Abstract
Background
Eosinophils have the capacity to secrete varied cytotoxic proteins. Among the proteins are the eosinophil-associated RNases (EARs): the human eosinophil-derived neurotoxin and eosinophilic cationic protein and their murine ortholog EARs, which have been shown to be involved in host defense, tissue remodeling and immunity regulation. However, the signal transduction that regulates EARs secretion in response to physiological stimuli, such as chemokines, has been little studied in human and scarcely in mouse eosinophils, the foremost animal model for eosinophil-associated human diseases.
Objective
In this study we aimed to understand the signal transduction involved in the secretion of enzymatically active EARs following chemokine stimulation.
Methods
Fresh mouse and human eosinophils were stimulated with CCL11 and CCL24 and the secretion of enzymatically active EARs was detected using an RNase activity assay. The involvement of signaling factors or integrins was probed using specific inhibitors and blocking antibodies. Adhesion was evaluated by microscopy.
Results
We found that secretion of mouse EARs in response to CCL11 and CCL24 was Gαi-dependent. Both mouse and human eosinophils required the activation of PI3K, ERK and p38 MAPK. In addition, the adhesion molecules β1 and β2 integrins were found to be crucial for EAR secretion, and we suggest a mechanism in which spreading is obligatory for EAR secretion.
Conclusions
Collectively, these data suggest a common CCR3-mediated signaling pathway that leads to EAR secretion in both mouse and human eosinophils. These findings are applicable for eosinophil-mediated host defense and eosinophil-associated diseases.
Keywords: adhesion, ECP, EDN, signaling
Introduction
Eosinophils are granulocytes that usually constitute up to 5% of peripheral blood leukocytes and reside principally in mucosal tissues. Over the years, eosinophils have been found to participate in various aspects of immune responses: as effectors in host defense responses against variable pathogens, as a rapid source of inflammatory mediators for immunomodulation and tissue remodeling and as professional antigen presenting cells (1). A defining feature of eosinophils is their specific granules, which contain basic proteins, including major basic protein (MBP), eosinophil peroxidase (EPO) and the eosinophil-associated RNases (EARs). Human EARs are eosinophil-derived neurotoxin (EDN) and eosinophilic cationic protein (ECP). The mouse EARs (mEARs) consist of 13 RNases, at least 6 of which have been shown to be localized in mouse eosinophil granules (2).
The mEARs as well as their human orthologous EARs (hEARs), ECP and EDN, are members of the RNase A superfamily of polymeric RNA ribonucleases, that when secreted from various cells can mediate innate immune roles in host defense (3). This family has endo- and/or exo-nucleolytic cleavage activity, which can mediate their anti-viral activity, in vivo and in vitro, in murine models of pneumonia virus of mice (PVM) and respiratory syncytial virus (RSV) infection (4), suggesting a role for EARs in the clearance of RNA viruses. In addition to anti-viral capacity, EARs exhibit anti-bacterial and anthelminthic cytotoxicity (5, 6), and can be found in anti-microbial extracellular DNA traps (7). Moreover, EARs are involved in the pathology of various eosinophil-associated diseases, such as asthma, atopic dermatitis and eosinophil esophagitis, and serve as biomarkers for disease severity (8).
Although the importance of EARs in host defense and eosinophil-associated diseases has been broadly studied in vitro and in vivo, the intracellular signaling involved in their secretion remains uncertain, especially in mice, the foremost in vivo model of infectious and allergic inflammation. Therefore, our study aimed to reveal the signal transduction mechanisms and the key factors required for the extracellular secretion of EARs in response to physiological stimuli.
In our studies we focused on eotaxin-1 (CCL11) and eotaxin-2 (CCL24) which bind the CCR3 G-protein coupled receptor. CCL11 is a major chemoattractant of human and mouse eosinophils. It was previously shown that CCL11 stimulates eosinophil degranulation in human eosinophils (9, 10). Recently, we have shown that CCL11 can induce secretion of enzymatically active EARs and other granule proteins from mouse eosinophils by means of piecemeal degranulation (11). Subcellular fractionation of mouse eosinophils has shown that almost 50% of total RNase activity is located in granule fractions with additional RNase activity in cytosolic and vesicle-containing fractions (11). Notably, in response to CCL11-elicited secretion of mEAR, granule fractions showed a decrease in RNase activity and cytosolic and vesicle low density fractions increased in RNase activity suggesting stimulated mobilization of granule mEARs into secretion competent lower density compartments (11).
By measuring RNase activity of EARs in supernatants of CCL11- and CCL24-stimulated mouse eosinophils, we have found that CCL24 can serve as a stimulator for mEARs secretion, similarly to our previous findings (11) with CCL11, and doing so in a Gαi dependent manner. In addition, EARs secretion required PI3K, ERK and p38 MAPK signaling in both human and mouse eosinophils. Finally, we found that both β1 and β2 integrins are involved in EAR secretion. The CCR3-mediated signal transduction increased β2 integrin expression and induced β2-mediated cell spreading, which was crucial for EAR secretion. These data suggest common signaling pathways required for EAR secretion in both mouse and human eosinophils.
Material and methods
Isolation of mouse eosinophils
IL-5 transgenic BALB/c mice (12), provided by Drs. Alison A. Humbles and Craig Gerard (Children’s Hospital Medical, Boston, MA, USA), were housed in a pathogen-free facility. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center, Boston, MA. Mouse eosinophils were isolated from mechanically disrupted spleens of IL-5 transgenic mice as previously described (13). Purity and viability of >98% were determined by microscopic analyses after Hema 3 staining and trypan blue exclusion, respectively.
Isolation of human eosinophils
Human eosinophils were purified from healthy donor blood by negative selection, as described (14). Written informed consent was obtained from donors in accordance with the Declaration of Helsinki, and Institutional Review Board (IRB) approval was obtained from the Beth Israel Deaconess Medical Center. Purity and viability of >98% were determined by microscopic analyses after Hema 3 staining and trypan blue exclusion, respectively.
