O’Malley JT, Burgess BJ, Jones DD, et al. Techniques of celloidin removal from temporal bone sections. Ann Otol Rhinol Laryngol. 2009;118:435–41. doi: 10.1177/000348940911800606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichenwald HF. A study of congenital toxoplasmosis, with particular emphasis on clinical manifestation, sequalae, and therapy. In: Siim JC, editor. Human Toxoplasmosis. Copenhagen: Munksgaard; 1960. p. 41. [Google Scholar]
- 8.Wilson CB, Remington JS, Stagno S, et al. Development of adverse squeal in children born with subclinical toxoplasma infection. Pediatrics. 1980;66:767–773. [PubMed] [Google Scholar]
- 9.Andrade GM, Resende LM, Goulart EM, et al. Hearing loss in congenital toxoplasmosis detected by newborn screening. Braz J Otorhinolaryngol. 2008;74:21–8. doi: 10.1016/S1808-8694(15)30746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelemen G. Toxoplasmosis and Congenital Deafness. Arch Otollaryngol. 1958;68:547–61. doi: 10.1001/archotol.1958.00730020569003. [DOI] [PubMed] [Google Scholar]
- 11.Skariah S, McIntyre MK, Mordue DG. Toxoplasma gondii: determinants of tachyzoite to bradyzoite conversion. Parasitol Res. 2010;107:253–60. doi: 10.1007/s00436-010-1899-6. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLeod R, Boyer K, Karrison T, et al. Toxoplasmosis Study Group. Outcome of treatment for congenital toxoplasmosis, 1981–2004: the National Collaborative Chicago-Based, Congenital Toxoplasmosis Study. Clin Infect Dis. 2006;42:1383–94. doi: 10.1086/501360. [DOI] [PubMed] [Google Scholar]
- 13.McGee T, Wolters C, Stein L, et al. Absence of sensorineural hearing loss in treated infants and children with congenital toxoplasmosis. Otolaryngol Head Neck Surg. 1992;106:75–80. doi: 10.1177/019459989210600131. [DOI] [PubMed] [Google Scholar]