Stimulation of eosinophils
Mouse and human eosinophils were resuspended in RPMI medium 1640 without phenol red (BioWhittaker, Walkersville, MD, USA), supplemented with 0.1% OVA (Ovalbumin, Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 106 cells/0.25 ml (unless otherwise mentioned, see legends of figures 1-3) and were stimulated at 37°C for 1 h in 48-well flat-bottom polystyrene tissue culture dish (Becton Dickinson (BD) Biosciences, San Jose, CA, USA), with 100 ng/ml of reecombinant mouse CCL11 or CCL24 (Peprotech, Rocky Hill, NJ, USA), or 300 ng/ml human CCL11 (R&D Systems, Inc., Minneapolis, MN, USA), respectively. The higher concentration of human CCL11 was used to compensate for donor variability. In some experiments, cells were pretreated 20 min before and during chemokine stimulation at 37°C with 5 mM EDTA; the Gαi inhibitor, pertussis toxin (PTX) (20 ng/ml, Calbiochem-EMD. Gibbstown, NJ, USA); the CCR3 antagonist, SB 328437 (1, 3 μM, Calbiochem-EMD); the PI3K inhibitors, LY294002 (1, 10 μM, Calbiochem-EMD) and wortmannin (0.1 μM, Calbiochem-EMD); the p38 MAPK inhibitor, SB 203580 (0.2, 2 μM, Calbiochem-EMD); or the MEK1 (MAP ERK kinase) inhibitors, PD98059, U0126 (10, 50 μM, Calbiochem-EMD), or PD184352 (1, 10 μM, Selleckchem, Houston, TX, USA). Human eosinophils were preincubated for 1-2 hr with 10 μM LY294002, 10 μM SB 203580, or 50 μM PD98059. As controls, cells were pretreated with equivalent concentrations of vehicle. Cell viability after inhibition and stimulation, as detected by trypan blue exclusion or propidium iodide staining, was >93%. Supernatants were obtained by centrifugation at 300 × g for 5 min at 4°C, followed by centrifugation at 20,000 × g for 10 min at 4°C.
Figure 1. mEARs are released from CCL11- and CCL24-stimulated mouse eosinophils.
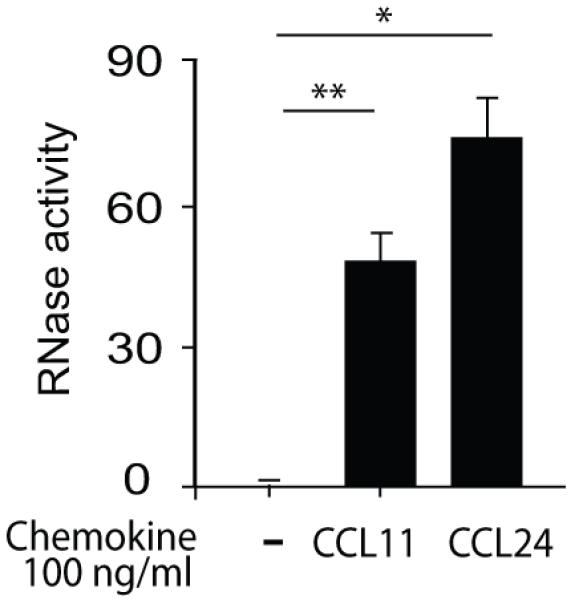
Mouse eosinophils (2 × 106) were stimulated with the indicated concentrations of CCL24 or CCL11 at 37°C for 1 h. Supernatants were measured for RNase enzymatic activity after incubation with the single strand RNA probe for 50 min at 37 °C. Data are means ± SD of relative fluorescence units acquired from duplicate wells and are representative of eight independent experiments. Values of stimulated eosinophils are after subtraction of non-stimulated eosinophils. P values of CCL11- or CCL24-stimulated eosinophils are compared with non-stimulated eosinophils. Asterisks represent P values < 0.05 (*), or < 0.01 (**).
Figure 3. CCL11- and CCL24-mediated mEAR secretion is PI3K-dependent.
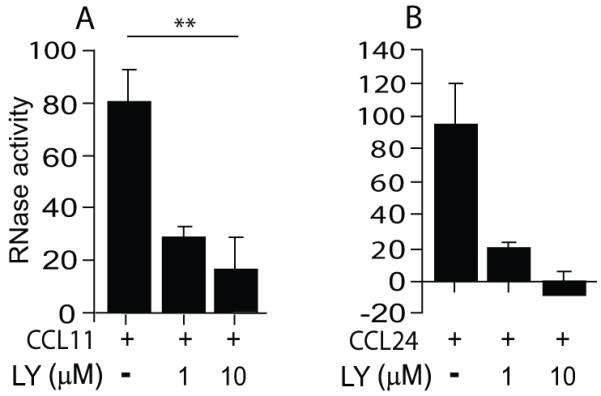
Mouse eosinophils (1.4× 106) were stimulated with 100 ng/ml of CCL11 (A) or CCL24 (B) in the presence of the PI3K inhibitor, LY294002 (LY), as described in Material and methods. Data are means ± SD from duplicate wells and are representative of three independent experiments. P values are compared between eosinophils stimulated with and without inhibitors. Asterisks represent P values < 0.05 (*), or < 0.01 (**).
Blockage of integrins in mouse eosinophils was performed by incubation of mouse eosinophils with rat anti-mouse CD16/CD32 (Fcγ III/II receptor blocking, 2.42G) antibodies (BioLegend, San Diego, CA, USA) and then with 10 μg/ml blocking antibodies against the mouse integrin β1 chain (BioLegend) or β2 chain (GAME-46, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or with equivalent azide-free isotype controls (BioLegend) for 10 min at 4°C prior to stimulation. The antibodies, known as blocking antibodies without activation effects (15, 16), remained with the cells during the stimulation with CCL11 or CCL24. To quantify the percentage of cell adhesion and spreading in response to chemokine and integrin blocking, we incubated mouse eosinophils (104/0.2 ml) in 8-well slide chamber (Lab-TEK, ThermoFisher Scientific, Pittsburgh, PA, USA) in OVA/RPMI medium in the presence of 100 ng/ml CCL11/OVA-RPMI and 10 μg/ml anti-integrin or isotype control antibodies up to 15 min. Images of arbitrary fields were taken using a Nikon inverted microscope (Nikon Eclipse TE300, Tokyo, Japan), fitted with a ×20 Plan objective, coupled to a Qimaging Retiga Exi fast 1394 cooled CCD camera (Qimaging, Surrey, Canada). Images were acquired using iVision software.
RNase activity assay
The human RNases, ECP and EDN, have a substrate preference for single-stranded RNA (17). Therefore, we measured the presence of mouse and human EARs in supernatants of stimulated mouse eosinophils by an RNase activity assay using a fluorescent single-stranded RNA oligonucleotide (RNaseAlert QC system, Ambion, Austin, TX, USA), according to the manufacturer’s instructions. Cleavage of the probe by RNases allows fluorescent emission that is detected by fluorometry. Data were acquired after 50 min (eosinophils) by the 7300 thermocycler (Applied Biosystems, Austin, TX, USA) and were within the linear range. All reactions were performed in duplicate or triplicate wells. Relative fluorescence units (×104 RFU) represent RNase activity levels from stimulated samples minus RNase activity levels from non-stimulated samples (except Supplemental Fig. S1 which shows the non-subtracted values). Ten × 104 relative fluorescence units represent enzyme activity that is equivalent to the activity of 4.0 ± 1.8 nano U RNases (~0.0398 pg), as calibrated with bovine pancreatic RNase A (Ambion). One unit is the amount of RNase equivalent to 0.1177 Kunitz Units (11).
Phospho-ERK multiplex analyses
Mouse eosinophils (4 × 106 cells/sample) were stimulated at 37°C for 10 min with 100 ng/ml CCL11 or CCL24 or OVA-RPMI medium alone. Cells were lysed with lysis buffer [0.5% hexadecyltrimethylammonium bromide (CTAB, Sigma-Aldrich), 10 mM Tris buffer (pH 7.4), 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 10% glycerol, 1 mM PMSF, protease inhibitors mixture (Sigma-Aldrich) and phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich)]. Total and phosphorylated ERK in supernatants of cell lysates were measured using the MILLIPLEX MAPK detecting multiplex kit according to the manufacturer’s (EMD-Millipore, Billerica, MA USA) instructions. Results are expressed as percentages of phosphorylated ERK versus total ERK.
Flow cytometry analyses of phospho-ERK and phospho-p38 MAPK
Mouse and human eosinophils were stimulated at 37°C for 2-3 min with 100 ng/ml murine CCL11 or 300 ng/ml human CCL11, respectively, in OVA-RPMI medium in the presence of MAPK inhibitors (as detailed above). Immediately after stimulation, cells were fixed with 3% paraformaldehyde, permeabilized and blocked with mouse FcγIII/II receptor blocking antibodies (BD Biosciences) for mouse cells and 2.5% human serum for human cells. Cells were incubated with antibodies against phosphorylated-ERK (Alexa Fluor 488-conjugated anti-ERK1/2, pT202/pY204), phosphorylated-p38 (Alexa Fluor 488-conjugated anti-p38 MAPK, pT180/pY182), or isotype controls (all from BD Biosciences). Data were acquired using the LSRII flow cytometer (BD Biosciences) and the analysis software, Flow Jo (Tree Star). ΔMFI (mean fluorescence intensity) was calculated by subtracting the geometric mean fluorescence of the samples stained with an isotype matched negative control antibody from that of the samples stained with specific antibodies. ΔMFI fold increases were calculated by the formula: ΔMFI fold increase = ΔMFI of the non-stimulated cell sample/ΔMFI of the stimulated cell samples.
Western blotting
Eosinophils were lysed in lysis buffer: Tris buffer (10 mM, pH 7.4) containing 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 10% glycerol, 1 mM PMSF, protease inhibitors mixture (Sigma-Aldrich), and phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich). Samples were loaded on 10% Bis-Tris gels (Invitrogen, Life Technologies, Grand Island, NY, USA) under denaturing conditions. Gels were transferred to PVDF membranes (Pierce Biotechnology, Rockford, IL, USA), blocked overnight with 5% non-fat dry milk (Bio-Rad, Life Science Research, Hercules, CA, USA), and probed with rabbit anti-phospho-ERK antibodies that detect dually phosphorylated Thr-202 and Tyr-204 (1:2000; Cell Signaling Technology, Inc., Danvers, MA USA), followed by anti-rabbit secondary antibodies conjugated to HRP (1:5,000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). Membranes were developed with SuperSignal West Femto chemiluminescence kits (Pierce Biotechnology). After membrane striping, membranes were re-blocked, and re-probed with rabbit anti-ERK antibodies (1:2000; Cell Signaling), as a load control.
Analyses of integrin expression by flow cytometry
Mouse eosinophils were stimulated at 37°C for 15 min with 100 ng/ml murine CCL11 in OVA-RPMI medium and placed on ice. Cells were blocked with mouse FcγIII/II receptor blocking antibodies (BD Biosciences). Antibodies against murine β1 and β2 integrins (Santa Cruz Biotechnology Inc, see above), or the 9EG antibody (BD Biosciences) against the β1 activation epitope, or isotype controls (BioLegend) were added to the cell suspension for further 30 min incubation on ice, followed by Alexa488-conjugated secondary antibody (Molecular Probes, Life Technologies). Data were acquired by flow cytometry and analyzed as detailed above. Percent fluorescence intensities were calculated by the formula: % fluorescence intensity = 100 × (ΔMFI of the stimulated cell sample/ΔMFI of the non-stimulated cell samples).
Statistical analysis
Levels of significance between groups were analyzed by two-tailed paired Student’s t tests. P values <0.05 were considered statistically significant. Asterisks represent P values <0.05 (*), or <0.01 (**).
Results
EARs are released from CCL11- and CCL24-stimulated mouse eosinophils
The chemokine CCL11, a chemoattractant and stimulator of eosinophils that express its receptor, CCR3, can induce mouse eosinophils to secrete preformed EARs, EPO, MBP and β-hexosaminidase (11). CCL24 (eotaxin-2) shares only 38.9% sequence identity with mouse CCL11 (18), but binds the same G protein-coupled receptor, CCR3. Although CCL24 induces migration of mouse eosinophils, it is not clear if CCL24 can elicit EAR or other granule protein secretion from mouse eosinophils. Therefore, we studied the capability of CCL24 to induce EAR secretion. Using RNase activity assay to measure the presence of EARs in supernatants, we found that CCL24-stimulated mouse eosinophils (1 h, 37°C) showed significant increase in extracellular mEAR secretion in a dose dependent manner (Fig. S1A). The increase of 1.5-fold at the lowest CCL24 concentration of 10 ng/ml (1.2 nM) and 2.5-fold at the higher CCL24 concentration of 100 ng/ml (11.9 nM) over that of non-stimulated cells was comparable to CCL11-mediated EAR secretion (Fig. S1B) (1.5-fold increase with 10 ng/ml and 1.8-fold with 100 ng/ml). This chemokine-mediated EAR secretion was not cytotoxic for eosinophils (>93% alive). Both mouse CCL11 and CCL24 had comparable potencies to stimulate mouse eosinophils (Fig. 1).
Chemokine-mediated secretion of mEARs is CCR3- and Gαi-dependent
As expected, pretreatment of mouse eosinophils with the highly potent and selective CCR3 antagonist, SB 328437, which acts as a competitive inhibitor of eotaxins (19), decreased CCL11- and CCL24-mediated secretion of mEARs, as measured by RNase activity (Fig. 2A). The CCR3 chemokine receptor belongs to the G-protein coupled receptor family that can exert its downstream signaling via Gα proteins. The Gαi inhibitor, pertussis toxin, also decreased CCL11- and CCL24-mediated secretion of mEARs (Fig. 2B). CCR3-initiated signaling accords with earlier observations (20) that Gαi is involved in CCR3-mediated migration of human eosinophils.
Figure 2. Chemokine-mediated mEAR secretion is CCR3- and Gαi-dependent.
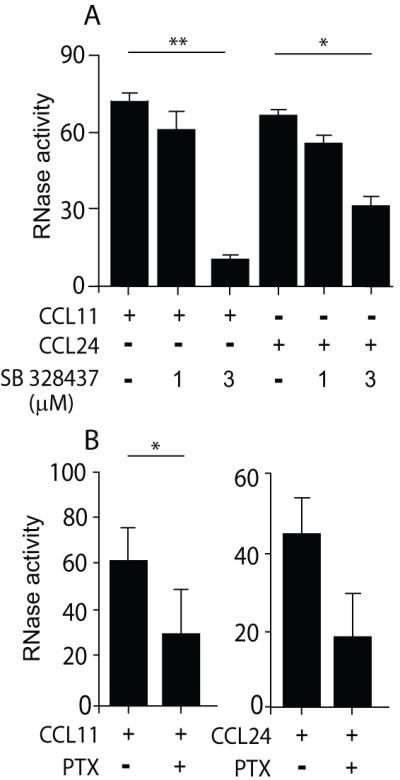
Mouse eosinophils (2 × 106) were stimulated with 100 ng/ml of CCL11 or CCL24 in the presence of the CCR3 antagonist, SB 328437 (A), or pertussis toxin (PTX) (B) as described in Material and methods. RNase activity was measured in supernatants. Data are means ± SD from duplicate wells and are representative of three independent experiments. P values are compared between eosinophils stimulated with and without inhibitors. Asterisks represent P values < 0.05 (*), or < 0.01 (**).
PI3K is involved in CCR3-mediated secretion of mEARs
To further investigate the signaling pathway involved in chemokine-mediated secretion of mEARs, we examined the downstream effector of G-protein-coupled receptor signaling, PI3K (21). It was previously shown in human eosinophils that survival and migration in vivo as well as toll-like receptor-mediated activation (22, 23) require the activity of PI3K. However, in vitro CCL11-mediated human eosinophil chemotaxis and PAF-induced leukotriene C4 secretion (24) were found to be PI3K independent. In a different study CCL11-mediated cytokine secretion from human eosinophils was found to be PI3K dependent (25). However, little is known about the involvement of PI3K in chemokine-mediated degranulation and EAR secretion in both human and mouse eosinophils. Therefore, we sought to delineate the involvement of PI3K in both CCL11-stimulated human and mouse eosinophils. Using a specific PI3K inhibitor, LY294002, we found both CCL11- and CCL24-mediated secretion of mEARs from mouse eosinophils to be PI3K-dependent (Fig. 3A, B) in a dose dependent manner as shown by at least a 5-fold decrease in mEAR secretion. CCL11 stimulation elicited RNase secretion also from human eosinophils, as detected by RNase activity assay (Fig. S2A) and was further confirmed by measuring the presence of human ECP in cell supernatants by ELISA assay (Fig. S2B). Measuring human EAR secretion in the presence of LY294002 by RNase activity assay also revealed that EAR secretion from human eosinophils was PI3K-dependent (Fig. S2A). We further confirmed these results using a different PI3K inhibitor, wortmannin, demonstrating that RNase secretion from both mouse and human eosinophils was inhibited in the presence of wortmannin (data not shown). These results indicate that, in addition to chemokine-mediated eosinophil migration, PI3K is also involved in chemokine-mediated EAR secretion from both human and mouse eosinophils.
p38 MAPK and ERK are involved in chemokine-stimulated secretion of mEARs
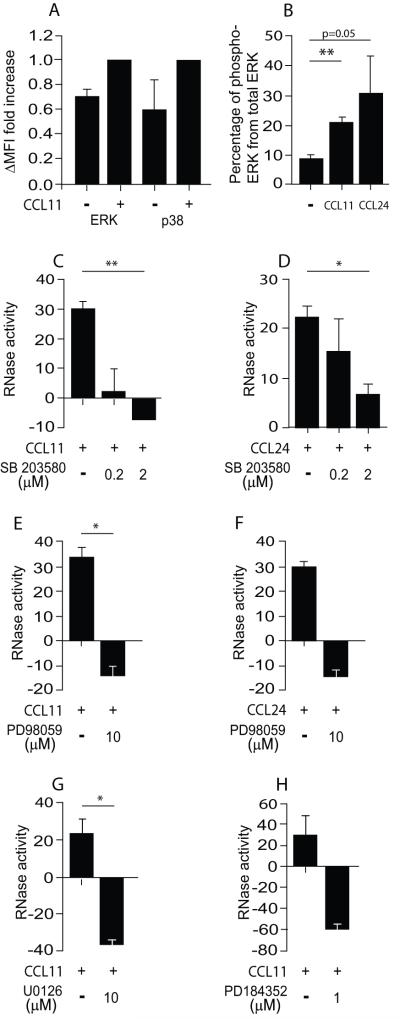
The MAPK kinase pathway is known to be activated upon CCR3 stimulation (10). In order to delineate the involvement of MAPK kinase pathways in mEAR secretion, we briefly stimulated mouse eosinophils with CCL11 (2 min, 37 °C) and evaluated the phosphorylation of both ERK and p38 MAPK by flow cytometry analyses with anti-phospho-ERK and phospho-p38 antibodies. Phosphorylation of both ERK and p38 MAPK was increased ~50% above the basal level (Fig. 4A) in mouse eosinophils. We further confirmed ERK phosphorylation in mouse eosinophils in response to CCL11 or CCL24 using multiplex signaling assays (Fig. 4B).
Figure 4. Chemokine-mediated mEAR secretion from mouse eosinophils is p38 MAPK- and ERK-dependent.
(A) Phosphorylation of ERK and p38 MAPK in CCL11-stimulated mouse eosinophils was measured using flow cytometry. ΔMFI fold increase of phosphorylated ERK or p38 MAPK in cells was calculated as described in Material and methods. Data are means ± SD of two independent experiments. (B) Phosphorylation of ERK in CCL11- or CCL24-stimulated (100 ng/ml) mouse eosinophils was measured using a multiplex signaling assay. Percentage of phosphorylated ERK from total ERK was calculated as described in Material and methods. Data are means ± SD of two independent experiments. P values are compared with non-stimulated eosinophils. (C-F) Mouse eosinophils were stimulated with 100 ng/ml of CCL11 or CCL24 in the presence of the p38 MAPK activation blocker, SB203580 (C, D), or the ERK activation inhibitors, PD98059 (E, F), PD184352 (G) and U0126 (H), as described in Material and methods. RNase activity was measured in supernatants. Data are means ± SD from triplicate wells and are representative of two-three independent experiments. P values are compared between eosinophils stimulated with and without inhibitors. Asterisks represent P values < 0.05 (*), or < 0.01 (**).
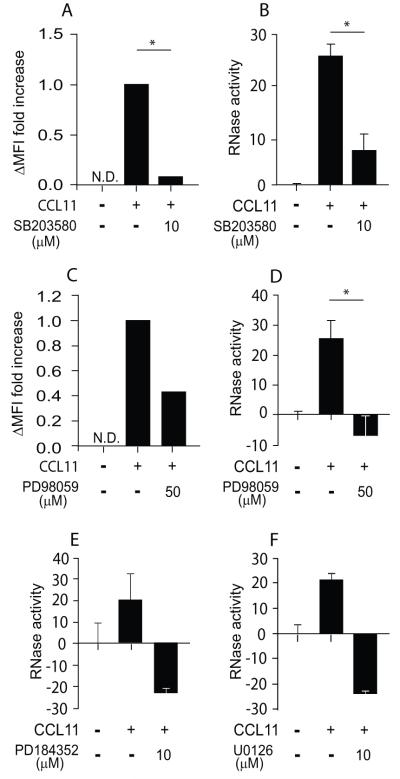
Using the p38 MAPK specific inhibitor, SB203580, we found significant blockage of both CCL11- and CCL24-elicited mEAR secretion from mouse eosinophils (Fig. 4C, D). Moreover, SB203580 affected the basal levels of secretion in the absence of chemokine as indicated by the negative values of inhibited cells (Fig. 4C, D). To evaluate ERK involvement, we blocked the upstream MAP ERK kinase (MEK1) that activates ERK by phosphorylation of tyrosine and threonine residues on ERK. Pretreatment of mouse eosinophils with the MEK1 inhibitor PD98059 inhibited ERK phosphorylation as detected by flow cytometry (Fig. S3 A) and Western blotting (Fig. S3 B) analyses. In the presence of PD98059, RNase secretion from mouse eosinophils was inhibited in response to CCL11 or CCL24 (Fig. 4 E-F). We confirmed these results with different MEK1 inhibitors, U0126 and the very potent and specific MEK1 inhibitor, PD184352. Functional concentrations that blocked ERK phosphorylation, as evaluated by Western blotting analysis (Fig. S3 B) and flow cytometry (Fig. S3 C), also inhibited CCL11-mediated EAR secretion from mouse eosinophils (Fig. 4 G-H). Therefore, ERK and p38 MAPK are required for CCR3-mediated secretion in mouse eosinophils.
Since the RNase assay allows us to detect the effect of inhibitors on secretion from mouse and human eosinophils, we further delineated the involvement of ERK in EAR secretion in human eosinophils. Similar to mouse eosinophils, the p38 MAPK inhibitor, SB203580, inhibited both the CCL11-mediated p38 MAPK phosphorylation (Fig. 5A) and CCL11-mediated hEAR secretion (Fig. 5B). The ERK inhibitor, PD98059, which decreased ERK phosphorylation evaluated by Western blotting analysis (Fig. S4A) and flow cytometry (Fig. 5C), further decreased hEAR secretion from human eosinophils (Fig. 5D). In addition, we further confirmed the requirement of ERK for EAR secretion using other ERK inhibitors, PD184352, and U0126 (Fig. S4A and Fig. 5E,F). Collectively, these results suggest that p38 MAPK and ERK are involved in CCR3-mediated EAR secretion in both mouse and human eosinophils.
Figure 5. Chemokine-mediated hEAR secretion from human eosinophils is p38 MAPK- and ERK-dependent.
(A, C) Phosphorylation of ERK and p38 MAPK in human eosinophils in response to CCL11 in the presence of p38 MAPK inhibitor, SB203580 (A), or ERK activation inhibitor, PD98059 (C), was measured using flow cytometry. ΔMFI fold increase of phosphorylated ERK in non-stimulated cells over stimulated cells was calculated as described in Material and methods. Data are representative of two (A, C) independent experiments. (B, D, E-F) Human eosinophils were stimulated with 300 ng/ml of CCL11 in the presence of the p38 MAPK activation blocker, SB203580 (B), or the ERK activation inhibitors, PD98059 (D), PD184352 (E) and U0126 (F), as described in Material and methods. RNase activity was measured in supernatants. Data are means ± SD from triplicate wells and are representative of five (B) and two-four (D-F) independent experiments. P values are compared between eosinophils stimulated with and without inhibitors. Asterisks represent P values < 0.05 (*), or < 0.01 (**).
CCL11-mediated EAR secretion requires intact integrins
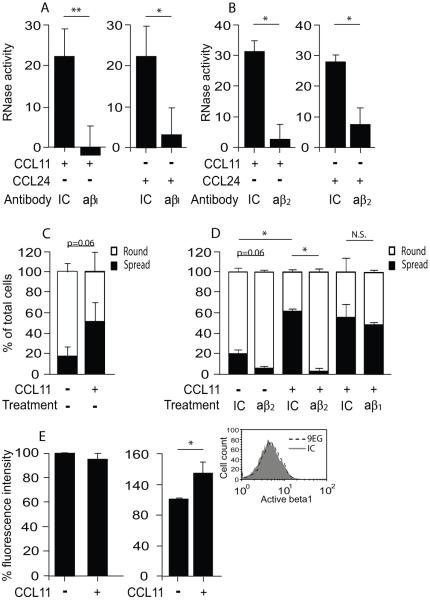
It was previously shown that functional β2 integrins are required for cytokine-mediated hEAR secretion (26), but such had not been evaluated for mouse eosinophils. Moreover, the involvement of β1 integrins in secretion from human eosinophils is still controversial (27-29), dependent on the stimulator and the secreted protein that was studied, and has not been studied in mouse eosinophils. Therefore, we blocked the major integrins expressed on mouse eosinophils by using blocking antibodies against the β1- and the β2 integrin chains and examined the effect on the CCR3-mediated secretion of mEARs from mouse eosinophils. These blocker antibodies were shown previously to effectively block the integrins without activating effects (15, 16). Both CCL11- and CCL24-mediated mEAR secretions were blocked by anti-β1 (Fig. 6A) as well as by anti-β2 integrin blocking antibodies (Fig. 6B), confirming that the secretion of mEARs requires functional β1 and 2 integrins. In order to understand the mechanism by which integrins affect the CCR3-mediated secretion, we examined cell adhesion and spreading in response to CCL11 and the effect of anti-integrin antibodies on adhesion and spreading. Our light microscopy observations showed an increase in cell adhesion in the presence of CCL11 (15 min) by 1.4 fold (± 0.2 SD, n=3), compared to non-stimulated mouse eosinophils. Moreover, cell spreading was also significantly increased by 2.8 fold in response to 15 min stimulation with CCL11 (Fig. 6C).
Figure 6. CCL11- and CCL24-mediated mEAR secretion from mouse eosinophils requires functional integrins.
Mouse eosinophils were stimulated with 100 ng/ml CCL11 or CCL24 in the presence of anti-β1 integrin (aβ1) blocking antibodies (A), anti-β2 integrin (aβ2) blocking antibodies (B) or isotype controls (IC). Data are means ± SD from duplicate wells and are representative of five (A) and four (B) independent experiments, respectively. P values are compared with stimulated, non-inhibited eosinophils. (C) Spreading of mouse eosinophils stimulated with 100 ng/ml CCL11was evaluated by microscopy. Numbers of round cells (white bars) vs. spread cells (black bars) are expressed as percentages of total adherent cells. Data are means ± SD of cells from two random fields from two-three independent experiments. P values of spread or round stimulated-eosinophils are compared with non-stimulated cells. (D) Spreading of mouse eosinophils stimulated with 100 ng/ml CCL11 in the presence of β1 and β2 integrin blocking antibodies (aβ1 or aβ2) or isotype control (IC) was evaluated by microscopy. Numbers of round cells (white bars) vs. spread cells (black bars) are expressed as percentages of total adherent cells. Data are means ± SD of cells from two random fields and are representative of two independent experiments. P values of spread or round stimulated-cells are compared with non-stimulated eosinophils, or of antibody-treated spread cells compared with isotype control treated eosinophils (D). (E) Surface expression of β1 integrins (left panel) or β2 integrins (right panel) in mouse eosinophils stimulated with 100 ng/ml CCL11 for 15 min. Surface expression of integrins was detected by flow cytometry using anti-β1 (left) or anti-β2 integrins (right) or isotope control (IC) antibodies. Percentage of integrin surface expression from basal expression found in non-stimulated cells was calculated as described in Material and methods. Data are means ± SD of three independent experiments. Inset: expression of the activation epitope in β1 integrins (9EG) or isotype control (IC) in the presence of CCL11 as above. Asterisks represent P values < 0.05 (*), or < 0.01 (**). N.S. stands for non-significant.
Interestingly, anti-β2 antibodies did not affect CCL11-mediated mouse eosinophil adhesion (1.1± 0.4 fold decrease with anti-β2 vs. isotype control (IC), n=3). However, anti-β2 antibodies inhibited cell spreading in both CCL11-stimulated and non-stimulated cells (Fig. 6D and Fig. S5A). Moreover, anti-β1 antibodies did not decrease CCL11-mediated mouse eosinophil adhesion (1.0± 0.05 fold decrease with anti-β1 vs. IC, n=2) or cell spreading (Fig. 6D and Fig. S5A). These results suggest that β2-mediated cell spreading may be required for mEAR secretion from mouse eosinophils.
We next examined how CCR3-mediated signaling contributes to this cell spreading by determining surface levels of integrins. Surface expression levels of β2, but not of β1, integrins on mouse eosinophils were significantly increased by ~30%, as measured by flow cytometry after 15 min stimulation with CCL11 (Fig. 6E). Measuring the β1 integrin activation epitope using the 9EG antibody (30) revealed negligible expression of this activation epitope in the absence or presence of CCL11 (Fig. 6E, Inset). Due to the lack of specific antibodies recognizing activation epitopes on murine β2 integrins, we could not evaluate whether a β2 integrin activation state is increased in response to CCL11.
Integrins have the capacity to signal “outside-in” and potentially can affect EAR secretion. Therefore, we measured the effect of β2 integrin blockage on ERK and p38 MAPK phosphorylation. Interestingly, CCL11-induced phosphorylations of p38 and ERK, in the presence of β2 integrin blocking antibodies, were not affected by this treatment (Fig. S5B,C). These results suggest that if integrin-mediated signaling occurs it was not via ERK and p38 MAPK pathways, since blocking of β2 integrins did not inhibit CCL11-mediated ERK or p38 MAPK phosphorylation.
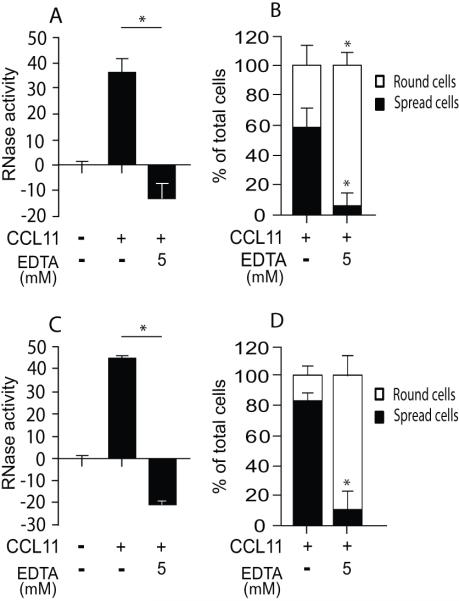
Divalent cations are essential for integrin functions, by stabilizing integrin structure and modulating integrin-ligand binding. The removal of divalent cations by EDTA inhibits integrin-ligand binding. To further confirm the necessity of integrin-mediated spreading, we pretreated mouse eosinophils with 5 mM EDTA to block integrin-mediated function and found a remarkable decrease in RNase secretion (Fig. 7A) together with inhibition of spreading (Fig. 7B). Adhesion (number of adherent eosinophils after washing) was not affected by EDTA (120±11.3 cells for CCL11-stimulated cells vs. 122±10 cells for EDTA and CCL11-treated cells). RNase secretion from human eosinophils was similarly robustly decreased by EDTA pretreatment (Fig. 7C), suggesting that both human and mouse eosinophils are required to spread via their integrins in order to release their EARs.
Figure 7. CCL11-mediated EAR secretion is inhibited in the presence of EDTA.
EAR secretion from mouse eosinophils (A) and human eosinophils (C) stimulated with CCL11 (100 ng/ml or 300 ng/ml, respectively) in the presence of 5 mM EDTA. Data are means ± SD from duplicate wells and are representative of two (A) and three (C) independent experiments, respectively. P values (< 0.05 (*) are compared with stimulated, non-inhibited eosinophils. (B, D) Spreading of mouse eosinophils (B) and human eosinophils (D) stimulated with CCL11 (100 ng/ml or 300 ng/ml, respectively) in the presence of 5 mM EDTA. The numbers of round cells (white bars) versus spread cells (black bars) is expressed as percentages of total adherent cells. Data are means ± SD of cells from two random fields from two- independent experiments. Asterisks represent P<0.05 of spread or round cells after EDTA pretreatment and CCL11 stimulation compared with stimulated but not EDTA-treated cells.
In summary, our unique assay to measure eosinophil EAR secretion allowed us to examine the signaling pathways involved in chemokine-mediated EAR secretion in both human and mouse eosinophils. Our results suggest that CCR3-mediated signaling through PI3K, ERK and p38 MAPK induces EAR secretion. In addition, CCR3-mediated signaling induces β2 integrin expression and β2-mediated cell spreading that is required for EAR secretion β1 integrins are also required for EAR secretion, although both β1 expression level and affinity did not increase upon CCL11 stimulation and blocking β1 integrins did not affect cell spreading.
Discussion
Our study identifies mechanisms by which CCL11 and CCL24 induce EAR secretion and reveals specific signaling pathways that are required for CCR3-mediated EAR secretion from mouse and human eosinophils. Upon binding of CCL11 or CCL24 to the CCR3 receptor, G-protein αi subunit is activated as confirmed by our studies with pertussis toxin. Activation of P13K, potentially achieved by binding to the released β-γ-subunits of the GTP-binding protein (31), is further required for the downstream signaling. ERK and p38 MAPK were found to be activated upon CCL11 and CCL24 stimulation and are crucial for EAR secretion in mouse and human eosinophils. The CCL11-initiated signaling leads to an increase in β2 integrin surface expression and cell spreading. As a result of this signal transduction, EARs are probably loaded into secretion vesicles and secreted extracellulary.
The involvement of ERK activation was previously found for increased leukotriene C4 biosynthesis in human eosinophils in response to fMLP (f-Met-Leu-Phe) (32). More specific for CCR3-mediated signaling, Kampen et al. (10) have shown that ERK and p38 MAPK activation are involved in hEAR secretion from human eosinophils in response to CCL11. Our findings are consistent with these earlier studies in human eosinophils and document the common involvement of ERK and p38 in EAR secretion from mouse eosinophils.
The links between adhesion and secretion in human eosinophils have been studied with human eosinophils indicating that β2 integrins are required for ECP secretion from human eosinophils (26, 33, 34). The requirement of β2 integrins for mEAR secretion that we found with mouse eosinophils demonstrates a commonality between mouse and human eosinophils.
Unlike β2 integrins, β1 integrins were previously found not to be required for EDN secretion from leukotriene D4-stimulated human eosinophils (29). Moreover with human eosinophils, blocking the α4 chain of VLA-4 integrins, although reducing eosinophil adhesion, enhanced (35) or had no affect on IL-5- or GM-CSF-mediated EDN secretion (36), but decreased secretion of leukotrienes and EPO (27, 28). With mouse eosinophils our results show robust inhibition of mEAR secretion from mouse cells with blocking of β1 integrins.
Integrins can mediate adhesion as well as “outside-in” signaling that hypothetically can affect EAR secretion. A previous study with human eosinophils has shown that upon integrin ligation a cascade of protein phosphorylation takes place together with secretion of EDN (37, 38). Walsh et al. (39) have shown that β1 integrin-mediated adhesion of human eosinophils to the extracellular matrix protein, laminin, induces autocrine secretion of survival cytokines, such as IL-5 and GM-CSF. Our experimental design could not distinguish whether integrin-mediated spreading or integrin-mediated cell signaling was required for EAR secretion. However, if indeed integrin-mediated signaling occurs it was not via ERK and p38 MAPK, since blocking of β2 integrins did not inhibit CCL11-mediated ERK or p38 MAPK phosphorylation.
Although the necessity of integrins for secretion has been known for a long time, mechanisms by which integrins affect secretion are not clear in human or mouse eosinophils. Our observations, as well as others, showed that in response to chemokines, such as CCL11, the expression level of β2 integrins increased. In our hands, although anti-β2 integrin antibodies blocked CCL11-mediated EAR secretion, they only mildly blocked adhesion. A similar effect was found in the presence of EDTA. This may suggest that additional surface receptors or a portion of integrin receptors not fully blocked by the antibodies or EDTA may be involved in cell adhesion. In contrast to adhesion, we found that cell spreading was blocked by β2 integrins and EDTA; and therefore, we suggest that cell spreading is required for EAR secretion. The observation that blocking β1 or β2 integrins inhibited mEAR secretion, but only β2 surface expression was increased upon CCL11 stimulation, and that spreading was affected only by β2 integrins, suggests that β1 and β2 integrins may contribute differentially to mEARs secretion.
Collectively, our data demonstrated the common involvement of cell-signaling effectors, such as Gαi subunit, PI3K, ERK and p38 MAPK, in CCR3-mediated EAR secretion from both mouse and human eosinophils. In addition, our results showed that the β1 and β2 integrins were required for EAR secretion and propose a mechanism by which integrins affect secretion. These results indicate that eosinophils from both mice and humans utilize similar mechanisms that control the capacities of eosinophils to secrete extracellularly their species specific EARs, a secretory response likely pertinent to anti-microbial host defense, allergy and other immune and homeostatic responses associated with eosinophils.
Supplementary Material
Acknowledgments
We thank Drs. Lisa A. Spencer and Ionita Ghiran for editorial assistance.
This study was funded by grants from NIH: R37 AI020241, R01 AI051645.
Abbreviations
- EARs
eosinophil-associated RNases
- ECP
eosinophilic cationic protein
- EDN
eosinophil-derived neurotoxin
- EPO
eosinophil peroxidase
- hEARs
human eosinophil-associated RNases
- IC
isotype control
- MBP
major basic protein
- MEK1
MAP ERK kinase
- mEARs
mouse eosinophil-associated RNases
- PI3K
Phosphoinositide 3-kinase
- PTX
pertussis toxin
- PVM
pneumonia virus of mice
- RSV
respiratory syncytial virus
Footnotes
Author contributions
R.S. designed and performed research, analyzed data and wrote the paper; K.M.Y. assisted in performing experiments; P.F.W. designed research and wrote the paper.
Conflict of interest
None of the authors has a conflict of interest respecting the study.
References
- 1.Shamri R, Xenakis JJ, Spencer LA. Eosinophils in innate immunity: an evolving story. Cell. Tissue Res. 2011;343:57–83. doi: 10.1007/s00441-010-1049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JJ, Lee NA. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin. Exp. Allergy. 2005;35:986–94. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- 3.Gupta SK, Haigh BJ, Griffin FJ, Wheeler TT. The mammalian secreted RNases: Mechanisms of action in host defence. Innate Immun. 19:86–97. doi: 10.1177/1753425912446955. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J. Leukoc. Biol. 2001;70:691–8. [PubMed] [Google Scholar]
- 5.McLaren DJ, McKean JR, Olsson I, Venges P, Kay AB. Morphological studies on the killing of schistosomula of Schistosoma mansoni by human eosinophil and neutrophil cationic proteins in vitro. Parasite. Immunol. 1981;3:359–73. doi: 10.1111/j.1365-3024.1981.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 6.Ackerman SJ, Gleich GJ, Loegering DA, Richardson BA, Butterworth AE. Comparative toxicity of purified human eosinophil granule cationic proteins for schistosomula of Schistosoma mansoni. Am. J. Trop. Med. Hyg. 1985;34:45. doi: 10.4269/ajtmh.1985.34.735. [DOI] [PubMed] [Google Scholar]
- 7.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008;14:949–53. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 8.Bystrom J, Amin K, Bishop-Bailey D. Analysing the eosinophil cationic protein-a clue to the function of the eosinophil granulocyte. Respir. Res. 2011;12:10. doi: 10.1186/1465-9921-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Shazly A, Masuyama K, Nakano K, Eura M, Samejima Y, Ishikawa T. Human eotaxin induces eosinophil-derived neurotoxin release from normal human eosinophils. Int. Arch. Allergy Immunol. 1998;117(Suppl 1):55–8. doi: 10.1159/000053573. [DOI] [PubMed] [Google Scholar]
- 10.Kampen GT, Stafford S, Adachi T, Jinquan T, Quan S, Grant JA, et al. Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood. 2000;95:1911–7. [PubMed] [Google Scholar]
- 11.Shamri R, Melo RC, Young KM, Bivas-Benita M, Xenakis JJ, Spencer LA, et al. CCL11 elicits secretion of RNases from mouse eosinophils and their cell-free granules. Faseb J. 2012;26:2084–93. doi: 10.1096/fj.11-200246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J. Exp. Med. 1990;172:1425–31. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J. Immunol. 2007;179:7585–92. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akuthota P, Shamri R, Weller PF. Isolation of human eosinophils. Curr Protoc Immunol. 2012 doi: 10.1002/0471142735.im0731s98. Chapter 7:Unit7 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driessens MH, van Hulten P, Zuurbier A, La Riviere G, Roos E. Inhibition and stimulation of LFA-1 and Mac-1 functions by antibodies against murine CD18. Evidence that the LFA-1 binding sites for ICAM-1, -2, and -3 are distinct. J. Leukoc. Biol. 1996;60:65. doi: 10.1002/jlb.60.6.758. [DOI] [PubMed] [Google Scholar]
- 16.Shamri R, Grabovsky V, Gauguet JM, Feigelson S, Manevich E, Kolanus W, et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat. Immunol. 2005;6:497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- 17.Boix E. Eosinophil cationic protein. Methods Enzymol. 2001;341:287–305. doi: 10.1016/s0076-6879(01)41159-1. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann N, Hogan SP, Mishra A, Brandt EB, Bodette TR, Pope SM, et al. Murine eotaxin-2: a constitutive eosinophil chemokine induced by allergen challenge and IL-4 overexpression. J. Immunol. 2000;165:5839–46. doi: 10.4049/jimmunol.165.10.5839. [DOI] [PubMed] [Google Scholar]
- 19.White JR, Lee JM, Dede K, Imburgia CS, Jurewicz AJ, Chan G, et al. Identification of potent, selective non-peptide CC chemokine receptor-3 antagonist that inhibits eotaxin-, eotaxin-2-, and monocyte chemotactic protein-4-induced eosinophil migration. J. Biol. Chem. 2000;275:36626–31. doi: 10.1074/jbc.M006613200. [DOI] [PubMed] [Google Scholar]
- 20.Boehme SA, Sullivan SK, Crowe PD, Santos M, Conlon PJ, Sriramarao P, et al. Activation of mitogen-activated protein kinase regulates eotaxin-induced eosinophil migration. J. Immunol. 1999;163:1611–8. [PubMed] [Google Scholar]
- 21.Curnock AP, Logan MK, Ward SG. Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases. Immunology. 2002;105:125–36. doi: 10.1046/j.1365-2567.2002.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinho V, Souza DG, Barsante MM, Hamer FP, De Freitas MS, Rossi AG, et al. Phosphoinositide-3 kinases critically regulate the recruitment and survival of eosinophils in vivo: importance for the resolution of allergic inflammation. J. Leukoc. Biol. 2005;77:800–10. doi: 10.1189/jlb.0704386. [DOI] [PubMed] [Google Scholar]
- 23.Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am. J. Respir. Cell Mol. Biol. 2007;37:85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- 24.Mishra RK, Scaife JE, Harb Z, Gray BC, Djukanovic R, Dent G. Differential dependence of eosinophil chemotactic responses on phosphoinositide 3-kinase (PI3K) Allergy. 2005;60:1204–7. doi: 10.1111/j.1398-9995.2005.00845.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamamura K, Adachi T, Masuda T, Kojima Y, Hara A, Toda T, et al. Intracellular protein phosphorylation in eosinophils and the functional relevance in cytokine production. Int. Arch. Allergy Immunol. 2009;149(Suppl 1):45–50. doi: 10.1159/000210653. [DOI] [PubMed] [Google Scholar]
- 26.Horie S, Kita H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony-stimulating factor and platelet-activating factor. J. Immunol. 1994;152:5457–67. [PubMed] [Google Scholar]
- 27.Anwar AR, Walsh GM, Cromwell O, Kay AB, Wardlaw AJ. Adhesion to fibronectin primes eosinophils via alpha 4 beta 1 (VLA-4) Immunology. 1994;82:222–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Munoz NM, Hamann KJ, Rabe KF, Sano H, Zhu X, Leff AR. Augmentation of eosinophil degranulation and LTC(4) secretion by integrin-mediated endothelial cell adhesion. Am. J. Physiol. 1999;277:L802–10. doi: 10.1152/ajplung.1999.277.4.L802. [DOI] [PubMed] [Google Scholar]
- 29.Saito K, Nagata M, Kikuchi I, Sakamoto Y. Leukotriene D4 and eosinophil transendothelial migration, superoxide generation, and degranulation via beta2 integrin. Ann. Allergy Asthma Immunol. 2004;93:600. doi: 10.1016/S1081-1206(10)61269-0. [DOI] [PubMed] [Google Scholar]
- 30.Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc. Natl. Acad. Sci. U S A. 1993;90:9051–5. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, et al. Roles of G beta gamma in membrane recruitment and activation of p110 gamma/p101 phosphoinositide 3-kinase gamma. J. Cell Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bates ME, Green VL, Bertics PJ. ERK1 and ERK2 activation by chemotactic factors in human eosinophils is interleukin 5-dependent and contributes to leukotriene C(4) biosynthesis. J. Biol. Chem. 2000;275:10968–75. doi: 10.1074/jbc.275.15.10968. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Hakansson L. Regulation of the release of eosinophil cationic protein by eosinophil adhesion. Clin. Exp. Allergy. 2000;30:794–806. doi: 10.1046/j.1365-2222.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 34.Adamko DJ, Wu Y, Ajamian F, Ilarraza R, Moqbel R, Gleich GJ. The effect of cationic charge on release of eosinophil mediators. J. Allergy Clin. Immunol. 2008;122:383–390. doi: 10.1016/j.jaci.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Sedgwick JB, Jansen KJ, Kennedy JD, Kita H, Busse WW. Effects of the very late adhesion molecule 4 antagonist WAY103 on human peripheral blood eosinophil vascular cell adhesion molecule 1-dependent functions. J. Allergy Clin. Immunol. 2005;116:812–9. doi: 10.1016/j.jaci.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko M, Horie S, Kato M, Gleich GJ, Kita H. A crucial role for beta 2 integrin in the activation of eosinophils stimulated by IgG. J. Immunol. 1995;155:2631–41. [PubMed] [Google Scholar]
- 37.Kato M, Abraham RT, Okada S, Kita H. Ligation of the beta2 integrin triggers activation and degranulation of human eosinophils. Am. J. Respir. Cell Mol. Biol. 1998;18:675–86. doi: 10.1165/ajrcmb.18.5.2885. [DOI] [PubMed] [Google Scholar]
- 38.Kato M, Kimura H, Tachibana A, Motegi Y, Tokuyama K, Kita H, et al. Intracellular signaling pathways in human eosinophil activation: role of a beta2 integrin, alphaMbeta2. Int. Arch. Allergy Immunol. 1999;120(Suppl 1):51–3. doi: 10.1159/000053595. [DOI] [PubMed] [Google Scholar]
- 39.Walsh GM, Wardlaw AJ. Dexamethasone inhibits prolonged survival and autocrine granulocyte-macrophage colony-stimulating factor production by human eosinophils cultured on laminin or tissue fibronectin. J. Allergy Clin. Immunol. 1997;100:208–15. doi: 10.1016/s0091-6749(97)70226-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.